Abstract
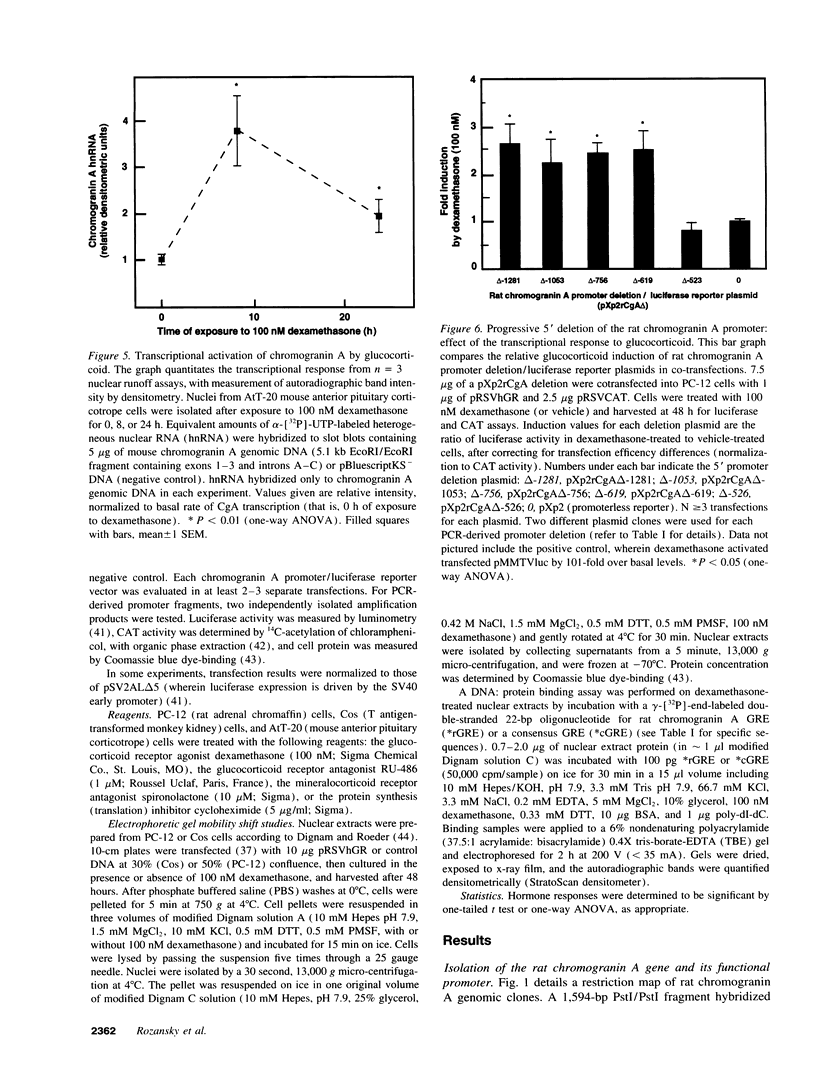
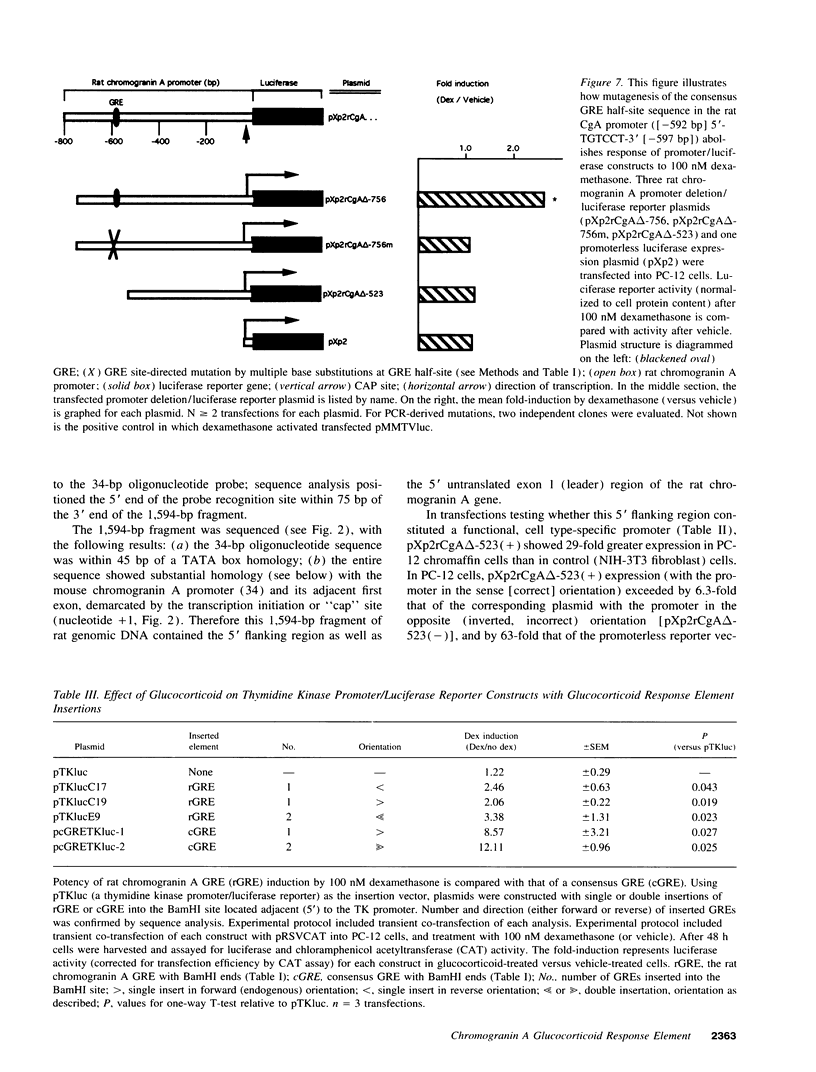
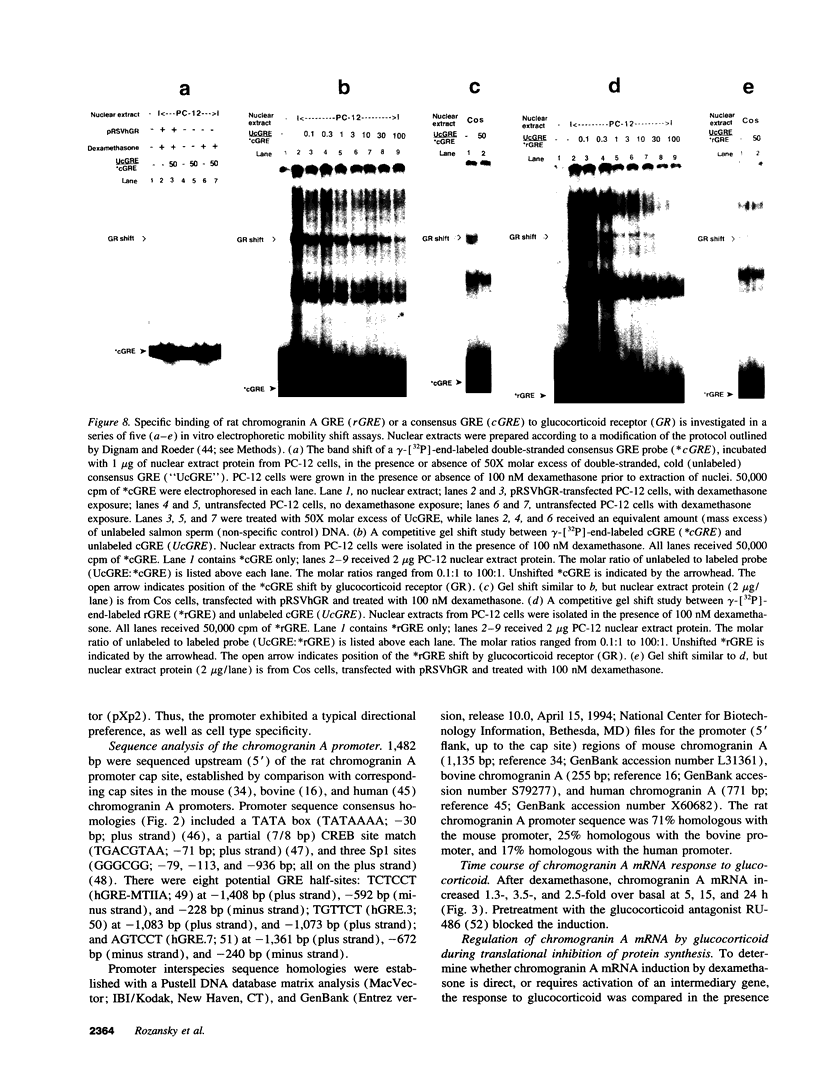
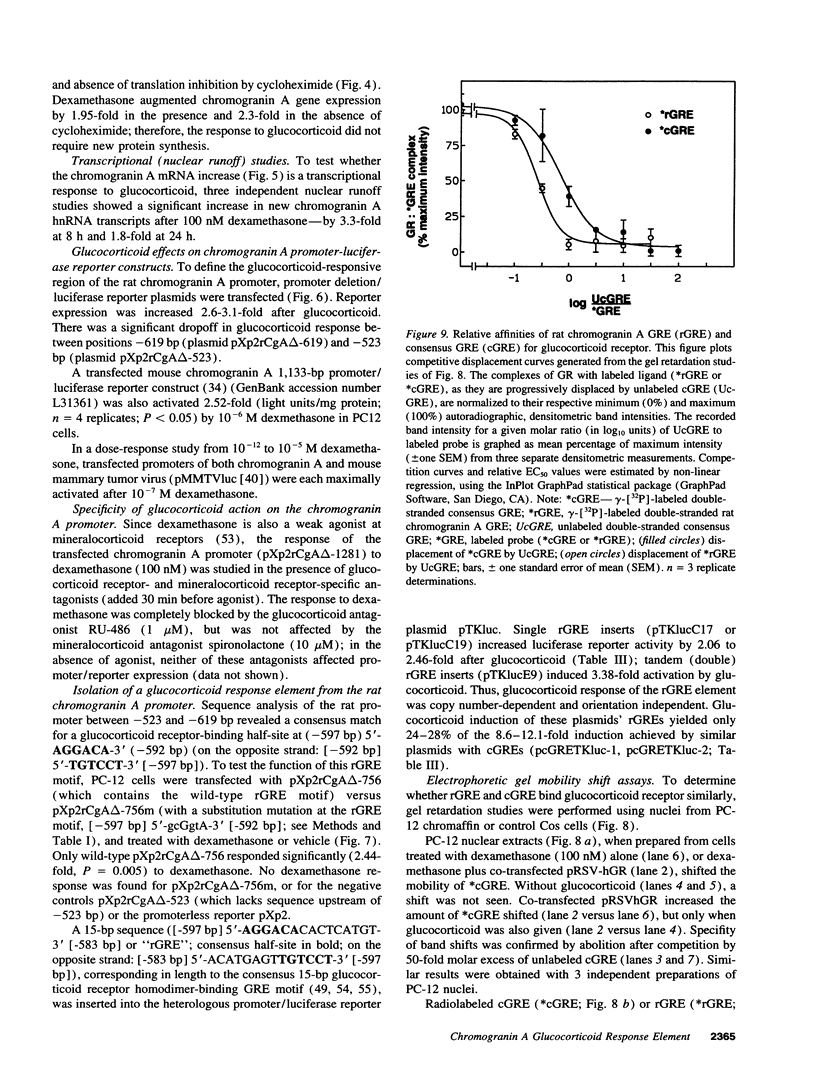
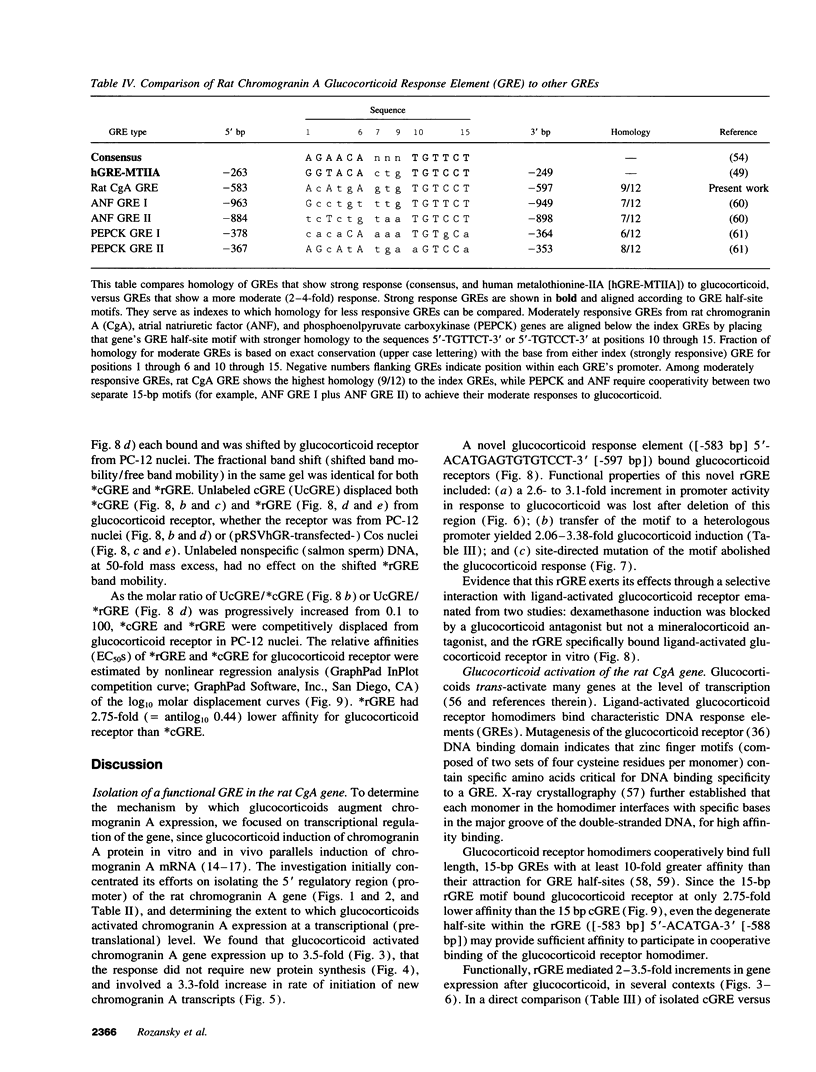
Glucocorticoids regulate catecholamine biosynthesis and storage at several sites. Chromogranin A, an abundant protein complexed with catecholamines in secretory vesicles of chromaffin cells and sympathetic axons, is also augmented by glucocorticoids. This study reports isolation of the rat chromogranin A promoter to elucidate transcriptional regulation of chromogranin A biosynthesis by glucocorticoids in neuroendocrine cells. Endogenous chromogranin A gene expression was activated up to 3.5-fold in chromaffin cells by glucocorticoid, in time-dependent fashion. Inhibition of new protein synthesis by cycloheximide did not alter the rise in chromogranin A mRNA, suggesting that glucocorticoids directly activate the chromogranin A promoter; nuclear runoff assays confirmed a 3.3-fold increased rate of initiation of new chromogranin A transcripts after glucocorticoid. Transfected rat chromogranin A promoter/luciferase reporter constructs were activated 2.6-3.1-fold by glucocorticoid, and selective agonist/antagonist studies determined that dexamethasone effects were mediated by glucocorticoid receptors. Both rat and mouse chromogranin A promoter/luciferase reporter constructs were activated by glucocorticoid. A series of promoter deletions narrowed the region of glucocorticoid action to a 93-bp section of the promoter, from position -526 to -619 bp upstream of the cap site. A 15-bp sequence ([-583 bp] 5'-ACATGAGTGTGTCCT-3' [-597 bp]) within this region showed partial homology to a glucocorticoid response element (GRE; half-site in italics) consensus sequence, and several lines of experimental