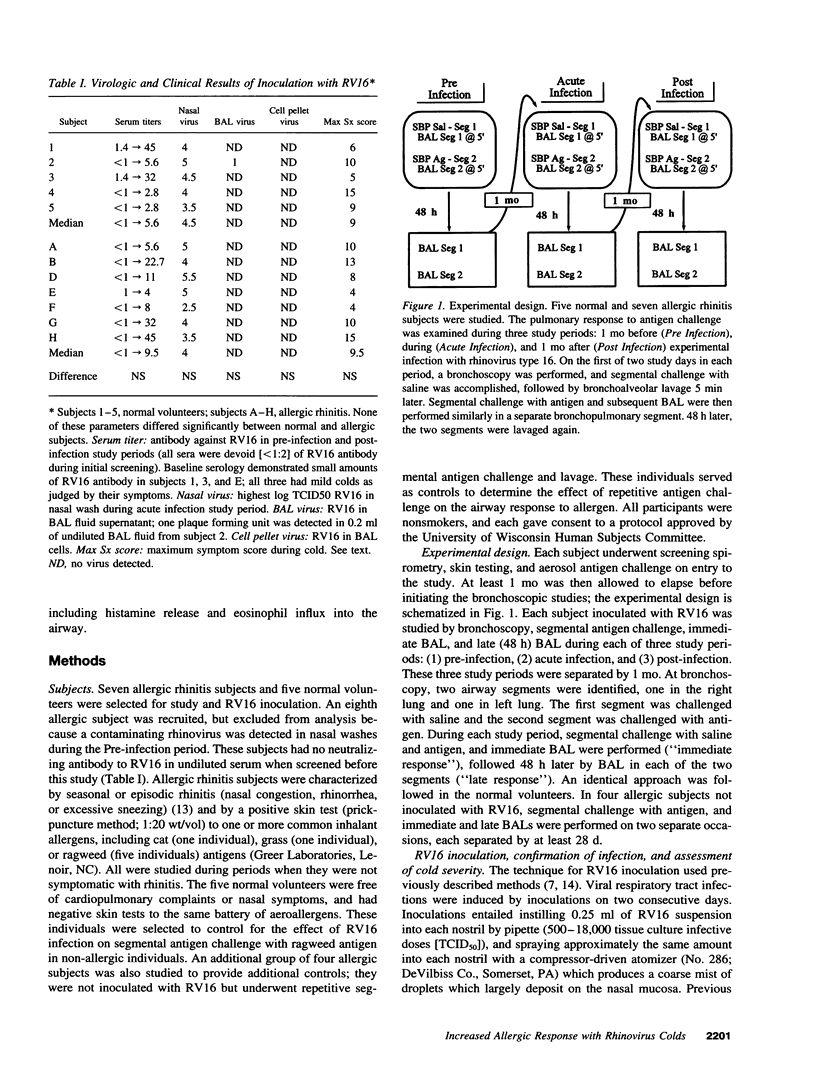
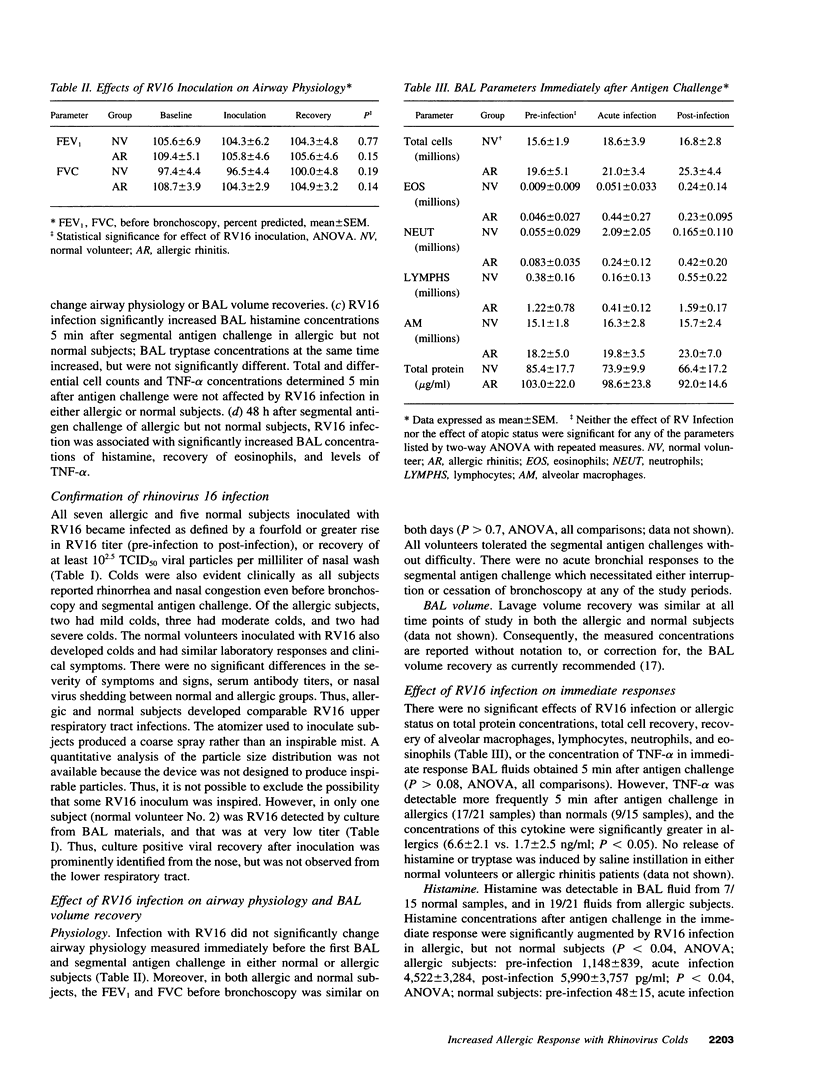
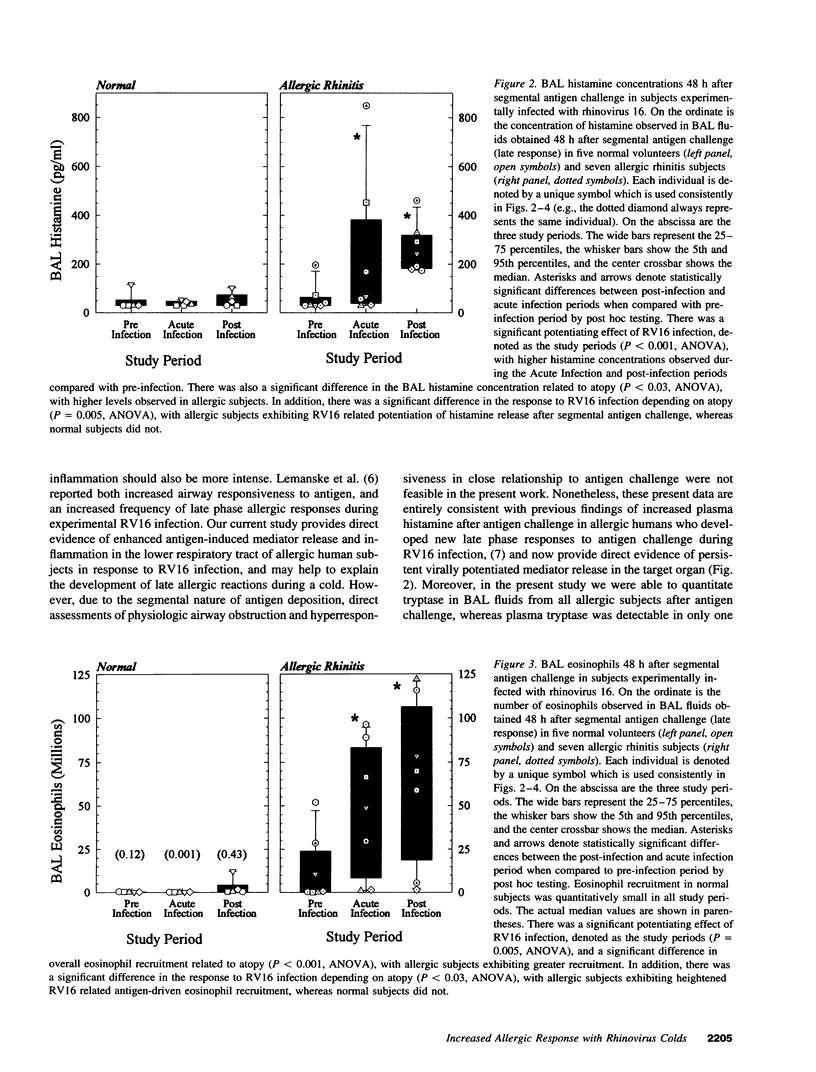
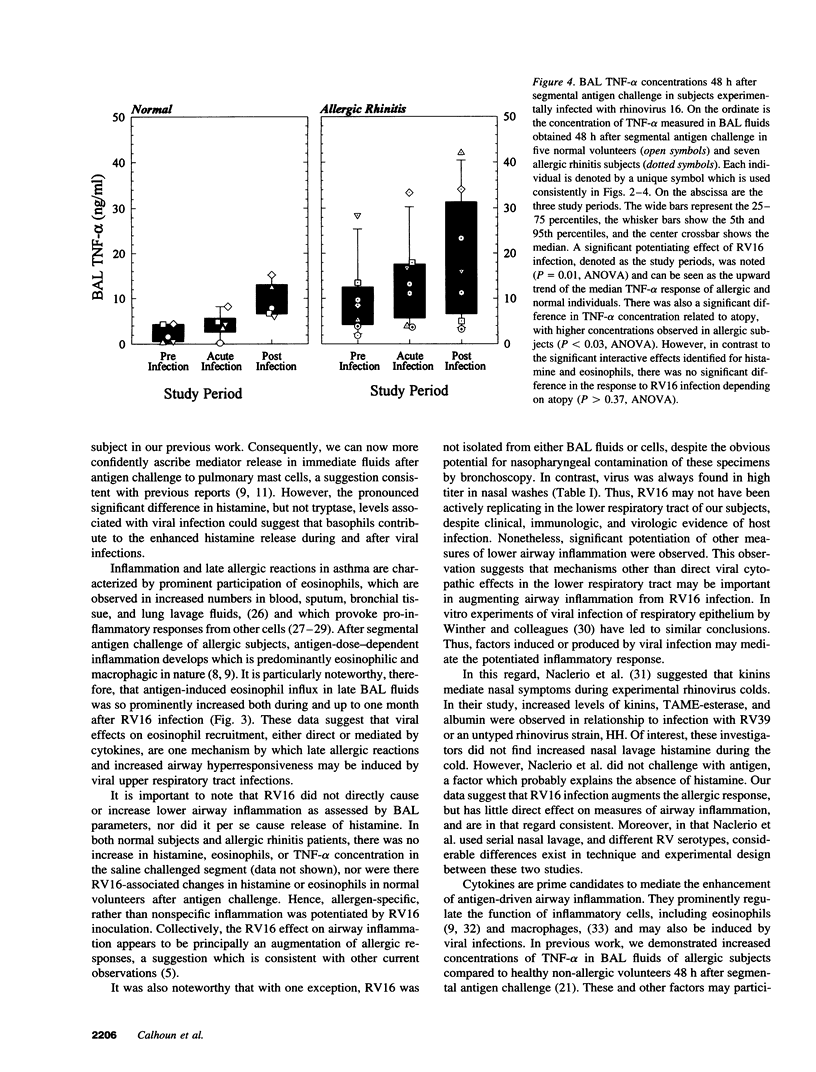
Abstract
Many patients with asthma have increased wheezing with colds. We hypothesized that rhinovirus colds might increase asthma by augmenting airway allergic responses (histamine release and eosinophil influx) after antigen challenge. Seven allergic rhinitis patients and five normal volunteers were infected with rhinovirus type 16 (RV16) and evaluated by segmental bronchoprovocation and bronchoalveolar lavage. Segmental challenge with saline and antigen was performed 1 mo before infection, during the acute infection, and 1 mo after infection. Lavage was performed immediately and 48 h after antigen challenge. Data were analyzed by two-way analysis of variance, and a P value of < or = 0.05 was considered to be significant. All volunteers inoculated with RV16 developed an acute respiratory infection. BAL fluid obtained from allergic rhinitis subjects during the acute viral infection, and 1 mo after infection, showed the following significant RV16-associated changes after antigen challenge: (a) an enhanced release of histamine immediately after local antigen challenge; (b) persistent histamine leak 48 h afterwards; and (c) a greater recruitment of eosinophils to the airway 48 h after challenge. These changes were not seen in non-allergic volunteers infected with RV16 and challenged with antigen, nor in allergic volunteers repetitively challenged with antigen but not infected with RV16, nor in RV16 infected allergic volunteers sham challenged with saline. We