Abstract
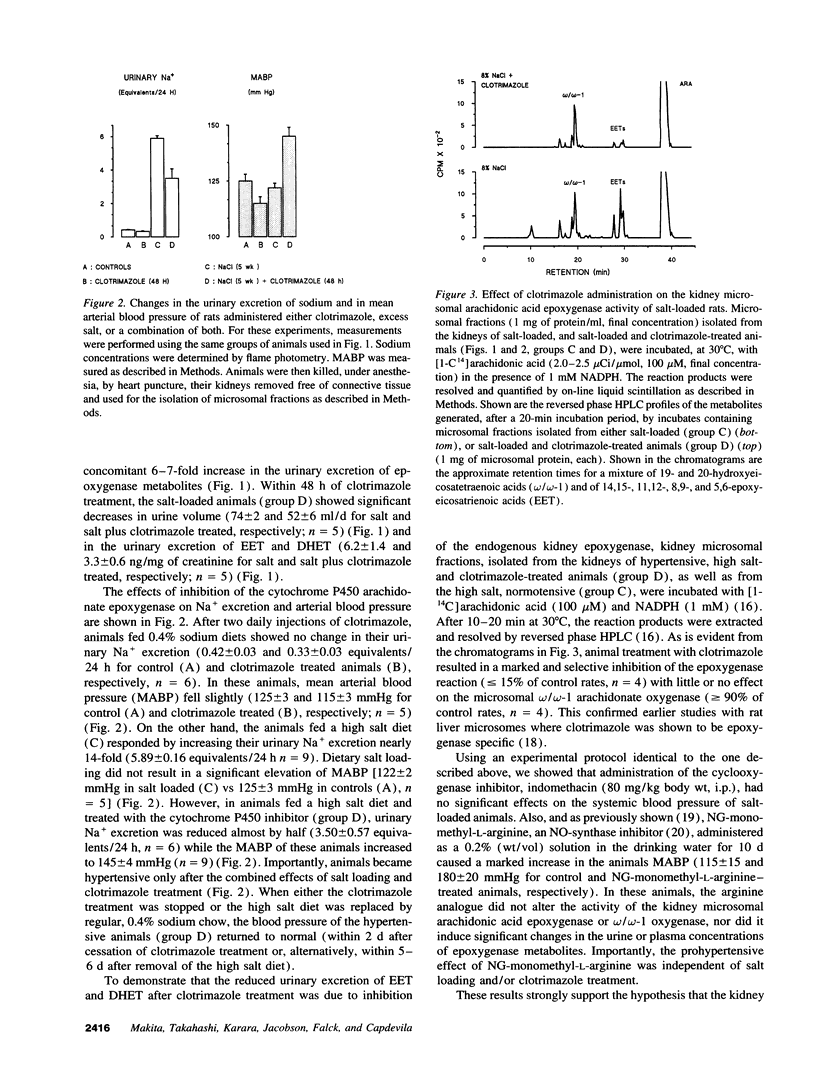
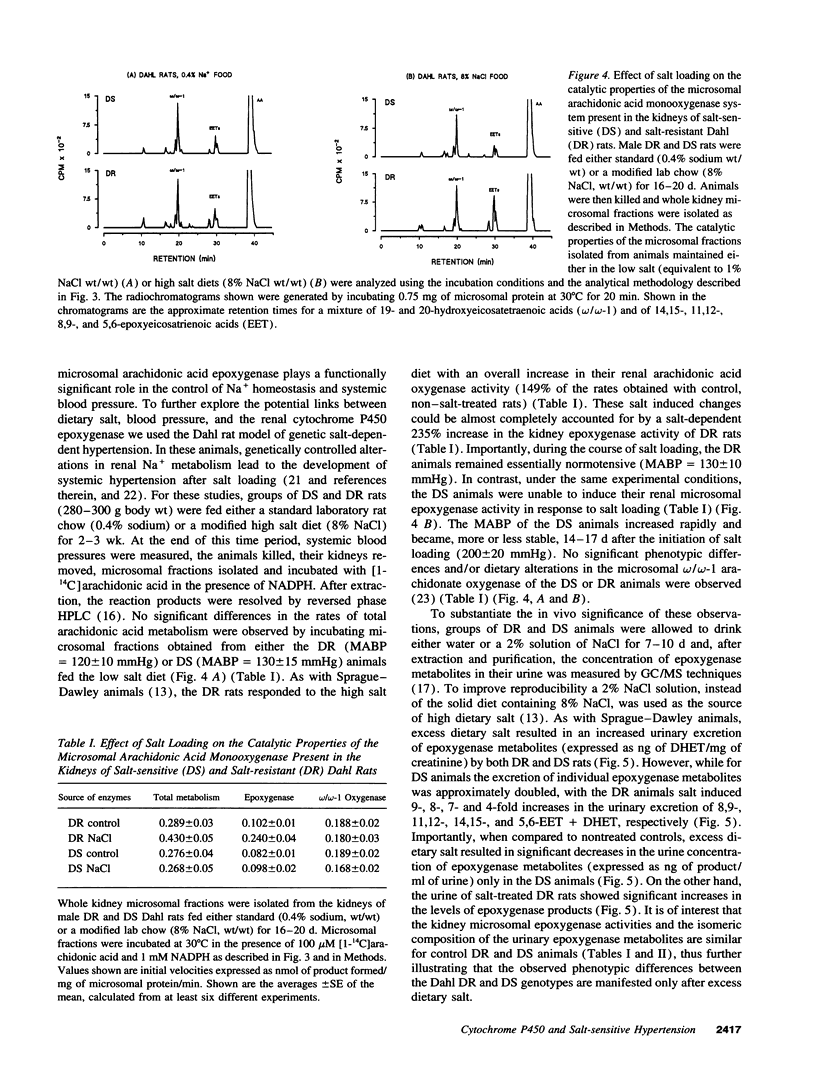
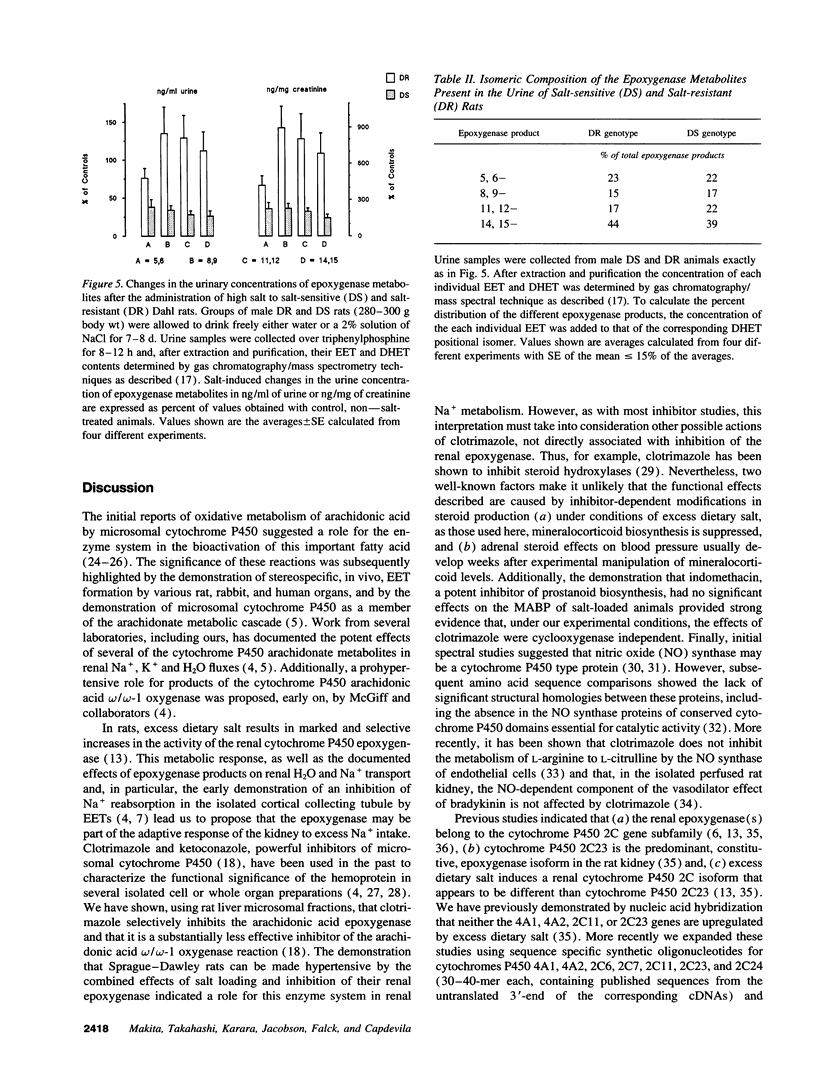
Excess dietary salt induces a cytochrome P450 arachidonic acid epoxygenase isoform in rat kidneys (Capdevila, J. H., S. Wei, J. Yang, A. Karara, H. R. Jacobson, J. R. Falck, F. P. Guengerich, and R. N. Dubois. 1992. J. Biol. Chem. 267:21720-21726). Treatment of rats on a high salt diet with the epoxygenase inhibitor, clotrimazole, produces significant increases in mean arterial blood pressure (122 +/- 2 and 145 +/- 4 mmHg for salt and salt- and clotrimazole-treated rats, respectively). The salt- and clotrimazole-dependent hypertension is accompanied by reductions in the urinary excretion of epoxygenase metabolites and by a selective inhibition of the renal microsomal epoxygenase reaction. The prohypertensive effects of clotrimazole are readily reversed when either the salt or clotrimazole treatment is discontinued. The indication that a salt-inducible renal epoxygenase protects against hypertension, are supported by studies with the Dahl rat model of genetic salt-sensitive hypertension. Dahl resistant animals responded to excess dietary salt by inducing the activity of their kidney microsomal epoxygenase(s) (0.102 +/- 0.01 and 0.240 +/- 0.04 nmol of products formed/min per mg of microsomal protein for control and salt-treated rats, respectively). Despite severe hypertension during excess dietary salt intake (200 +/- 20 mmHg), Dahl salt-sensitive rats demonstrated no increase in renal epoxygenase activity. These studies indicate that acquired or inherited abnormalities in renal epoxygenase activities and/or regulation can be related to salt-sensitive hypertension in rodents. Studies on the human renal epoxygenase and its relationship to salt hypertension may prove useful.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Montero M., Garcia-Sancho J. Cytochrome P450 may regulate plasma membrane Ca2+ permeability according to the filling state of the intracellular Ca2+ stores. FASEB J. 1992 Jan 6;6(2):786–792. doi: 10.1096/fasebj.6.2.1537469. [DOI] [PubMed] [Google Scholar]
