Abstract
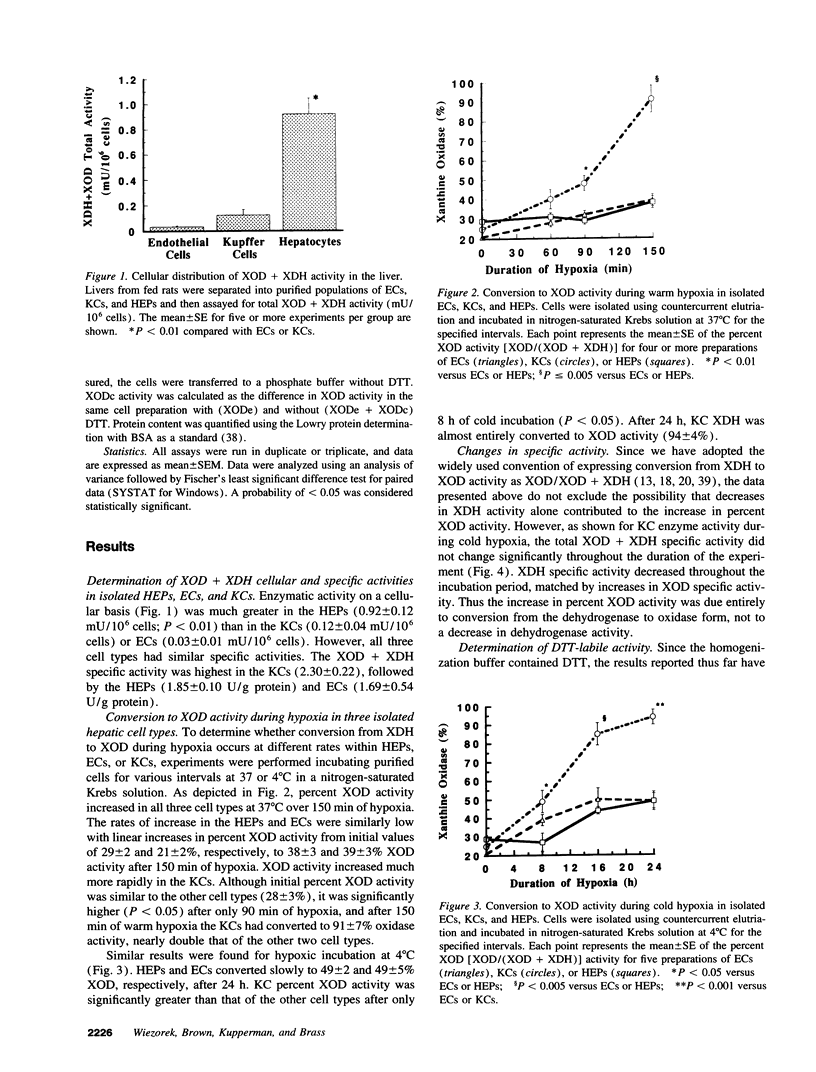
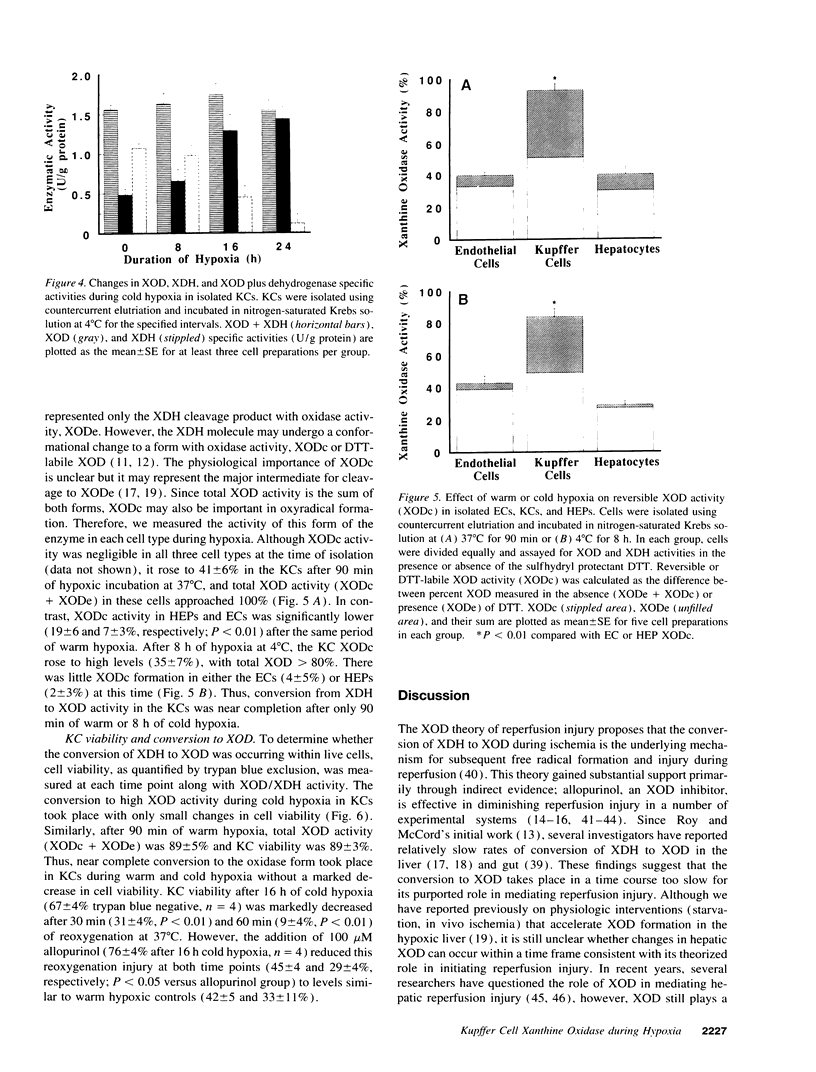
It has been widely postulated that the central mechanism of hepatic reperfusion injury involves the conversion, during ischemia, of the enzyme xanthine dehydrogenase (XDH) to its free radical-producing form, xanthine oxidase (XOD). However, this theory has been questioned because (a) XDH to XOD conversion in whole liver occurs very slowly; (b) the cellular distribution of XDH/XOD is unclear; and (c) the direct demonstration of XDH to XOD conversion in viable cells is lacking. In this paper, we address all three issues by measuring XDH to XOD conversion and cell viability in purified populations of hepatic endothelial cells (EC), Kupffer cells (KC), and hepatocytes (HEP). Although XDH/XOD activity on a cellular basis was greater in hepatocytes (0.92 +/- 0.12 mU/10(6) cells) than ECs (0.03 +/- 0.01) or KCs (0.12 +/- 0.04), XDH + XOD specific activity was similar in all three cell types (HEP 1.85 +/- 0.10 U/g protein; EC 1.69 +/- 0.54; KC 2.30 +/- 0.22). Over 150 min of warm (37 degrees C) or 24 h of cold (4 degrees C) hypoxia, percent XOD activity increased slowly in ECs, from 21 +/- 2% (basal) to 39 +/- 3% (warm) and 49 +/- 5% (cold) and in HEPs (29 +/- 2% to 38 +/- 3% and 49 +/- 2%), but converted significantly faster in KCs (28 +/- 3% to 91 +/- 7% and 94 +/- 4%). The dramatic changes in Kupffer cell XOD during cold hypoxia occurred despite only minor changes in cell viability. When hypoxic KCs were reoxygenated after 16 h of cold hypoxia, there was a marked increase in cell death that was significantly blocked by allopurinol. These data suggest that significant conversion to the free radical-producing state occurs within viable KCs, and that Kupffer cell XOD may play an important role in mediating reperfusion injury in the liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angermüller S., Bruder G., Völkl A., Wesch H., Fahimi H. D. Localization of xanthine oxidase in crystalline cores of peroxisomes. A cytochemical and biochemical study. Eur J Cell Biol. 1987 Dec;45(1):137–144. [PubMed] [Google Scholar]
- Atalla S. L., Toledo-Pereyra L. H., MacKenzie G. H., Cederna J. P. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985 Dec;40(6):584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Auscher C., Amory N. The histochemical localization of xanthine oxidase in the rat liver. Biomedicine. 1976 Feb 10;25(1):37–38. [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Ferrari F. K., Simmons R. L. Evidence that activation of Kupffer cells results in production of L-arginine metabolites that release cell-associated iron and inhibit hepatocyte protein synthesis. Surgery. 1989 Aug;106(2):364–372. [PubMed] [Google Scholar]
