Abstract
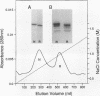
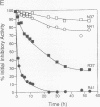
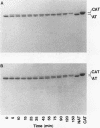
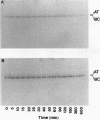
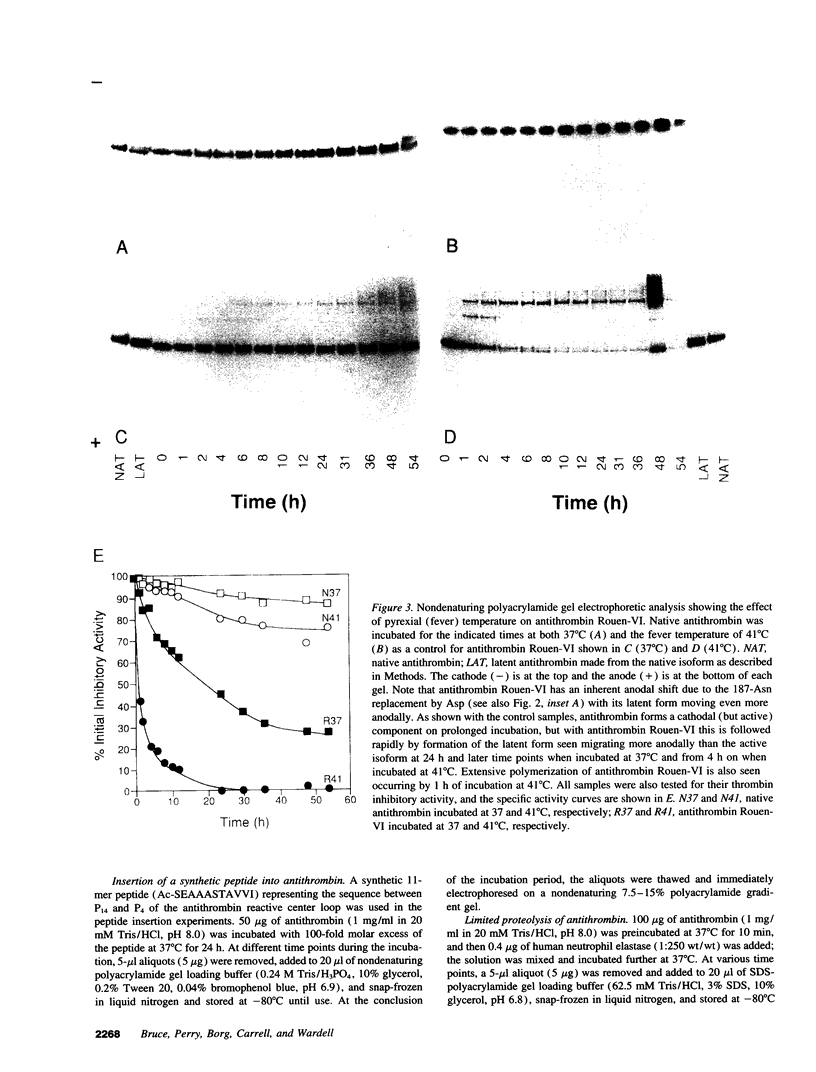
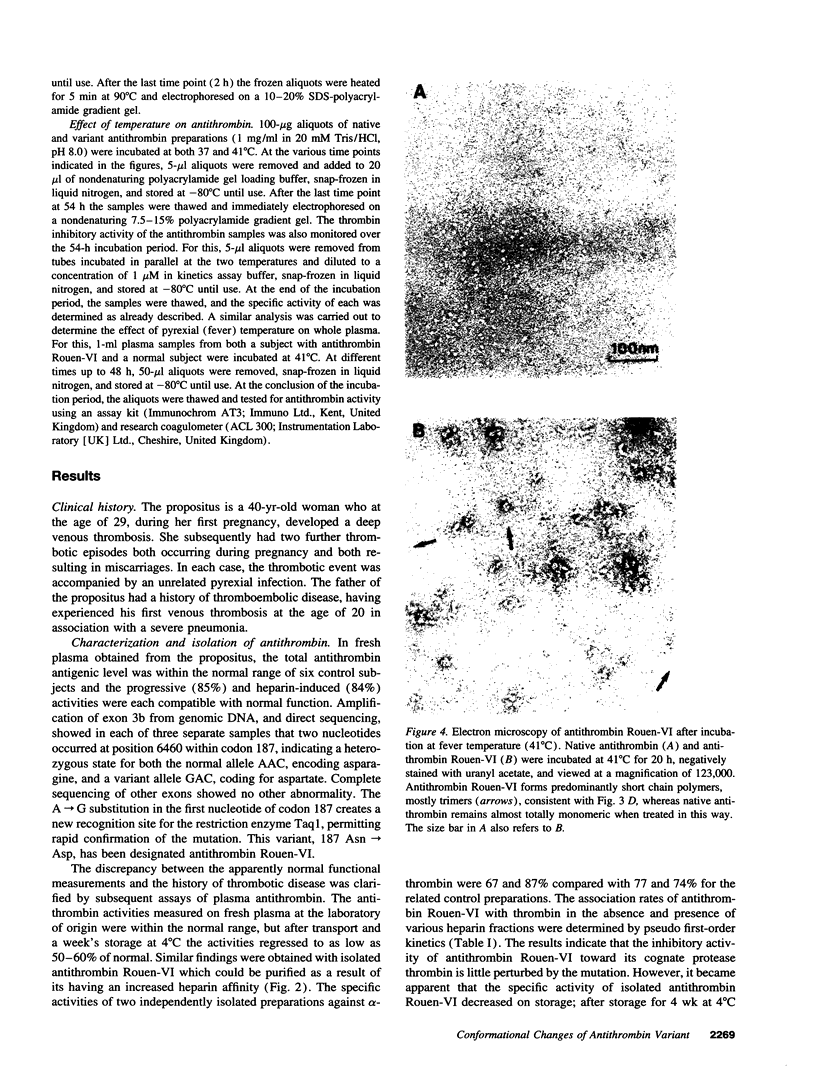
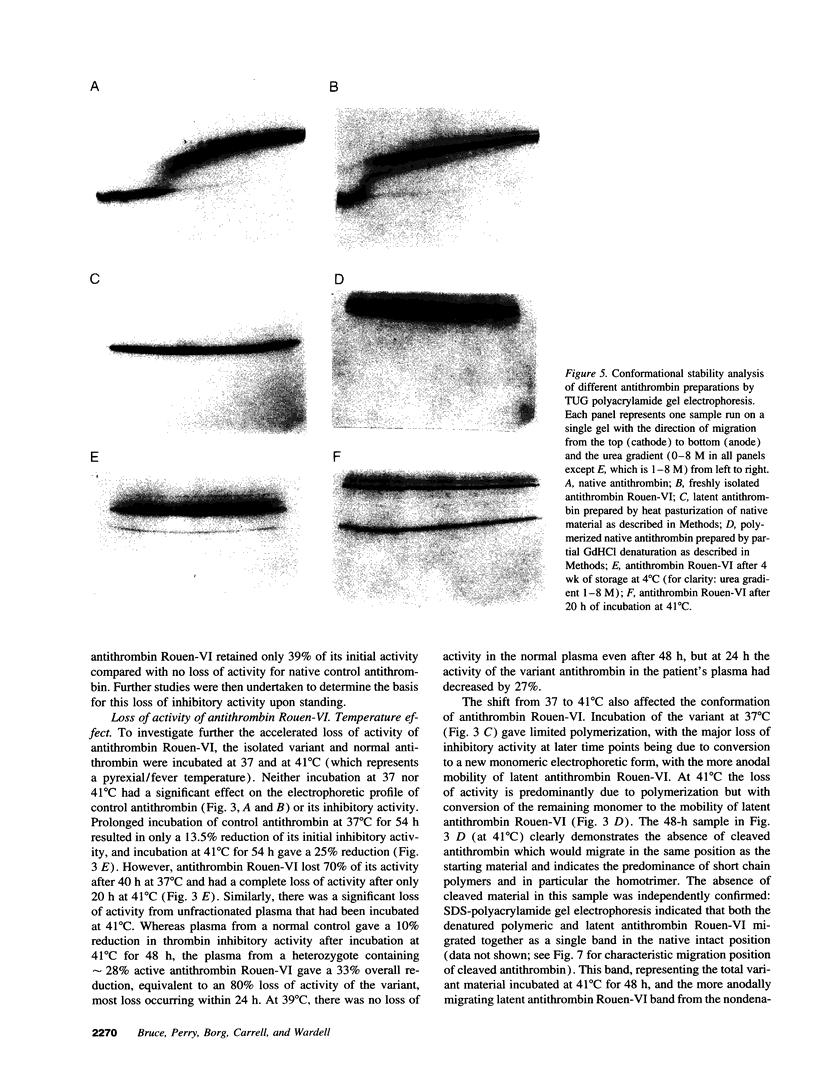
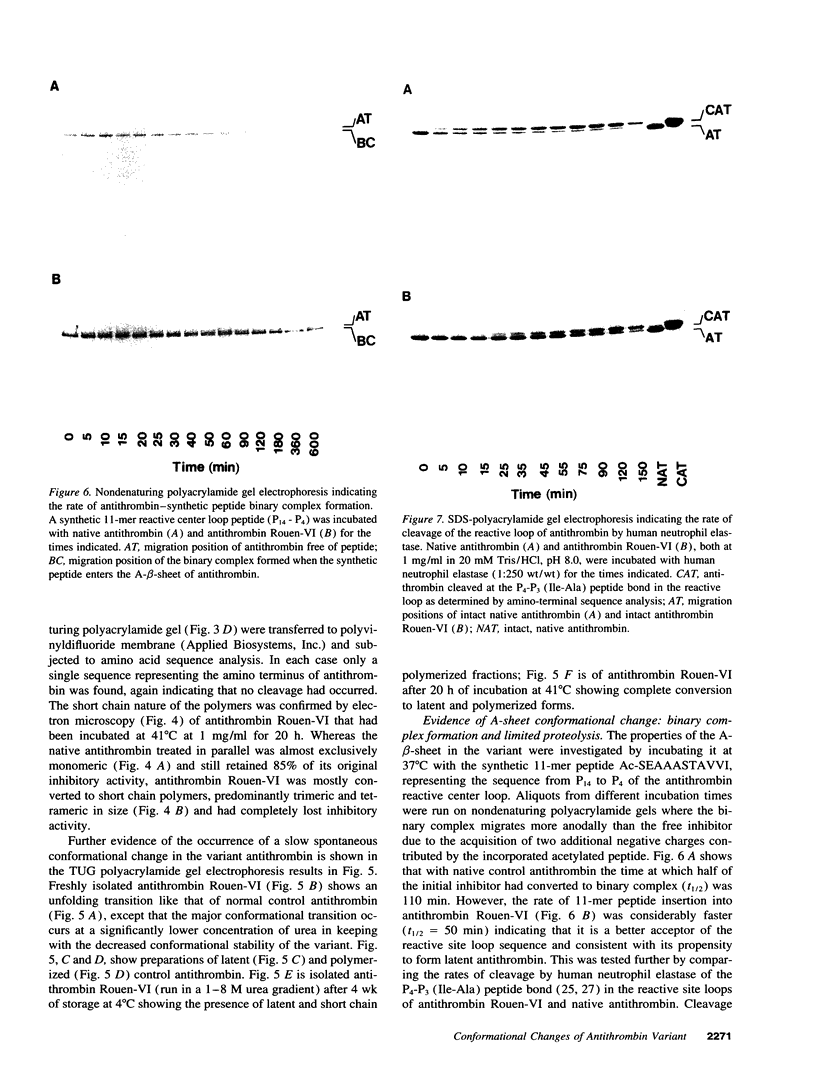
A new variant of antithrombin (Rouen-VI, 187 Asn-->Asp) with increased heparin affinity was shown to have normal inhibitory activity which decreased slowly at 4 degrees C and rapidly at 41 degrees C. On electrophoresis the freshly isolated variant had an anodal shift relative to native antithrombin due to the mutation. A further anodal transition occurred after either prolonged storage at 4 degrees C or incubation at 41 degrees C due to the formation of a new inactive uncleaved component with properties characteristic of L-form (latent) antithrombin. At the same time, polymerization also occurred with a predominance of di-, tri-, and tetra-mers. These findings fit with the observed mutation of the conserved asparagine (187) in the F-helix destabilizing the underlying A-sheet of the molecule. Evidence of A-sheet perturbation is provided by the increased rate of peptide insertion into the A-sheet and by the decreased vulnerability of the reactive loop to proteolysis. The spontaneous formation of both L-antithrombin and polymers is consistent with our crystal structure of intact antithrombin where L-form and active antithrombin are linked together as dimers. The nature of this linkage favors a mechanism of polymerization whereby the opening of the A-sheet, to give incorporation of the reactive center loop, is accompanied by the bonding of the loop of one molecule to the C-sheet of the next. The accelerated lability of antithrombin Rouen-VI at 41 versus 37 degrees C provides an explanation for the clinical observation that episodes of thrombosis were preceded by unrelated pyrexias.