- Capdevila J. H., Dishman E., Karara A., Falck J. R. Cytochrome P450 arachidonic acid epoxygenase: stereochemical characterization of epoxyeicosatrienoic acids. Methods Enzymol. 1991;206:441–453. doi: 10.1016/0076-6879(91)06113-h. [DOI] [PubMed] [Google Scholar]
- Capdevila J. H., Falck J. R., Dishman E., Karara A. Cytochrome P-450 arachidonate oxygenase. Methods Enzymol. 1990;187:385–394. doi: 10.1016/0076-6879(90)87045-5. [DOI] [PubMed] [Google Scholar]
- Capdevila J. H., Falck J. R., Estabrook R. W. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992 Jan 6;6(2):731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- Capdevila J. H., Karara A., Waxman D. J., Martin M. V., Falck J. R., Guenguerich F. P. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem. 1990 Jul 5;265(19):10865–10871. [PubMed] [Google Scholar]
- Capdevila J. H., Wei S., Yan J., Karara A., Jacobson H. R., Falck J. R., Guengerich F. P., DuBois R. N. Cytochrome P-450 arachidonic acid epoxygenase. Regulatory control of the renal epoxygenase by dietary salt loading. J Biol Chem. 1992 Oct 25;267(30):21720–21726. [PubMed] [Google Scholar]
- Capdevila J., Chacos N., Werringloer J., Prough R. A., Estabrook R. W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Gil L., Orellana M., Marnett L. J., Mason J. I., Yadagiri P., Falck J. R. Inhibitors of cytochrome P-450-dependent arachidonic acid metabolism. Arch Biochem Biophys. 1988 Mar;261(2):257–263. doi: 10.1016/0003-9861(88)90340-2. [DOI] [PubMed] [Google Scholar]
- Catella F., Lawson J. A., Fitzgerald D. J., FitzGerald G. A. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5893–5897. doi: 10.1073/pnas.87.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl L. K., Heine M., Thompson K. Genetic influence of the kidneys on blood pressure. Evidence from chronic renal homografts in rats with opposite predispositions to hypertension. Circ Res. 1974 Jan;34(1):94–101. doi: 10.1161/01.res.40.4.94. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Murphy R. C. Cytochrome P-450 metabolism of arachidonic acid: formation and biological actions of "epoxygenase"-derived eicosanoids. Pharmacol Rev. 1988 Dec;40(4):229–241. [PubMed] [Google Scholar]
