Abstract
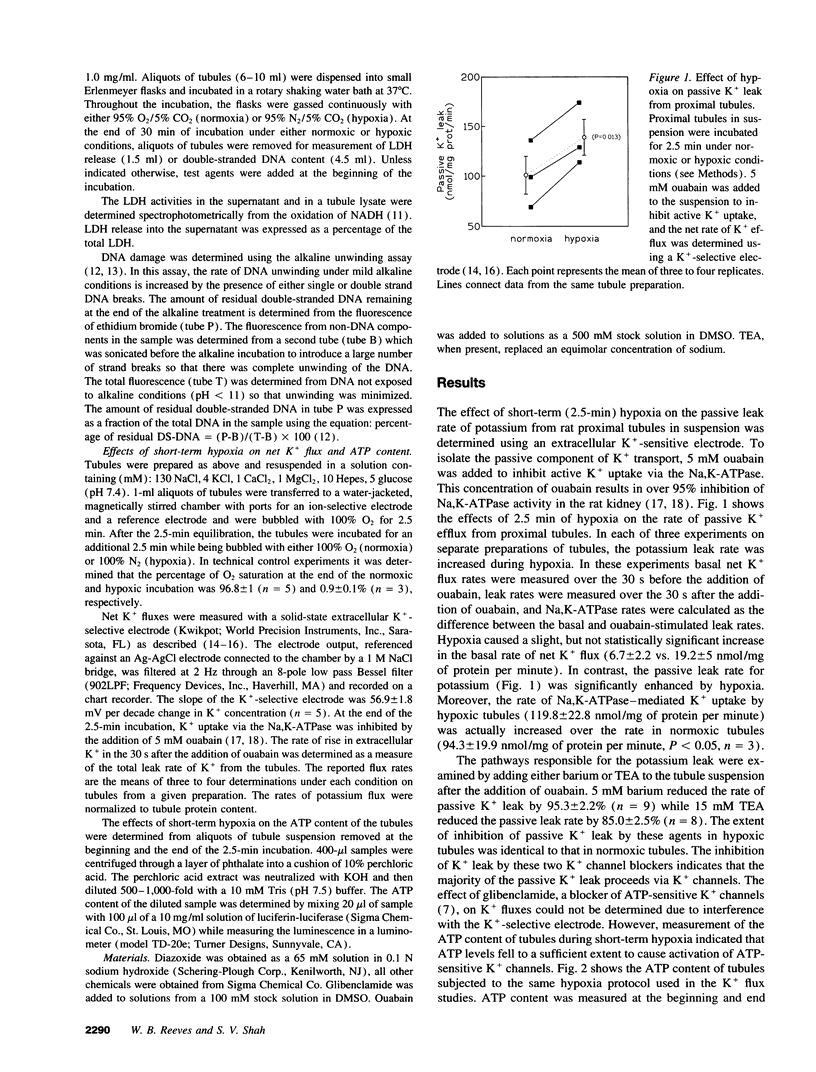
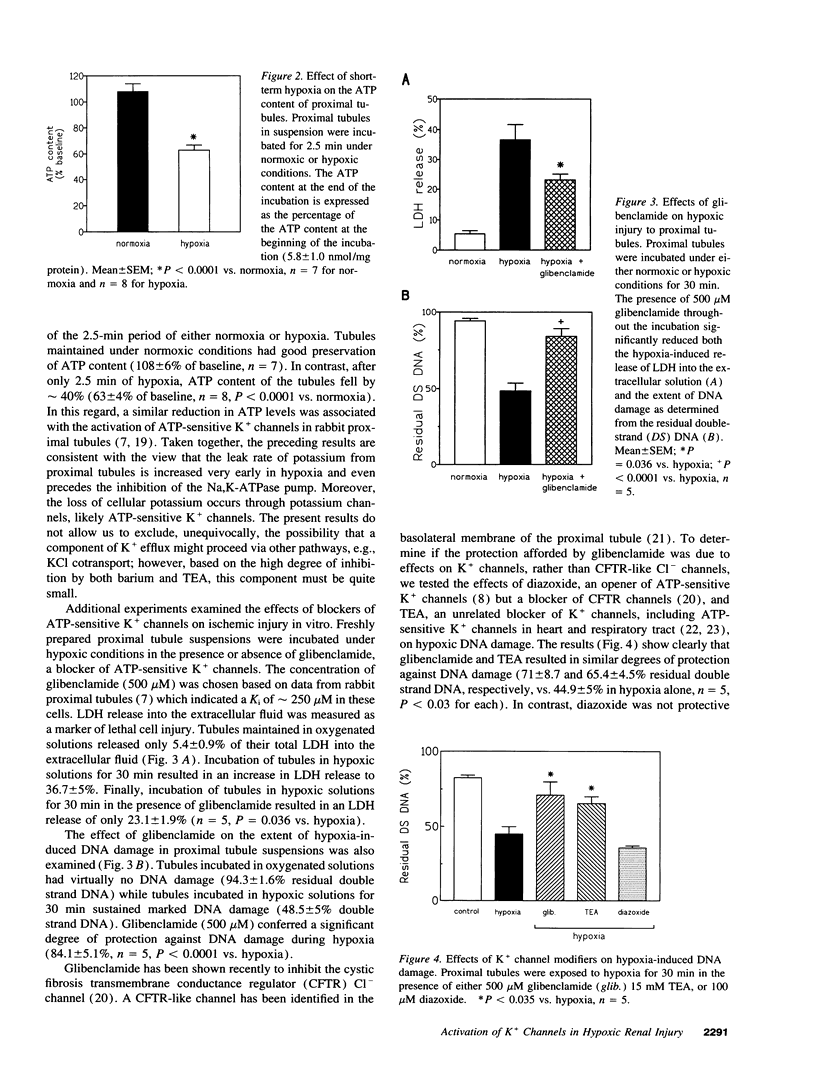
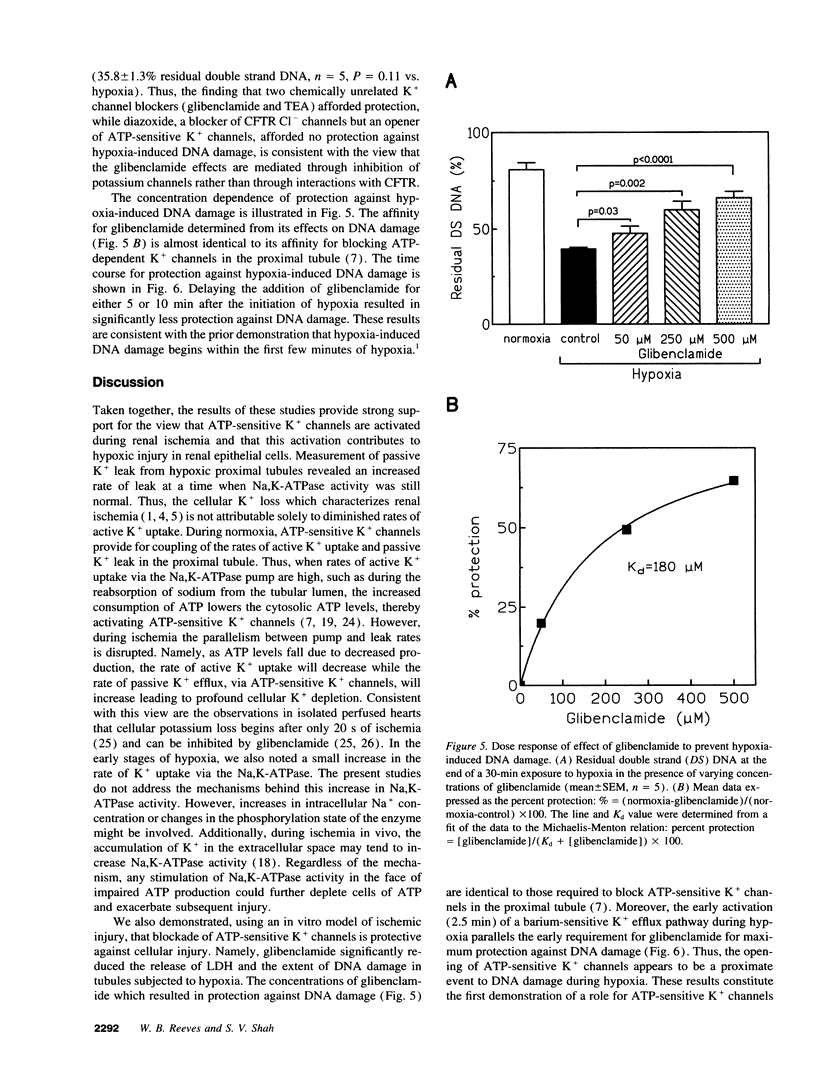
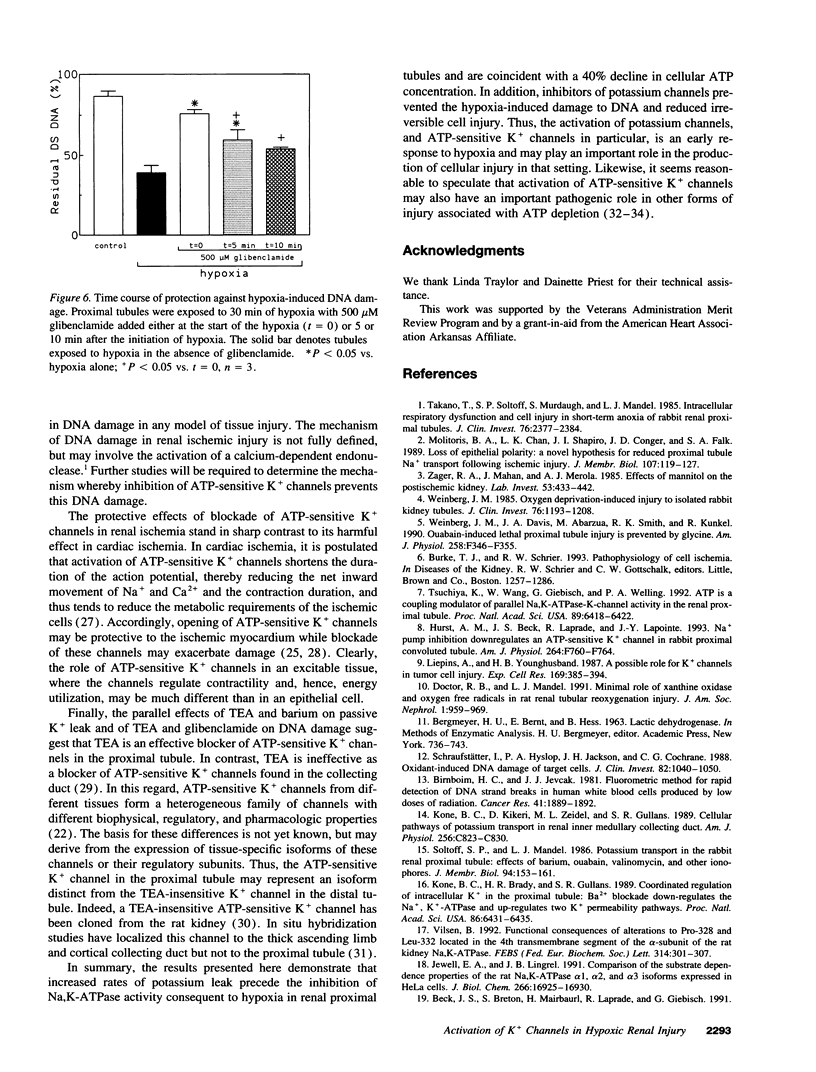
The mechanisms responsible for the loss of cell potassium during renal ischemia are poorly understood. The present studies examined the hypothesis that potassium channels are activated as an early response to hypoxia and contribute to potassium loss independent from an inhibition of active K+ uptake. Potassium flux in suspensions of freshly isolated rat proximal tubules was measured using an ion-selective electrode. Exposure of the tubules to hypoxia for only 2.5 min resulted in a rise in the passive leak rate of K+ but no decrease in active K+ uptake. The passive leak of K+ was associated with a 40% decrease in cell ATP content. The passive K+ efflux was inhibited by 5 mM Ba2+ (95%) and by 15 mM tetraethylammonium (85%) suggesting that K+ channels were the primary route of K+ movement. The effects of K+ channel blockade on the development of hypoxic injury were also examined. Tetraethylammonium and glibenclamide, an inhibitor of ATP-sensitive K+ channels, reduced hypoxic injury as assessed by the release of lactate dehydrogenase or measurement of DNA damage. These results suggest that activation of K+ channels is an early response to hypoxia and contributes to hypoxic renal injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida A. R., Wetzels J. F., Bunnachak D., Burke