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U., Lie M., Odegård O. R. Antithrombin (heparin cofactor) assay with "new" chromogenic substrates (S-2238 and Chromozym TH). Thromb Res. 1977 Oct;11(4):549–553. doi: 10.1016/0049-3848(77)90208-0. [DOI] [PubMed] [Google Scholar]
- Aulak K. S., Eldering E., Hack C. E., Lubbers Y. P., Harrison R. A., Mast A., Cicardi M., Davis A. E., 3rd A hinge region mutation in C1-inhibitor (Ala436-->Thr) results in nonsubstrate-like behavior and in polymerization of the molecule. J Biol Chem. 1993 Aug 25;268(24):18088–18094. [PubMed] [Google Scholar]
- Baumann U., Huber R., Bode W., Grosse D., Lesjak M., Laurell C. B. Crystal structure of cleaved human alpha 1-antichymotrypsin at 2.7 A resolution and its comparison with other serpins. J Mol Biol. 1991 Apr 5;218(3):595–606. doi: 10.1016/0022-2836(91)90704-a. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Evans D. L., Stein P. E. Mobile reactive centre of serpins and the control of thrombosis. Nature. 1991 Oct 10;353(6344):576–578. doi: 10.1038/353576a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Owen M. C. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature. 1985 Oct 24;317(6039):730–732. doi: 10.1038/317730a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Stein P. E., Fermi G., Wardell M. R. Biological implications of a 3 A structure of dimeric antithrombin. Structure. 