Abstract
Gelatinases, belonging to the matrix metalloproteases, contribute to tissue destruction in inflammatory demyelinating disorders of the central nervous system such as multiple sclerosis. We used experimental autoimmune encephalomyelitis (EAE) as an animal model to evaluate the effect of a hydroxamate matrix metalloprotease inhibitor (GM 6001) on inflammatory demyelination. A single dose of the inhibitor, given intraperitoneally, provided sufficient levels in the cerebrospinal fluid of animals with EAE to induce at least a partial inhibition of the gelatinase activity in the cerebrospinal fluid. When administered daily either from the time of disease induction or from the onset of clinical signs, GM 6001 suppressed the development or reversed clinical EAE in a dose-dependent way, respectively. Animals returned to the same clinical course as the nontreated group after cessation of treatment. Animals treated from the onset of clinical signs had normal permeability of the blood-brain barrier, compared with the enhanced permeability in nontreated animals. These results indicate that matrix metalloprotease inhibition can reverse ongoing EAE. This effect appears to be mediated mainly through restoration of the damaged blood-brain barrier in the inflammatory phase of the disease, since, the degree of demyelination and inflammation did not differ between the treatment groups.
Full text
PDF
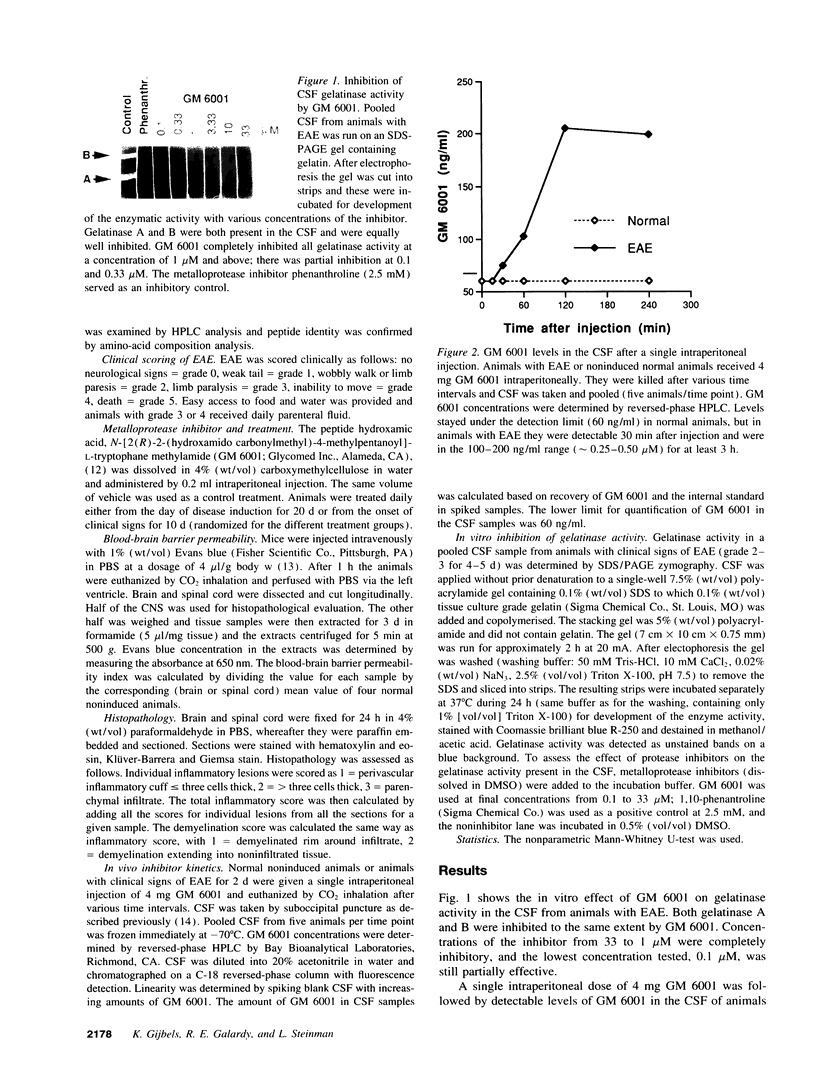




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banik N. L. Pathogenesis of myelin breakdown in demyelinating diseases: role of proteolytic enzymes. Crit Rev Neurobiol. 1992;6(4):257–271. [PubMed] [Google Scholar]
- Boehme D. H., Umezawa H., Hashim G., Marks N. Treatment of experimental allergic encephalomyelitis with an inhibitor of cathepsin D (pepstatin). Neurochem Res. 1978 Apr;3(2):185–194. doi: 10.1007/BF00964059. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Cammer W., Norton W. T., Bloom B. R. Proteinase inhibitors suppress the development of experimental allergic encephalomyelitis. Nature. 1980 May 22;285(5762):235–237. doi: 10.1038/285235a0. [DOI] [PubMed] [Google Scholar]
- Farrell C. L., Stewart P. A., Del Maestro R. F. A new glioma model in rat: the C6 spheroid implantation technique permeability and vascular characterization. J Neurooncol. 1987;4(4):403–415. doi: 10.1007/BF00195612. [DOI] [PubMed] [Google Scholar]
- Gijbels K., Masure S., Carton H., Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992 Nov;41(1):29–34. doi: 10.1016/0165-5728(92)90192-n. [DOI] [PubMed] [Google Scholar]
- Gijbels K., Proost P., Masure S., Carton H., Billiau A., Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993 Nov 1;36(4):432–440. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- Gijbels K., Van Damme J., Proost P., Put W., Carton H., Billiau A. Interleukin 6 production in the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 1990 Jan;20(1):233–235. doi: 10.1002/eji.1830200134. [DOI] [PubMed] [Google Scholar]