T. J., Chaimovitz C., Hammond W. S., Schrier R. W. Acute phosphate depletion and in vitro rat proximal tubule injury: protection by glycine and acidosis. Kidney Int. 1992 Jun;41(6):1494–1500. doi: 10.1038/ki.1992.218. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Auchampach J. A., Maruyama M., Cavero I., Gross G. J. The new K+ channel opener Aprikalim (RP 52891) reduces experimental infarct size in dogs in the absence of hemodynamic changes. J Pharmacol Exp Ther. 1991 Dec;259(3):961–967. [PubMed] [Google Scholar]
- Beck J. S., Breton S., Mairbäurl H., Laprade R., Giebisch G. Relationship between sodium transport and intracellular ATP in isolated perfused rabbit proximal convoluted tubule. Am J Physiol. 1991 Oct;261(4 Pt 2):F634–F639. doi: 10.1152/ajprenal.1991.261.4.F634. [DOI] [PubMed] [Google Scholar]
- Beck J. S., Hurst A. M., Lapointe J. Y., Laprade R. Regulation of basolateral K channels in proximal tubule studied during continuous microperfusion. Am J Physiol. 1993 Mar;264(3 Pt 2):F496–F501. doi: 10.1152/ajprenal.1993.264.3.F496. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Doctor R. B., Mandel L. J. Minimal role of xanthine oxidase and oxygen free radicals in rat renal tubular reoxygenation injury. J Am Soc Nephrol. 1991 Jan;1(7):959–969. doi: 10.1681/ASN.V17959. [DOI] [PubMed] [Google Scholar]