1994 Apr 15;2(4):257–270. doi: 10.1016/s0969-2126(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Hardman K. D. Computer-generated schematic diagrams of protein structures. Science. 1982 Apr 30;216(4545):539–540. doi: 10.1126/science.7071602. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Lomas D. A., Evans D. L., Stone S. R., Chang W. S., Carrell R. W. Effect of the Z mutation on the physical and inhibitory properties of alpha 1-antitrypsin. Biochemistry. 1993 Jan 19;32(2):500–508. doi: 10.1021/bi00053a014. [DOI] [PubMed] [Google Scholar]
- Lomas D. A., Finch J. T., Seyama K., Nukiwa T., Carrell R. W. Alpha 1-antitrypsin Siiyama (Ser53-->Phe). Further evidence for intracellular loop-sheet polymerization. J Biol Chem. 1993 Jul 25;268(21):15333–15335. [PubMed] [Google Scholar]
- Mast A. E., Enghild J. J., Pizzo S. V., Salvesen G. Analysis of the plasma elimination kinetics and conformational stabilities of native, proteinase-complexed, and reactive site cleaved serpins: comparison of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, antithrombin III, alpha 2-antiplasmin, angiotensinogen, and ovalbumin. Biochemistry. 1991 Feb 12;30(6):1723–1730. doi: 10.1021/bi00220a039. [DOI] [PubMed] [Google Scholar]
- Mast A. E., Enghild J. J., Salvesen G. Conformation of the reactive site loop of alpha 1-proteinase inhibitor probed by limited proteolysis. Biochemistry. 1992 Mar 17;31(10):2720–2728. doi: 10.1021/bi00125a012. [DOI] [PubMed] [Google Scholar]
- McKay E. J. A simple two-step procedure for the isolation of antithrombin III from biological fluids. 1981 Feb 15-Mar 1Thromb Res. 21(4-5):375–382. doi: 10.1016/0049-3848(81)90138-9. [DOI] [PubMed] [Google Scholar]