conclude that rhinovirus upper respiratory infection significantly augments immediate and late allergic responses in the airways of allergic individuals after local antigen challenge. These data suggest that one mechanism of increased asthma during a cold is an accentuation of allergic responses in the airway which may then contribute to bronchial inflammation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bousquet J., Chanez P., Lacoste J. Y., Barnéon G., Ghavanian N., Enander I., Venge P., Ahlstedt S., Simony-Lafontaine J., Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Bowsher R. R., Verburg K. M., Henry D. P. Rat histamine N-methyltransferase. Quantification, tissue distribution, purification, and immunologic properties. J Biol Chem. 1983 Oct 25;258(20):12215–12220. [PubMed] [Google Scholar]
- Calhoun W. J., Bush R. K. Enhanced reactive oxygen species metabolism of airspace cells and airway inflammation follow antigen challenge in human asthma. J Allergy Clin Immunol. 1990 Sep;86(3 Pt 1):306–313. doi: 10.1016/s0091-6749(05)80092-2. [DOI] [PubMed] [Google Scholar]
- Calhoun W. J., Reed H. E., Moest D. R., Stevens C. A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992 Feb;145(2 Pt 1):317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- Calhoun W. J., Swenson C. A., Dick E. C., Schwartz L. B., Lemanske R. F., Jr, Busse W. W. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1991 Dec;144(6):1267–1273. doi: 10.1164/ajrccm/144.6.1267. [DOI] [PubMed] [Google Scholar]