- Groves C. E., Schnellmann R. G., Sokol P. P., Steffens T. G., Lock E. A. Pentachlorobutadienyl-L-cysteine (PCBC) toxicity: the importance of mitochondrial dysfunction. J Biochem Toxicol. 1991 Winter;6(4):253–260. doi: 10.1002/jbt.2570060404. [DOI] [PubMed] [Google Scholar]
- Hurst A. M., Beck J. S., Laprade R., Lapointe J. Y. Na+ pump inhibition downregulates an ATP-sensitive K+ channel in rabbit proximal convoluted tubule. Am J Physiol. 1993 Apr;264(4 Pt 2):F760–F764. doi: 10.1152/ajprenal.1993.264.4.F760. [DOI] [PubMed] [Google Scholar]
- Jewell E. A., Lingrel J. B. Comparison of the substrate dependence properties of the rat Na,K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J Biol Chem. 1991 Sep 5;266(25):16925–16930. [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor P. F., Coetzee W. A., Carmeliet E. E., Dennis S. C., Opie L. H. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res. 1990 Feb;66(2):478–485. doi: 10.1161/01.res.66.2.478. [DOI] [PubMed] [Google Scholar]
- Kone B. C., Brady H. R., Gullans S. R. Coordinated regulation of intracellular K+ in the proximal tubule: Ba2+ blockade down-regulates the Na+,K+-ATPase and up-regulates two K+ permeability pathways. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6431–6435. doi: 10.1073/pnas.86.16.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kone B. C., Kikeri D., Zeidel M. L., Gullans S. R. Cellular pathways of potassium transport in renal inner medullary collecting duct. Am J Physiol. 1989 Apr;256(4 Pt 1):C823–C830. doi: 10.1152/ajpcell.1989.256.4.C823. [DOI] [PubMed] [Google Scholar]