- Grobelny D., Poncz L., Galardy R. E. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992 Aug 11;31(31):7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- Inuzuka T., Sato S., Baba H., Miyatake T. Suppressive effect of camostat mesilate (FOY 305) on acute experimental allergic encephalomyelitis (EAE). Neurochem Res. 1988 Mar;13(3):225–228. doi: 10.1007/BF00971537. [DOI] [PubMed] [Google Scholar]
- Kleiner D. E., Jr, Stetler-Stevenson W. G. Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol. 1993 Oct;5(5):891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Hartzler J. L., Pelina M. D., Thorgeirsson U. P. Studies on the ability of 65-kDa and 92-kDa tumor cell gelatinases to degrade type IV collagen. J Biol Chem. 1990 Dec 15;265(35):21929–21934. [PubMed] [Google Scholar]
- McDonald W. I., Miller D. H., Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992 Aug;18(4):319–334. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Atkinson S., Ward R., Gavrilovic J., Reynolds J. J. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann N Y Acad Sci. 1992 Dec 4;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- Rasminsky M. Pathophysiology of demyelination. Ann N Y Acad Sci. 1984;436:68–85. doi: 10.1111/j.1749-6632.1984.tb14776.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. A., Kornfeld M., Estrada E., Kelley R. O., Liotta L. A., Stetler-Stevenson W. G. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res. 1992 Apr 3;576(2):203–207. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., Strelow S., Stern G. A., Chegini N., Grant M. B., Galardy R. E., Grobelny D., Rowsey J. J., Stonecipher K., Parmley V. Treatment of alkali-injured rabbit corneas with a synthetic inhibitor of matrix metalloproteinases. Invest Ophthalmol Vis Sci. 1992 Nov;33(12):3325–3331. [PubMed] [Google Scholar]
- Sibley W. A., Kiernat J., Laguna J. F. The modification of experimental allergic encephalomyelitis with epsilon aminocaproic acid. Neurology. 1978 Sep;28(9 Pt 2):102–105. doi: 10.1212/wnl.28.9_part_2.102. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Amaducci L. A. Observations on the effects of protease inhibitors on the suppression of experimental allergic encephalomyelitis. Neurochem Res. 1982 May;7(5):541–554. doi: 10.1007/BF00965121. [DOI] [PubMed] [Google Scholar]
- Steinman L. Autoimmune disease. Sci Am. 1993 Sep;269(3):106–114. doi: 10.1038/scientificamerican0993-106. [DOI] [PubMed] [Google Scholar]
- Steinman L., Miller A., Bernard C. C., Oksenberg J. R. The epigenetics of multiple sclerosis: clues to etiology and a rationale for immune therapy. Annu Rev Neurosci. 1994;17:247–265. doi: 10.1146/annurev.ne.17.030194.001335. [DOI] [PubMed] [Google Scholar]
- Steinman L. The development of rational strategies for selective immunotherapy against autoimmune demyelinating disease. Adv Immunol. 1991;49:357–379. doi: 10.1016/s0065-2776(08)60779-8. [DOI] [PubMed] [Google Scholar]
- Waxman S. G. Clinical course and electrophysiology of multiple sclerosis. Adv Neurol. 1988;47:157–184. [PubMed] [Google Scholar]
- Youl B. D., Turano G., Miller D. H., Towell A. D., MacManus D. G., Moore S. G., Jones S. J., Barrett G., Kendall B. E., Moseley I. F. The pathophysiology of acute optic neuritis. An association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991 Dec;114(Pt 6):2437–2450. doi: 10.1093/brain/114.6.2437. [DOI] [PubMed] [Google Scholar]