evidence confirmed its function as a GRE: (a) site-directed mutation of this GRE prevented glucocorticoid activation of a chromogranin A promoter/reporter; (b) transfer of this GRE to a heterologous (thymidine kinase) promoter/reporter conferred activation by glucocorticoid, in copy number-dependent and orientation-independent fashion; and (c) electrophoretic gel mobility shifts demonstrated binding of this GRE by ligand-activated glucocorticoid receptor, though at 2.75-fold lower affinity than the glucocorticoid receptor interaction with a consensus GRE. The rat chromogranin A GRE showed functional and structural similarities to GREs in other genes proportionally regulated by glucocorticoids. We conclude that a discrete domain of the chromogranin A promoter is both necessary and sufficient to confer glucocorticoid regulation onto the gene, and that the activity of this region also explains the degree of activation of the endogenous gene by glucocorticoid.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy I., Freedman L. P. DNA binding analysis of glucocorticoid receptor specificity mutants. Nucleic Acids Res. 1992 Mar 11;20(5):1045–1052. doi: 10.1093/nar/20.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Michelsohn A. Role of glucocorticoids in the chromaffin-neuron developmental decision. Int J Dev Neurosci. 1989;7(5):475–487. doi: 10.1016/0736-5748(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Argentin S., Sun Y. L., Lihrmann I., Schmidt T. J., Drouin J., Nemer M. Distal cis-acting promoter sequences mediate glucocorticoid stimulation of cardiac atrial natriuretic factor gene transcription. J Biol Chem. 1991 Dec 5;266(34):23315–23322. [PubMed] [Google Scholar]
- Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Barbosa J. A., Gill B. M., Takiyyuddin M. A., O'Connor D. T. Chromogranin A: posttranslational modifications in secretory granules. Endocrinology. 1991 Jan;128(1):174–190. doi: 10.1210/endo-128-1-174. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. The steroid hormone antagonist RU486. Mechanism at the cellular level and clinical applications. Endocrinol Metab Clin North Am. 1991 Dec;20(4):873–891. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Geisse S., Wenz M., Westphal H. M., Beato M. The nucleotide sequences recognized by the glucocorticoid receptor in the rabbit uteroglobin gene region are located far upstream from the initiation of transcription. EMBO J. 1984 Dec 1;3(12):2771–2778. doi: 10.1002/j.1460-2075.1984.tb02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Cooper G. M. Transformation of NIH/3T3 mouse cells by DNA of Rous sarcoma virus. Cell. 1979 Aug;17(4):993–1002. doi: 10.1016/0092-8674(79)90338-6. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson I. M., Mains R. E. Cell-type specific posttranslational processing of peptides by different pituitary cell lines. Endocrinology. 1990 Jul;127(1):133–140. doi: 10.1210/endo-127-1-133. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Huttner W. B., Mallet J., O'Connor D. T., Winkler H., Zanini A. A nomenclature proposal for the chromogranin/secretogranin proteins. Neuroscience. 1987 Jun;21(3):1019–1021. doi: 10.1016/0306-4522(87)90056-x. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Lewis K., Rothenberg B. E. High efficiency vectors for cosmid microcloning and genomic analysis. Gene. 1989 Jun 30;79(1):9–20. doi: 10.1016/0378-1119(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Wahl G. M. Cosmid vectors for genomic walking and rapid restriction mapping. Methods Enzymol. 1987;152:604–610. doi: 10.1016/0076-6879(87)52068-7. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Iacangelo A., Eiden L. E. Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci U S A. 1988 May;85(9):3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härd T., Dahlman K., Carlstedt-Duke J., Gustafsson J. A., Rigler R. Cooperativity and specificity in the interactions between DNA and the glucocorticoid receptor DNA-binding domain. Biochemistry. 1990 Jun 5;29(22):5358–5364. doi: 10.1021/bi00474a022. [DOI] [PubMed] [Google Scholar]
- Iacangelo A. L., Fischer-Colbrie R., Koller K. J., Brownstein M. J., Eiden L. E. The sequence of porcine chromogranin A messenger RNA demonstrates chromogranin A can serve as the precursor for the biologically active hormone, pancreastatin. Endocrinology. 1988 May;122(5):2339–2341. doi: 10.1210/endo-122-5-2339. [DOI] [PubMed] [Google Scholar]
- Iacangelo A. L., Grimes M., Eiden L. E. The bovine chromogranin A gene: structural basis for hormone regulation and generation of biologically active peptides. Mol Endocrinol. 1991 Nov;5(11):1651–1660. doi: 10.1210/mend-5-11-1651. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Okayama H., Eiden L. E. Primary structure of rat chromogranin A and distribution of its mRNA. FEBS Lett. 1988 Jan 25;227(2):115–121. doi: 10.1016/0014-5793(88)80880-9. [DOI] [PubMed] [Google Scholar]
- Imai E., Stromstedt P. E., Quinn P. G., Carlstedt-Duke J., Gustafsson J. A., Granner D. K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Sep;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus 'immediate-early' gene transcription in vitro. Nature. 1985 Sep 12;317(6033):179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Lad R. P., Smith M. A., Hilt D. C. Molecular cloning and regional distribution of rat brain cyclophilin. Brain Res Mol Brain Res. 1991 Feb;9(3):239–244. doi: 10.1016/0169-328x(91)90007-k. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouland A. J., Bevan S., White J. H., Hendy G. N. Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression. J Biol Chem. 1994 Mar 4;269(9):6918–6926. [PubMed] [Google Scholar]