- Liepins A., Younghusband H. B. A possible role for K+ channels in tumor cell injury. Membrane vesicle shedding and nuclear DNA fragmentation. Exp Cell Res. 1987 Apr;169(2):385–394. doi: 10.1016/0014-4827(87)90199-6. [DOI] [PubMed] [Google Scholar]
- Mitani A., Kinoshita K., Fukamachi K., Sakamoto M., Kurisu K., Tsuruhara Y., Fukumura F., Nakashima A., Tokunaga K. Effects of glibenclamide and nicorandil on cardiac function during ischemia and reperfusion in isolated perfused rat hearts. Am J Physiol. 1991 Dec;261(6 Pt 2):H1864–H1871. doi: 10.1152/ajpheart.1991.261.6.H1864. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Chan L. K., Shapiro J. I., Conger J. D., Falk S. A. Loss of epithelial polarity: a novel hypothesis for reduced proximal tubule Na+ transport following ischemic injury. J Membr Biol. 1989 Feb;107(2):119–127. doi: 10.1007/BF01871717. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Ewell F. P., Sgambati M., Mandel L. J. Mitochondrial toxicity of 2-bromohydroquinone in rabbit renal proximal tubules. Toxicol Appl Pharmacol. 1987 Sep 30;90(3):420–426. doi: 10.1016/0041-008x(87)90134-7. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Potassium transport in the rabbit renal proximal tubule: effects of barium, ouabain, valinomycin, and other ionophores. J Membr Biol. 1986;94(2):153–161. doi: 10.1007/BF01871195. [DOI] [PubMed] [Google Scholar]
