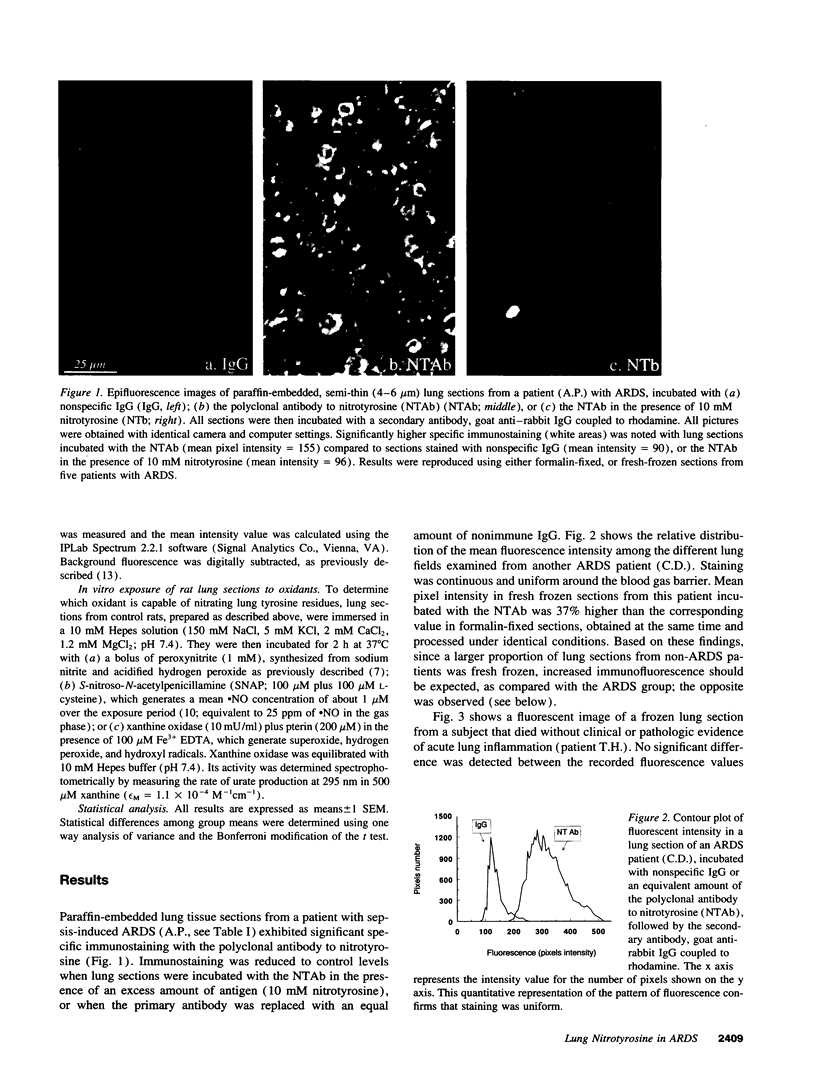
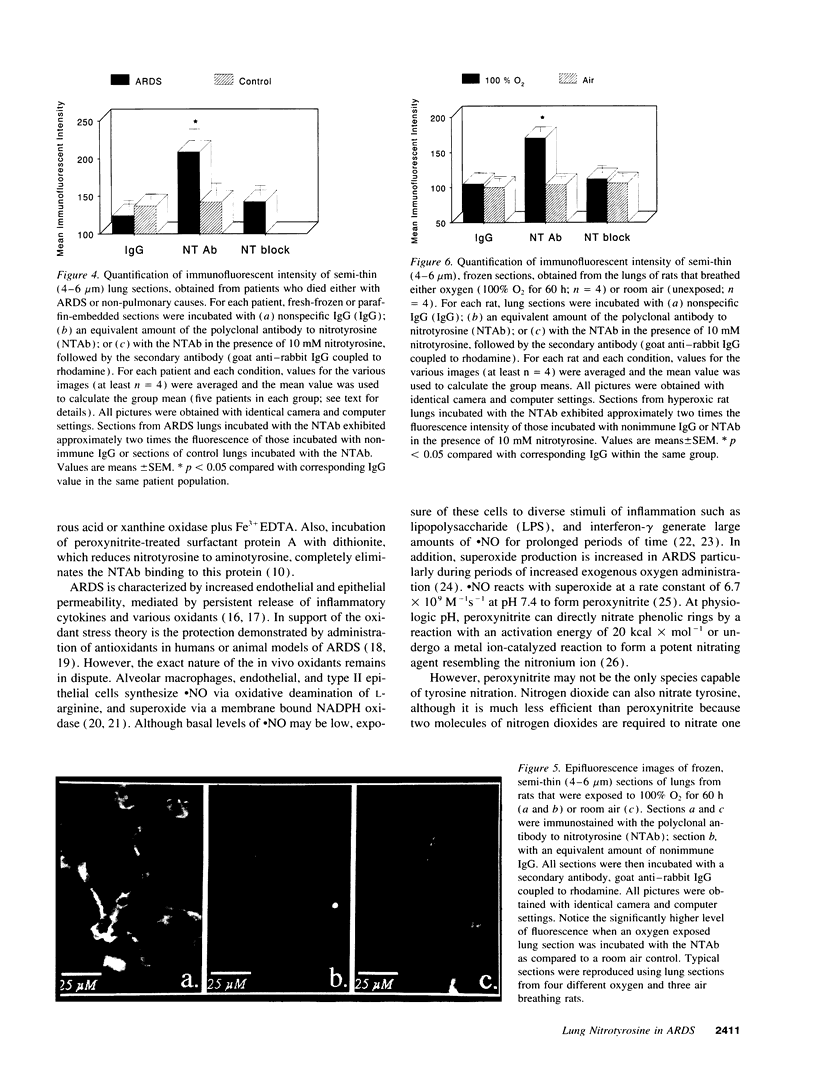
Abstract
Activated alveolar macrophages and epithelial type II cells release both nitric oxide and superoxide which react at near diffusion-limited rate (6.7 x 10(9) M-1s-1) to form peroxynitrite, a potent oxidant capable of damaging the alveolar epithelium and pulmonary surfactant. Peroxynitrite, but not nitric oxide or superoxide, readily nitrates phenolic rings including tyrosine. We quantified the presence of nitrotyrosine in the lungs of patients with the adult respiratory distress syndrome (ARDS) and in the lungs of rats exposed to hyperoxia (100% O2 for 60 h) using quantitative immunofluorescence. Fresh frozen or paraffin-embedded lung sections were incubated with a polyclonal antibody to nitrotyrosine, followed by goat anti-rabbit IgG coupled to rhodamine. Sections from patients with ARDS (n = 5), or from rats exposed to hyperoxia (n = 4), exhibited a twofold increase of specific binding over controls. This binding was blocked by the addition of an excess amount of nitrotyrosine and was absent when the nitrotyrosine antibody was replaced with nonimmune IgG. In additional experiments we demonstrated nitrotyrosine formation in rat lung sections incubated in vitro with peroxynitrite, but not nitric oxide or reactive oxygen species. These data suggest that toxic levels of peroxynitrite may be formed in the lungs of patients with acute lung injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Ischiropoulos H., Zhu L., van der Woerd M., Smith C., Chen J., Harrison J., Martin J. C., Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Donnelly T. J., Meade P., Jagels M., Cryer H. G., Law M. M., Hugli T. E., Shoemaker W. C., Abraham E. Cytokine, complement, and endotoxin profiles associated with the development of the adult respiratory distress syndrome after severe injury. Crit Care Med. 1994 May;22(5):768–776. doi: 10.1097/00003246-199405000-00010. [DOI] [PubMed] [Google Scholar]