- Fulton D., McGiff J. C., Quilley J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br J Pharmacol. 1992 Nov;107(3):722–725. doi: 10.1111/j.1476-5381.1992.tb14513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., Bennett T., Palmer R. M., Moncada S. Nitric oxide synthase inhibitors cause sustained, but reversible, hypertension and hindquarters vasoconstriction in Brattleboro rats. Eur J Pharmacol. 1992 Mar 31;213(3):449–451. doi: 10.1016/0014-2999(92)90636-i. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Herrera V. L., Ruiz-Opazo N. Alteration of alpha 1 Na+,K(+)-ATPase 86Rb+ influx by a single amino acid substitution. Science. 1990 Aug 31;249(4972):1023–1026. doi: 10.1126/science.1975705. [DOI] [PubMed] [Google Scholar]
- Hirt D. L., Capdevila J., Falck J. R., Breyer M. D., Jacobson H. R. Cytochrome P450 metabolites of arachidonic acid are potent inhibitors of vasopressin action on rabbit cortical collecting duct. J Clin Invest. 1989 Dec;84(6):1805–1812. doi: 10.1172/JCI114365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai N., Inagami T. Isolation of preferentially expressed genes in the kidneys of hypertensive rats. Hypertension. 1991 Feb;17(2):161–169. doi: 10.1161/01.hyp.17.2.161. [DOI] [PubMed] [Google Scholar]
- Junier M. P., Dray F., Blair I., Capdevila J., Dishman E., Falck J. R., Ojeda S. R. Epoxygenase products of arachidonic acid are endogenous constituents of the hypothalamus involved in D2 receptor-mediated, dopamine-induced release of somatostatin. Endocrinology. 1990 Mar;126(3):1534–1540. doi: 10.1210/endo-126-3-1534. [DOI] [PubMed] [Google Scholar]
- Karara A., Makita K., Jacobson H. R., Falck J. R., Guengerich F. P., DuBois R. N., Capdevila J. H. Molecular cloning, expression, and enzymatic characterization of the rat kidney cytochrome P-450 arachidonic acid epoxygenase. J Biol Chem. 1993 Jun 25;268(18):13565–13570. [PubMed] [Google Scholar]