- Mottonen J., Strand A., Symersky J., Sweet R. M., Danley D. E., Geoghegan K. F., Gerard R. D., Goldsmith E. J. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992 Jan 16;355(6357):270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- Mourey L., Samama J. P., Delarue M., Petitou M., Choay J., Moras D. Crystal structure of cleaved bovine antithrombin III at 3.2 A resolution. J Mol Biol. 1993 Jul 5;232(1):223–241. doi: 10.1006/jmbi.1993.1378. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Björk I. Binding of low-affinity and high-affinity heparin to antithrombin. Ultraviolet difference spectroscopy and circular dichroism studies. Biochemistry. 1978 Aug 8;17(16):3339–3344. doi: 10.1021/bi00609a026. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Nyström C., Björk I. The size and shape of human and bovine antithrombin III. Eur J Biochem. 1977 Aug 15;78(1):195–203. doi: 10.1111/j.1432-1033.1977.tb11730.x. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Srinivasan K. R., Björk I., Shore J. D. Binding of high affinity heparin to antithrombin III. Stopped flow kinetic studies of the binding interaction. J Biol Chem. 1981 Nov 10;256(21):11073–11079. [PubMed] [Google Scholar]
- Perry D. J., Harper P. L., Fairham S., Daly M., Carrell R. W. Antithrombin Cambridge, 384 Ala to Pro: a new variant identified using the polymerase chain reaction. FEBS Lett. 1989 Aug 28;254(1-2):174–176. doi: 10.1016/0014-5793(89)81033-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Rovelli G., Stone S. R., Guidolin A., Sommer J., Monard D. Characterization of the heparin-binding site of glia-derived nexin/protease nexin-1. Biochemistry. 1992 Apr 7;31(13):3542–3549. doi: 10.1021/bi00128a031. [DOI] [PubMed] [Google Scholar]