- Takano T., Soltoff S. P., Murdaugh S., Mandel L. J. Intracellular respiratory dysfunction and cell injury in short-term anoxia of rabbit renal proximal tubules. J Clin Invest. 1985 Dec;76(6):2377–2384. doi: 10.1172/JCI112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Wang W., Giebisch G., Welling P. A. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsen B. Functional consequences of alterations to Pro328 and Leu332 located in the 4th transmembrane segment of the alpha-subunit of the rat kidney Na+,K(+)-ATPase. FEBS Lett. 1992 Dec 21;314(3):301–307. doi: 10.1016/0014-5793(92)81494-7. [DOI] [PubMed] [Google Scholar]
- Wang W., Sackin H., Giebisch G. Renal potassium channels and their regulation. Annu Rev Physiol. 1992;54:81–96. doi: 10.1146/annurev.ph.54.030192.000501. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Smith R. K., Kunkel R. Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol. 1990 Feb;258(2 Pt 2):F346–F355. doi: 10.1152/ajprenal.1990.258.2.F346. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985 Sep;76(3):1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager R. A., Mahan J., Merola A. J. Effects of mannitol on the postischemic kidney. Biochemical, functional, and morphologic assessments. Lab Invest. 1985 Oct;53(4):433–442. [PubMed] [Google Scholar]
- ten Eick R. E., Whalley D. W., Rasmussen H. H. Connections: heart disease, cellular electrophysiology, and ion channels. FASEB J. 1992 May;6(8):2568–2580. doi: 10.1096/fasebj.6.8.1375569. [DOI] [PubMed] [Google Scholar]



