Abstract
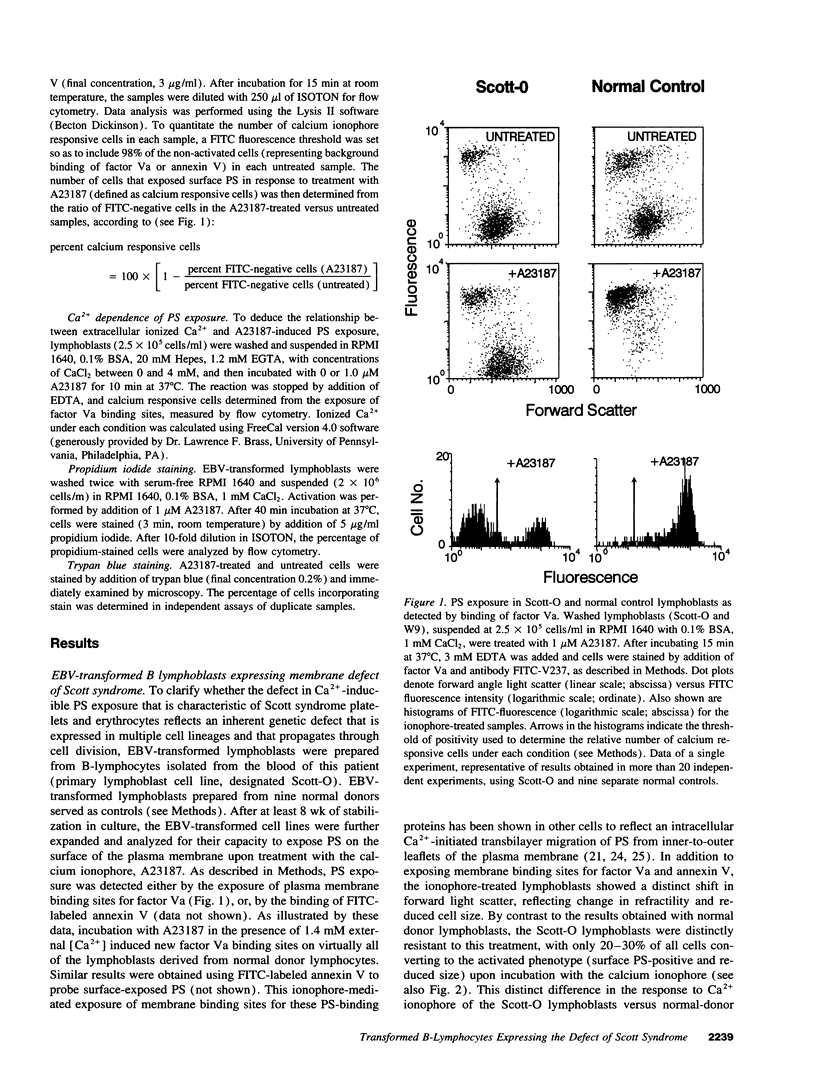
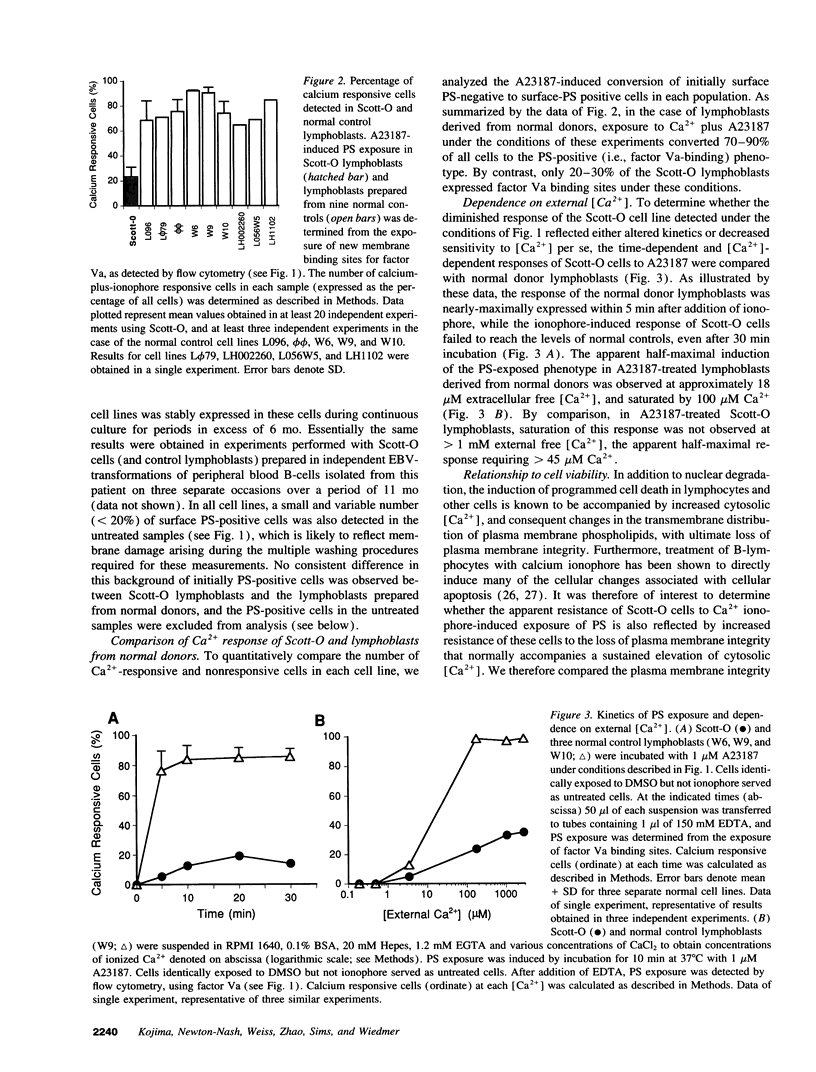
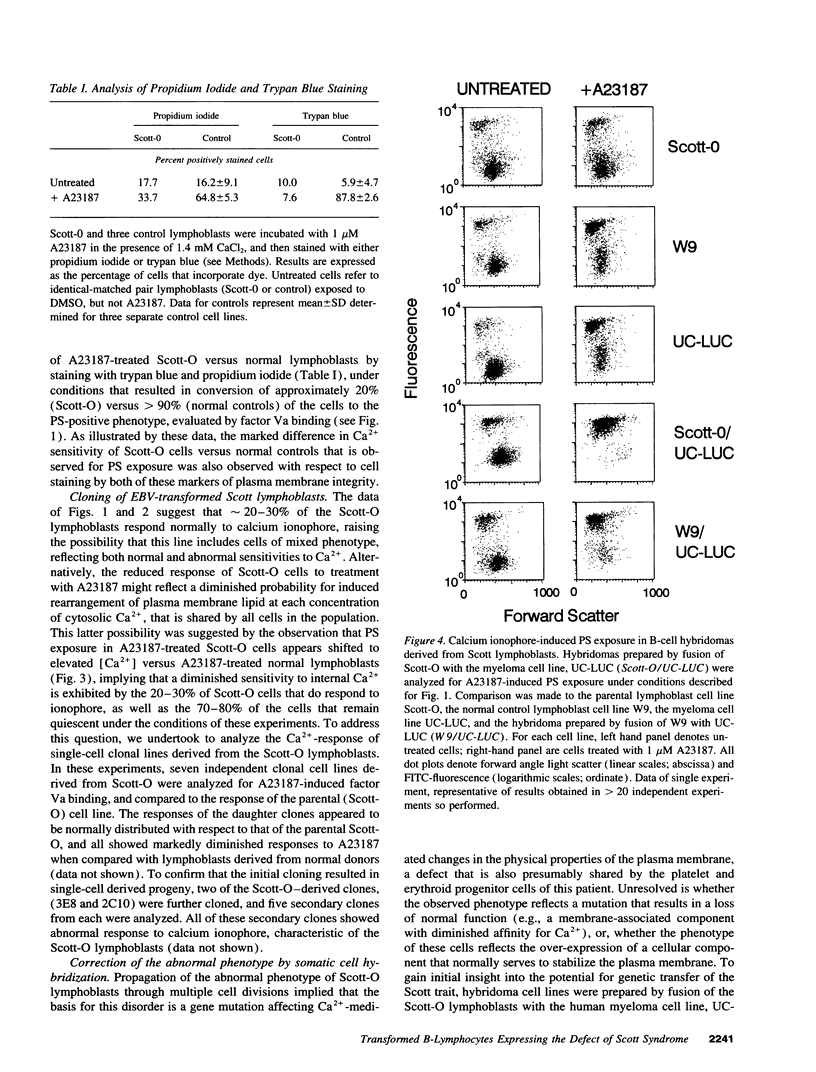
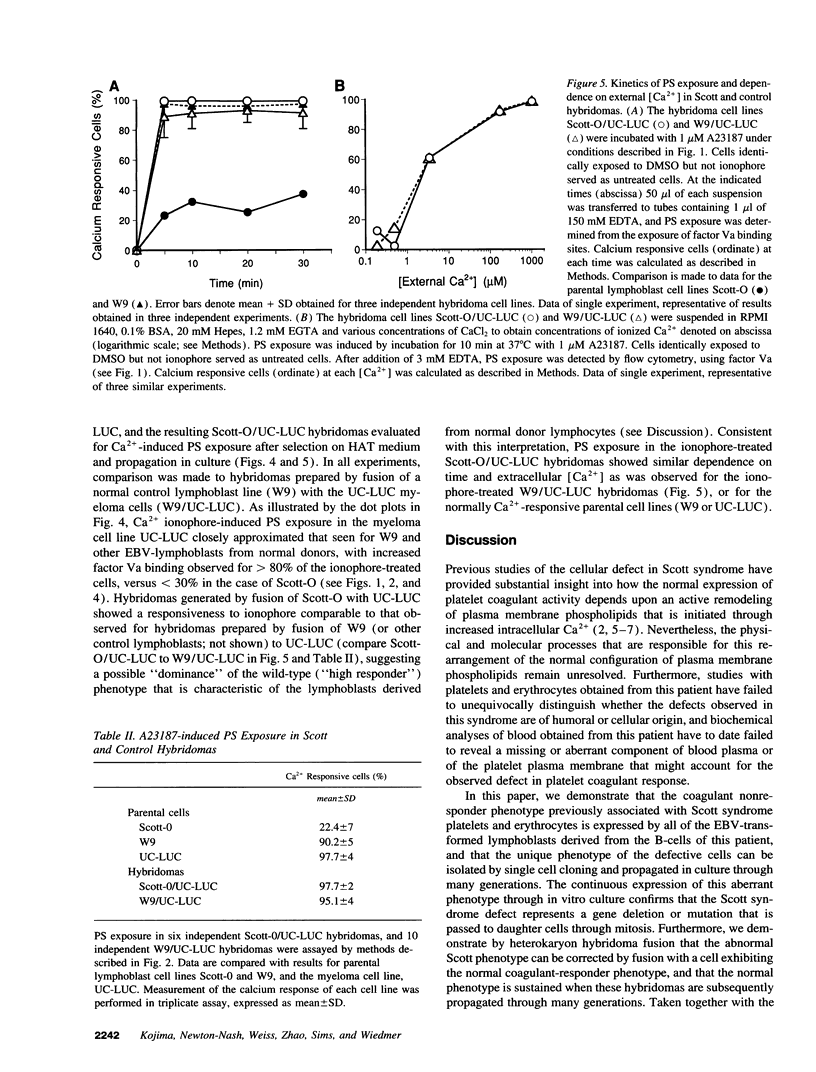
Scott syndrome is a bleeding disorder associated with an isolated defect in expression of membrane coagulant activity by stimulated platelets. This defect represents a decrease in platelet membrane binding sites for coagulation factors Va and VIIIa, reflecting diminished surface exposure of phosphatidylserine (PS). To gain insight into the cellular and genetic basis for this disorder, B-lymphocytes from a patient with Scott syndrome and from normal donors were immortalized by EBV-transformation, and tested for their capacity to expose plasma membrane PS in response to the Ca2+ ionophore, A23187. Upon incubation with A23187, EBV-lymphoblasts derived from normal donors consistently induced surface expression of PS in > 70% of all cells, as detected by membrane association of the PS-binding proteins, factor Va or annexin V. PS exposure in these cells was maximal after 5 min, and saturated at < 100 microM external free [Ca2+]. By contrast, < 30% of Scott syndrome lymphoblasts exposed PS, and saturation was not observed at > 1 mM external free [Ca2+]. Single-cell clones derived from the Scott lymphoblasts all exhibited a diminished response to A23187 comparable with that of the parental cells, suggesting that all lymphocytes from this patient share this membrane abnormality. Hybridomas prepared by fusion of Scott lymphoblasts with the myeloma cell line UC-LUC showed responses to Ca2+ ionophore comparable to those observed for normal lymphoblasts and for hybridomas prepared by fusion of normal lymphoblasts with UC-LUC. This correction of the Scott abnormality suggests possible complementation of an aberrant gene(s) responsible for this disorder.