- Schreuder H. A., de Boer B., Dijkema R., Mulders J., Theunissen H. J., Grootenhuis P. D., Hol W. G. The intact and cleaved human antithrombin III complex as a model for serpin-proteinase interactions. Nat Struct Biol. 1994 Jan;1(1):48–54. doi: 10.1038/nsb0194-48. [DOI] [PubMed] [Google Scholar]
- Stein P. E., Leslie A. G., Finch J. T., Turnell W. G., McLaughlin P. J., Carrell R. W. Crystal structure of ovalbumin as a model for the reactive centre of serpins. Nature. 1990 Sep 6;347(6288):99–102. doi: 10.1038/347099a0. [DOI] [PubMed] [Google Scholar]
- Stein P., Chothia C. Serpin tertiary structure transformation. J Mol Biol. 1991 Sep 20;221(2):615–621. doi: 10.1016/0022-2836(91)80076-7. [DOI] [PubMed] [Google Scholar]
- Verpy E., Couture-Tosi E., Tosi M. C1 inhibitor mutations which affect intracellular transport and secretion in type I hereditary angioedema. Behring Inst Mitt. 1993 Dec;(93):120–124. [PubMed] [Google Scholar]
- Wardell M. R., Abrahams J. P., Bruce D., Skinner R., Leslie A. G. Crystallization and preliminary X-ray diffraction analysis of two conformations of intact human antithrombin. J Mol Biol. 1993 Dec 20;234(4):1253–1258. doi: 10.1006/jmbi.1993.1676. [DOI] [PubMed] [Google Scholar]
- Wei A., Rubin H., Cooperman B. S., Christianson D. W. Crystal structure of an uncleaved serpin reveals the conformation of an inhibitory reactive loop. Nat Struct Biol. 1994 Apr;1(4):251–258. doi: 10.1038/nsb0494-251. [DOI] [PubMed] [Google Scholar]
- Wright H. T., Qian H. X., Huber R. Crystal structure of plakalbumin, a proteolytically nicked form of ovalbumin. Its relationship to the structure of cleaved alpha-1-proteinase inhibitor. J Mol Biol. 1990 Jun 5;213(3):513–528. doi: 10.1016/s0022-2836(05)80212-8. [DOI] [PubMed] [Google Scholar]