- Chai H., Farr R. S., Froehlich L. A., Mathison D. A., McLean J. A., Rosenthal R. R., Sheffer A. L., Spector S. L., Townley R. G. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975 Oct;56(4):323–327. doi: 10.1016/0091-6749(75)90107-4. [DOI] [PubMed] [Google Scholar]
- Hall W. J., Hall C. B., Speers D. M. Respiratory syncytial virus infection in adults: clinical, virologic, and serial pulmonary function studies. Ann Intern Med. 1978 Feb;88(2):203–205. doi: 10.7326/0003-4819-88-2-203. [DOI] [PubMed] [Google Scholar]
- Hsia J., Goldstein A. L., Simon G. L., Sztein M., Hayden F. G. Peripheral blood mononuclear cell interleukin-2 and interferon-gamma production, cytotoxicity, and antigen-stimulated blastogenesis during experimental rhinovirus infection. J Infect Dis. 1990 Sep;162(3):591–597. doi: 10.1093/infdis/162.3.591. [DOI] [PubMed] [Google Scholar]
- Lemanske R. F., Jr, Dick E. C., Swenson C. A., Vrtis R. F., Busse W. W. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989 Jan;83(1):1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. C., Hubbard W. C., Proud D., Stealey B. A., Galli S. J., Kagey-Sobotka A., Bleecker E. R., Lichtenstein L. M. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am Rev Respir Dis. 1991 Jul;144(1):51–58. doi: 10.1164/ajrccm/144.1.51. [DOI] [PubMed] [Google Scholar]
- Macy E., Kemeny M., Saxon A. Enhanced ELISA: how to measure less than 10 picograms of a specific protein (immunoglobulin) in less than 8 hours. FASEB J. 1988 Nov;2(14):3003–3009. doi: 10.1096/fasebj.2.14.3263291. [DOI] [PubMed] [Google Scholar]