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S. S., Rawala-Sheikh R., Ashby B., Walsh P. N. Platelet receptor-mediated factor X activation by factor IXa. High-affinity factor IXa receptors induced by factor VIII are deficient on platelets in Scott syndrome. J Clin Invest. 1989 Sep;84(3):824–828. doi: 10.1172/JCI114242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassé F., Gaffet P., Rendu F., Bienvenüe A. Translocation of spin-labeled phospholipids through plasma membrane during thrombin- and ionophore A23187-induced platelet activation. Biochemistry. 1993 Mar 9;32(9):2337–2344. doi: 10.1021/bi00060a027. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Wiedmer T., Comfurius P., Shattil S. J., Weiss H. J., Zwaal R. F., Sims P. J. Defective Ca(2+)-induced microvesiculation and deficient expression of procoagulant activity in erythrocytes from a patient with a bleeding disorder: a study of the red blood cells of Scott syndrome. Blood. 1992 Jan 15;79(2):380–388. [PubMed] [Google Scholar]
- Bratton D. L. Release of platelet activation factor from activated neutrophils. Transglutaminase-dependent enhancement of transbilayer movement across the plasma membrane. J Biol Chem. 1993 Feb 15;268(5):3364–3373. [PubMed] [Google Scholar]
- Chang C. P., Zhao J., Wiedmer T., Sims P. J. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J Biol Chem. 1993 Apr 5;268(10):7171–7178. [PubMed] [Google Scholar]
- Comfurius P., Senden J. M., Tilly R. H., Schroit A. J., Bevers E. M., Zwaal R. F. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim Biophys Acta. 1990 Jul 24;1026(2):153–160. doi: 10.1016/0005-2736(90)90058-v. [DOI] [PubMed] [Google Scholar]