- Muller S. R., Sullivan P. D., Clegg D. O., Feinstein S. C. Efficient transfection and expression of heterologous genes in PC12 cells. DNA Cell Biol. 1990 Apr;9(3):221–229. doi: 10.1089/dna.1990.9.221. [DOI] [PubMed] [Google Scholar]
- Nawata H., Yanase T., Higuchi K., Kato K., Ibayashi H. Epinephrine and norepinephrine syntheses are regulated by a glucocorticoid receptor-mediated mechanism in the bovine adrenal medulla. Life Sci. 1985 May 20;36(20):1957–1966. doi: 10.1016/0024-3205(85)90445-x. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- O'Connor D. T., Subramani S. Do transcriptional enhancers also augment DNA replication? Nucleic Acids Res. 1988 Dec 9;16(23):11207–11222. doi: 10.1093/nar/16.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmer R. J., Koop A. H., Handa M. T., O'Connor D. T. Molecular cloning of chromogranin A from rat pheochromocytoma cells. Hypertension. 1989 Oct;14(4):435–444. doi: 10.1161/01.hyp.14.4.435. [DOI] [PubMed] [Google Scholar]
- Rausch D. M., Iacangelo A. L., Eiden L. E. Glucocorticoid- and nerve growth factor-induced changes in chromogranin A expression define two different neuronal phenotypes in PC12 cells. Mol Endocrinol. 1988 Oct;2(10):921–927. doi: 10.1210/mend-2-10-921. [DOI] [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Chromogranins, widespread in endocrine and nervous tissue, bind Ca2+. FEBS Lett. 1986 Jan 20;195(1-2):327–330. doi: 10.1016/0014-5793(86)80187-9. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Ross M. E., Evinger M. J., Hyman S. E., Carroll J. M., Mucke L., Comb M., Reis D. J., Joh T. H., Goodman H. M. Identification of a functional glucocorticoid response element in the phenylethanolamine N-methyltransferase promoter using fusion genes introduced into chromaffin cells in primary culture. J Neurosci. 1990 Feb;10(2):520–530. doi: 10.1523/JNEUROSCI.10-02-00520.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Hendy G. N., Hamelin J., Paquin J., Lazure C., Metters K. M., Rossier J., Chrétien M. Chromogranin A can act as a reversible processing enzyme inhibitor. Evidence from the inhibition of the IRCM-serine protease 1 cleavage of pro-enkephalin and ACTH at pairs of basic amino acids. FEBS Lett. 1987 Jan 26;211(2):144–150. doi: 10.1016/0014-5793(87)81425-4. [DOI] [PubMed] [Google Scholar]
- Sietzen M., Schober M., Fischer-Colbrie R., Scherman D., Sperk G., Winkler H. Rat adrenal medulla: levels of chromogranins, enkephalins, dopamine beta-hydroxylase and of the amine transporter are changed by nervous activity and hypophysectomy. Neuroscience. 1987 Jul;22(1):131–139. doi: 10.1016/0306-4522(87)90203-x. [DOI] [PubMed] [Google Scholar]
- Simon J. P., Bader M. F., Aunis D. Secretion from chromaffin cells is controlled by chromogranin A-derived peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1712–1716. doi: 10.1073/pnas.85.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak M. K., Hong J. S., Viveros O. H. Coordinate and differential regulation of phenylethanolamine N-methyltransferase, tyrosine hydroxylase and proenkephalin mRNAs by neural and hormonal mechanisms in cultured bovine adrenal medullary cells. Brain Res. 1990 Mar 5;510(2):277–288. doi: 10.1016/0006-8993(90)91378-t. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Perlman R. L., Morse G. M., Sheard B. E. Glucocorticoids increase catecholamine synthesis and storage in PC12 pheochromocytoma cell cultures. J Neurochem. 