- Haddad I. Y., Crow J. P., Hu P., Ye Y., Beckman J., Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol. 1994 Sep;267(3 Pt 1):L242–L249. doi: 10.1152/ajplung.1994.267.3.L242. [DOI] [PubMed] [Google Scholar]
- Haddad I. Y., Ischiropoulos H., Holm B. A., Beckman J. S., Baker J. R., Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol. 1993 Dec;265(6 Pt 1):L555–L564. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- Hu P., Ischiropoulos H., Beckman J. S., Matalon S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am J Physiol. 1994 Jun;266(6 Pt 1):L628–L634. doi: 10.1152/ajplung.1994.266.6.L628. [DOI] [PubMed] [Google Scholar]
- Huie R. E., Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Jänig G. R., Kraft R., Blanck J., Ristau O., Rabe H., Ruckpaul K. Chemical modification of cytochrome P-450 LM4. Identification of functionally linked tyrosine residues. Biochim Biophys Acta. 1987 Dec 18;916(3):512–523. doi: 10.1016/0167-4838(87)90198-1. [DOI] [PubMed] [Google Scholar]
- Kinsella J. P., Neish S. R., Shaffer E., Abman S. H. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992 Oct 3;340(8823):819–820. doi: 10.1016/0140-6736(92)92687-b. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993 Oct;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Stuehr D. J., Nathan C. F. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991 Oct 1;174(4):761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L., Wu D., Jakes S., Graves D. J. Chemical influences on the specificity of tyrosine phosphorylation. J Biol Chem. 1990 May 5;265(13):7108–7111. [PubMed] [Google Scholar]
- Matalon S., Kirk K. L., Bubien J. K., Oh Y., Hu P., Yue G., Shoemaker R., Cragoe E. J., Jr, Benos D. J. Immunocytochemical and functional characterization of Na+ conductance in adult alveolar pneumocytes. Am J Physiol. 1992 May;262(5 Pt 1):C1228–C1238. doi: 10.1152/ajpcell.1992.262.5.C1228. [DOI] [PubMed] [Google Scholar]