- Connor J., Gillum K., Schroit A. J. Maintenance of lipid asymmetry in red blood cells and ghosts: effect of divalent cations and serum albumin on the transbilayer distribution of phosphatidylserine. Biochim Biophys Acta. 1990 Jun 11;1025(1):82–86. doi: 10.1016/0005-2736(90)90193-r. [DOI] [PubMed] [Google Scholar]
- Dachary-Prigent J., Freyssinet J. M., Pasquet J. M., Carron J. C., Nurden A. T. Annexin V as a probe of aminophospholipid exposure and platelet membrane vesiculation: a flow cytometry study showing a role for free sulfhydryl groups. Blood. 1993 May 15;81(10):2554–2565. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Austin C. D. The role of calpain in stimulus-response coupling: evidence that calpain mediates agonist-induced expression of procoagulant activity in platelets. Blood. 1990 Dec 15;76(12):2510–2519. [PubMed] [Google Scholar]
- Franck P. F., Bevers E. M., Lubin B. H., Comfurius P., Chiu D. T., Op den Kamp J. A., Zwaal R. F., van Deenen L. L., Roelofsen B. Uncoupling of the membrane skeleton from the lipid bilayer. The cause of accelerated phospholipid flip-flop leading to an enhanced procoagulant activity of sickled cells. J Clin Invest. 1985 Jan;75(1):183–190. doi: 10.1172/JCI111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T., Heimark R. L., Hendrickson L. E., McMullen B. A., Fujikawa K. Human placental anticoagulant protein: isolation and characterization. Biochemistry. 1987 Aug 25;26(17):5572–5578. doi: 10.1021/bi00391a053. [DOI] [PubMed] [Google Scholar]
- Giblin P. A., Leahy D. J., Mennone J., Kavathas P. B. The role of charge and multiple faces of the CD8 alpha/alpha homodimer in binding to major histocompatibility complex class I molecules: support for a bivalent model. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1716–1720. doi: 10.1073/pnas.91.