- Meschievitz C. K., Schultz S. B., Dick E. C. A model for obtaining predictable natural transmission of rhinoviruses in human volunteers. J Infect Dis. 1984 Aug;150(2):195–201. doi: 10.1093/infdis/150.2.195. [DOI] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., DeMeo A. N., Ouellette J. J., Cohen M., Reed C. E. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974 Jan 21;227(3):292–298. [PubMed] [Google Scholar]
- Moy J. N., Gleich G. J., Thomas L. L. Noncytotoxic activation of neutrophils by eosinophil granule major basic protein. Effect on superoxide anion generation and lysosomal enzyme release. J Immunol. 1990 Oct 15;145(8):2626–2632. [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Lichtenstein L. M., Kagey-Sobotka A., Hendley J. O., Sorrentino J., Gwaltney J. M. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988 Jan;157(1):133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- Nadel J. A. Bronchial reactivity. Adv Intern Med. 1983;28:207–223. [PubMed] [Google Scholar]
- Nicholson K. G., Kent J., Ireland D. C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993 Oct 16;307(6910):982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M. C., Ackerman S. J., Gleich G. J., Thomas L. L. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med. 1983 Jun 1;157(6):1981–1991. doi: 10.1084/jem.157.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Kita H., Weiler D., Sur S., Sedgwick J. B., Calhoun W. J., Busse W. W., Abrams J. S., Gleich G. J. IL-5 is the predominant eosinophil-active cytokine in the antigen-induced pulmonary late-phase reaction. Am Rev Respir Dis. 1993 Apr;147(4):901–907. doi: 10.1164/ajrccm/147.4.901. [DOI] [PubMed] [Google Scholar]
- Rankin J. A., Harris P., Ackerman S. J. The effects of eosinophil-granule major basic protein on lung-macrophage superoxide anion generation. J Allergy Clin Immunol. 1992 Mar;89(3):746–752. doi: 10.1016/0091-6749(92)90383-d. [DOI] [PubMed] [Google Scholar]