- McCall M. N., Easterbrook-Smith S. B. Comparison of the role of tyrosine residues in human IgG and rabbit IgG in binding of complement subcomponent C1q. Biochem J. 1989 Feb 1;257(3):845–851. doi: 10.1042/bj2570845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991 Aug;21(4):361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fernández M. A., Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992 Feb;22(2):301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- Ohshima H., Friesen M., Brouet I., Bartsch H. Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem Toxicol. 1990 Sep;28(9):647–652. doi: 10.1016/0278-6915(90)90173-k. [DOI] [PubMed] [Google Scholar]
- Padgett E. L., Pruett S. B. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1992 Jul 31;186(2):775–781. doi: 10.1016/0006-291x(92)90813-z. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R. V., Gudapaty R., Liener I. E., Schwartz B. A., Hoidal J. R. Protection against pulmonary oxygen toxicity in rats by the intratracheal administration of liposome-encapsulated superoxide dismutase or catalase. Am Rev Respir Dis. 1985 Jul;132(1):164–167. doi: 10.1164/arrd.1985.132.1.164. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Rossaint R., Falke K. J., López F., Slama K., Pison U., Zapol W. M. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993 Feb 11;328(6):399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Loro M. L., Wong V. Z., Tashkin D. P. Cytokine- and Pneumocystis carinii- induced L-arginine oxidation by murine and human pulmonary alveolar macrophages. J Protozool. 1991 Nov-Dec;38(6):234S–236S. [PubMed] [Google Scholar]
- Stadler J., Billiar T. R., Curran R. D., Stuehr D. J., Ochoa J. B., Simmons R. L. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991 May;260(5 Pt 1):C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- Suter P. M., Domenighetti G., Schaller M. D., Laverrière M. C., Ritz R., Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest. 1994 Jan;105(1):190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]