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich J. P., Kane W. H., Hofmann S. L., Stanford N., Majerus P. W. Deficiency of factor Xa-factor Va binding sites on the platelets of a patient with a bleeding disorder. Blood. 1979 Nov;54(5):1015–1022. [PubMed] [Google Scholar]
- Ning Z. Q., Murphy J. J. Calcium ionophore-induced apoptosis of human B cells is preceded by the induced expression of early response genes. Eur J Immunol. 1993 Dec;23(12):3369–3372. doi: 10.1002/eji.1830231247. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Nicotera P. Role of calcium in toxic and programmed cell death. Adv Exp Med Biol. 1991;283:419–425. doi: 10.1007/978-1-4684-5877-0_57. [DOI] [PubMed] [Google Scholar]
- Rosing J., Bevers E. M., Comfurius P., Hemker H. C., van Dieijen G., Weiss H. J., Zwaal R. F. Impaired factor X and prothrombin activation associated with decreased phospholipid exposure in platelets from a patient with a bleeding disorder. Blood. 1985 Jun;65(6):1557–1561. [PubMed] [Google Scholar]
- Sims P. J., Faioni E. M., Wiedmer T., Shattil S. J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988 Dec 5;263(34):18205–18212. [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T., Esmon C. T., Weiss H. J., Shattil S. J. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989 Oct 15;264(29):17049–17057. [PubMed] [Google Scholar]
- Sulpice J. C., Zachowski A., Devaux P. F., Giraud F. Requirement for phosphatidylinositol 4,5-bisphosphate in the Ca(2+)-induced phospholipid redistribution in the human erythrocyte membrane. J Biol Chem. 1994 Mar 4;269(9):6347–6354. [PubMed] [Google Scholar]
- Thiagarajan P., Tait J. F. Collagen-induced exposure of anionic phospholipid in platelets and platelet-derived microparticles. J Biol Chem. 1991 Dec 25;266(36):24302–24307. [PubMed] [Google Scholar]