- Brass C. A., Narciso J., Gollan J. L. Enhanced activity of the free radical producing enzyme xanthine oxidase in hypoxic rat liver. Regulation and pathophysiologic significance. J Clin Invest. 1991 Feb;87(2):424–431. doi: 10.1172/JCI115013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Terada L. S., Grosso M. A., Whitmann G. J., Velasco S. E., Patt A., Harken A. H., Repine J. E. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest. 1988 Apr;81(4):1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell-Kenkel J. C., Currin R. T., Tanaka Y., Thurman R. G., Lemasters J. J. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991 Jan;13(1):83–95. [PubMed] [Google Scholar]
- Caldwell-Kenkel J. C., Currin R. T., Tanaka Y., Thurman R. G., Lemasters J. J. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology. 1989 Sep;10(3):292–299. doi: 10.1002/hep.1840100307. [DOI] [PubMed] [Google Scholar]
- Clare D. A., Blakistone B. A., Swaisgood H. E., Horton H. R. Sulfhydryl oxidase-catalyzed conversion of xanthine dehydrogenase to xanthine oxidase. Arch Biochem Biophys. 1981 Oct 1;211(1):44–47. doi: 10.1016/0003-9861(81)90427-6. [DOI] [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Girotti A. W., Thomas J. P., Jordan J. E. Xanthine oxidase-catalyzed crosslinking of cell membrane proteins. Arch Biochem Biophys. 1986 Dec;251(2):639–653. doi: 10.1016/0003-9861(86)90374-7. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Kvietys P. R., Perry M. A. Leukocyte--endothelial cell adhesion induced by ischemia and reperfusion. Can J Physiol Pharmacol. 1993 Jan;71(1):67–75. doi: 10.1139/y93-011. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Holloway C. M., Harvey P. R., Strasberg S. M. Viability of sinusoidal lining cells in cold-preserved rat liver allografts. Transplantation. 1990 Jan;49(1):225–229. [PubMed] [Google Scholar]
- Holmes R. S., Vandeberg J. L. Aldehyde dehydrogenases, aldehyde oxidase and xanthine oxidase from baboon tissues: phenotypic variability and subcellular distribution in liver and brain. Alcohol. 1986 May-Jun;3(3):205–214. doi: 10.1016/0741-8329(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Im M. J., Hoopes J. E., Yoshimura Y., Manson P. N., Bulkley G. B. Xanthine:acceptor oxidoreductase activities in ischemic rat skin flaps. J Surg Res. 1989 Mar;46(3):230–234. doi: 10.1016/0022-4804(89)90062-0. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991 Mar;260(3 Pt 1):G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991 Mar;260(3 Pt 1):G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Farhood A., Smith C. W. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990 Dec;4(15):3355–3359. [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide as index of oxidant stress in rat liver during hypoxia. Am J Physiol. 1990 Apr;258(4 Pt 1):G499–G505. doi: 10.1152/ajpgi.1990.258.4.G499. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Smith C. V., Mitchell J. R. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988 Apr;81(4):1240–1246. doi: 10.1172/JCI113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Karwinski W., Drange A., Farstad M., Ulvik R., Søreide O. 60 min normothermic liver ischemia in rats: allopurinol improves energy status and bile flow during reperfusion. Eur Surg Res. 1990;22(1):27–33. doi: 10.1159/000129079. [DOI] [PubMed] [Google Scholar]
- Koo A., Komatsu H., Tao G., Inoue M., Guth P. H., Kaplowitz N. Contribution of no-reflow phenomenon to hepatic injury after ischemia-reperfusion: evidence for a role for superoxide anion. Hepatology. 1992 Mar;15(3):507–514. doi: 10.1002/hep.1840150325. [DOI] [PubMed] [Google Scholar]
- Kooij A., Frederiks W. M., Gossrau R., Van Noorden C. J. Localization of xanthine oxidoreductase activity using the tissue protectant polyvinyl alcohol and final electron acceptor Tetranitro BT. J Histochem Cytochem. 1991 Jan;39(1):87–93. doi: 10.1177/39.1.1983876. [DOI] [PubMed] [Google Scholar]
- Kuiper J., Casteleyn E., Van Berkel T. J. Regulation of liver metabolism by intercellular communication. Adv Enzyme Regul. 1988;27:193–208. doi: 10.1016/0065-2571(88)90017-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemaster J. J., Thurman R. G. Hypoxia and reperfusion injury to liver. Prog Liver Dis. 1993;11:85–114. [PubMed] [Google Scholar]
- Linas S. L., Shanley P. F., Whittenburg D., Berger E., Repine J. E. Neutrophils accentuate ischemia-reperfusion injury in isolated perfused rat kidneys. Am J Physiol. 1988 Oct;255(4 Pt 2):F728–F735. doi: 10.1152/ajprenal.1988.255.4.F728. [DOI] [PubMed] [Google Scholar]
- Marotto M. E., Thurman R. G., Lemasters J. J. Early midzonal cell death during low-flow hypoxia in the isolated, perfused rat liver: protection by allopurinol. Hepatology. 1988 May-Jun;8(3):585–590. doi: 10.1002/hep.1840080325. [DOI] [PubMed] [Google Scholar]
- Marubayashi S., Dohi K., Yamada K., Kawasaki T. Role of conversion of xanthine dehydrogenase to oxidase in ischemic rat liver cell injury. Surgery. 1991 Sep;110(3):537–543. [PubMed] [Google Scholar]
- Marzi I., Cowper K., Takei Y., Lindert K., Lemasters J. J., Thurman R. G. Methyl palmitate prevents Kupffer cell activation and improves survival after orthotopic liver transplantation in the rat. Transpl Int. 1991 Dec;4(4):215–220. doi: 10.1007/BF00649106. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McKelvey T. G., Höllwarth M. E., Granger D. N., Engerson T. D., Landler U., Jones H. P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988 May;254(5 Pt 1):G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- Metzger J., Lauterburg B. H. Effect of allopurinol on oxidant stress and hepatic function following ischemia and reperfusion in the rat. Liver. 