- Reed C. E. New therapeutic approaches in asthma. J Allergy Clin Immunol. 1986 Apr;77(4):537–543. doi: 10.1016/0091-6749(86)90342-8. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R., Lee D. C., Chlebowski J. F. Immunologic and physicochemical evidence for conformational changes occurring on conversion of human mast cell tryptase from active tetramer to inactive monomer. Production of monoclonal antibodies recognizing active tryptase. J Immunol. 1990 Mar 15;144(6):2304–2311. [PubMed] [Google Scholar]
- Sedgwick J. B., Calhoun W. J., Gleich G. J., Kita H., Abrams J. S., Schwartz L. B., Volovitz B., Ben-Yaakov M., Busse W. W. Immediate and late airway response of allergic rhinitis patients to segmental antigen challenge. Characterization of eosinophil and mast cell mediators. Am Rev Respir Dis. 1991 Dec;144(6):1274–1281. doi: 10.1164/ajrccm/144.6.1274. [DOI] [PubMed] [Google Scholar]
- Silberstein D. S., Austen K. F., Owen W. F., Jr Hemopoietins for eosinophils. Glycoprotein hormones that regulate the development of inflammation in eosinophilia-associated disease. Hematol Oncol Clin North Am. 1989 Sep;3(3):511–533. [PubMed] [Google Scholar]
- Wenzel S. E., Fowler A. A., 3rd, Schwartz L. B. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988 May;137(5):1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- Winther B., Gwaltney J. M., Hendley J. O. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):839–845. doi: 10.1164/ajrccm/141.4_Pt_1.839. [DOI] [PubMed] [Google Scholar]