1983 Feb;40(2):364–370. doi: 10.1111/j.1471-4159.1983.tb11291.x. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Perlman R. L., Morse G. M., Sheard B. E. Glucocorticoids increase catecholamine synthesis and storage in PC12 pheochromocytoma cell cultures. J Neurochem. 1983 Feb;40(2):364–370. doi: 10.1111/j.1471-4159.1983.tb11291.x. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Perlman R. L., Nunnemacher G., Morse G. M., DeLellis R. A., Wolfe H. J., Sheard B. E. Long-term effects of dexamethasone and nerve growth factor on adrenal medullary cells cultured from young adult rats. Cell Tissue Res. 1982;225(3):525–542. doi: 10.1007/BF00214802. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Videen J. S., Mezger M. S., Chang Y. M., O'Connor D. T. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992 Feb 15;267(5):3066–3073. [PubMed] [Google Scholar]
- Winkler H., Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992 Aug;49(3):497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. P., Zilliacus J., McEwan I. J., Dahlman-Wright K., Almlöf T., Carlstedt-Duke J., Gustafsson J. A. Structure and function of the glucocorticoid receptor. J Steroid Biochem Mol Biol. 1993 Dec;47(1-6):11–19. doi: 10.1016/0960-0760(93)90052-x. [DOI] [PubMed] [Google Scholar]
- Wu H. J., Rozansky D. J., Parmer R. J., Gill B. M., O'Connor D. T. Structure and function of the chromogranin A gene. Clues to evolution and tissue-specific expression. J Biol Chem. 1991 Jul 15;266(20):13130–13134. [PubMed] [Google Scholar]
- Zhang J. X., Fasciotto B. H., Cohn D. V. Dexamethasone and calcium interact in the regulation of parathormone and chromogranin-A secretion and messenger ribonucleic acid levels in parathyroid cells. Endocrinology. 1993 Jul;133(1):152–158. doi: 10.1210/endo.133.1.8319562. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]