- Weiss H. J. Scott syndrome: a disorder of platelet coagulant activity. Semin Hematol. 1994 Oct;31(4):312–319. [PubMed] [Google Scholar]
- Weiss H. J., Vicic W. J., Lages B. A., Rogers J. Isolated deficiency of platelet procoagulant activity. Am J Med. 1979 Aug;67(2):206–213. doi: 10.1016/0002-9343(79)90392-9. [DOI] [PubMed] [Google Scholar]
- Wiedmer T., Shattil S. J., Cunningham M., Sims P. J. Role of calcium and calpain in complement-induced vesiculation of the platelet plasma membrane and in the exposure of the platelet factor Va receptor. Biochemistry. 1990 Jan 23;29(3):623–632. doi: 10.1021/bi00455a005. [DOI] [PubMed] [Google Scholar]
- Wiedmer T., Sims P. J. Participation of protein kinases in complement C5b-9-induced shedding of platelet plasma membrane vesicles. Blood. 1991 Dec 1;78(11):2880–2886. [PubMed] [Google Scholar]
- Williamson P., Kulick A., Zachowski A., Schlegel R. A., Devaux P. F. Ca2+ induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry. 1992 Jul 14;31(27):6355–6360. doi: 10.1021/bi00142a027. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Bevers E. M., Comfurius P., Rosing J., Tilly R. H., Verhallen P. F. Loss of membrane phospholipid asymmetry during activation of blood platelets and sickled red cells; mechanisms and physiological significance. 1989 Nov 23-Dec 19Mol Cell Biochem. 91(1-2):23–31. doi: 10.1007/BF00228075. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., Bevers E. M. Platelet procoagulant activity and microvesicle formation. Its putative role in hemostasis and thrombosis. Biochim Biophys Acta. 1992 Oct 13;1180(1):1–8. doi: 10.1016/0925-4439(92)90019-j. [DOI] [PubMed] [Google Scholar]