1988 Dec;8(6):344–349. doi: 10.1111/j.1600-0676.1988.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Mora N. P., Klintmalm G. B., Cofer J. B., Poplawski S. S., Goldstein R. M., Gonwa T. A., Husberg B. S. Results after liver retransplantation (RETx): a comparative study between "elective" vs "nonelective" RETx. Transplant Proc. 1990 Aug;22(4):1509–1511. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Owens M. L., Lazarus H. M., Wolcott M. W., Maxwell J. G., Taylor J. B. Allopurinol and hypoxanthine pretreatment of canine kidney donors. Transplantation. 1974 Apr;17(4):424–427. doi: 10.1097/00007890-197404000-00015. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Parks D. A., Williams T. K., Beckman J. S. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988 May;254(5 Pt 1):G768–G774. doi: 10.1152/ajpgi.1988.254.5.G768. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratych R. E., Chuknyiska R. S., Bulkley G. B. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987 Aug;102(2):122–131. [PubMed] [Google Scholar]
- Rymsa B., Becker H. D., Lauchart W., de Groot H. Hypoxia/reoxygenation injury in liver: Kupffer cells are much more vulnerable to reoxygenation than to hypoxia. Res Commun Chem Pathol Pharmacol. 1990 May;68(2):263–266. [PubMed] [Google Scholar]
- Rymsa B., Wang J. F., de Groot H. O2-. release by activated Kupffer cells upon hypoxia-reoxygenation. Am J Physiol. 1991 Oct;261(4 Pt 1):G602–G607. doi: 10.1152/ajpgi.1991.261.4.G602. [DOI] [PubMed] [Google Scholar]
- Takei Y., Marzi I., Gao W. S., Gores G. J., Lemasters J. J., Thurman R. G. Leukocyte adhesion and cell death following orthotopic liver transplantation in the rat. Transplantation. 1991 May;51(5):959–965. doi: 10.1097/00007890-199105000-00005. [DOI] [PubMed] [Google Scholar]
- Walsh T. R., Rao P. N., Makowka L., Starzl T. E. Lipid peroxidation is a nonparenchymal cell event with reperfusion after prolonged liver ischemia. J Surg Res. 1990 Jul;49(1):18–22. doi: 10.1016/0022-4804(90)90104-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976 Feb;172(2):354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Cotran R. S., Fahimi H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972 Jan;52(1):159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Lemasters J. J., Thurman R. G. Role of purines and xanthine oxidase in reperfusion injury in perfused rat liver. J Pharmacol Exp Ther. 1989 Aug;250(2):470–475. [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkel T. J., Koster J. F., Hülsmann W. C. Distribution of L- and M-type pyruvate kinase between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1972 Aug 28;276(2):425–429. doi: 10.1016/0005-2744(72)91003-0. [DOI] [PubMed] [Google Scholar]