- Katoh T., Takahashi K., Capdevila J., Karara A., Falck J. R., Jacobson H. R., Badr K. F. Glomerular stereospecific synthesis and hemodynamic actions of 8,9-epoxyeicosatrienoic acid in rat kidney. Am J Physiol. 1991 Oct;261(4 Pt 2):F578–F586. doi: 10.1152/ajprenal.1991.261.4.F578. [DOI] [PubMed] [Google Scholar]
- Laethem R. M., Laethem C. L., Koop D. R. Purification and properties of a cytochrome P450 arachidonic acid epoxygenase from rabbit renal cortex. J Biol Chem. 1992 Mar 15;267(8):5552–5559. [PubMed] [Google Scholar]
- Matsukawa N., Nonaka Y., Higaki J., Nagano M., Mikami H., Ogihara T., Okamoto M. Dahl's salt-resistant normotensive rat has mutations in cytochrome P450(11 beta), but the salt-sensitive hypertensive rat does not. J Biol Chem. 1993 Apr 25;268(12):9117–9121. [PubMed] [Google Scholar]
- McGiff J. C. Cytochrome P-450 metabolism of arachidonic acid. Annu Rev Pharmacol Toxicol. 1991;31:339–369. doi: 10.1146/annurev.pa.31.040191.002011. [DOI] [PubMed] [Google Scholar]
- McMillan K., Bredt D. S., Hirsch D. J., Snyder S. H., Clark J. E., Masters B. S. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. R., Pascoe N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7375–7378. doi: 10.1073/pnas.78.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Nishi A., Bertorello A. M., Aperia A. High salt diet down-regulates proximal tubule Na+, K(+)-ATPase activity in Dahl salt-resistant but not in Dahl salt-sensitive rats: evidence of defective dopamine regulation. Acta Physiol Scand. 1992 Mar;144(3):263–267. doi: 10.1111/j.1748-1716.1992.tb09295.x. [DOI] [PubMed] [Google Scholar]
- Oliw E. H., Oates J. A. Oxygenation of arachidonic acid by hepatic microsomes of the rabbit. Mechanism of biosynthesis of two vicinal dihydroxyeicosatrienoic acids. Biochim Biophys Acta. 1981 Dec 23;666(3):327–340. doi: 10.1016/0005-2760(81)90291-5. [DOI] [PubMed] [Google Scholar]
- Oyekan A. O., McGiff J. C., Rosencrantz-Weiss P., Quilley J. Relaxant responses of rabbit aorta: influence of cytochrome P450 inhibitors. J Pharmacol Exp Ther. 1994 Jan;268(1):262–269. [PubMed] [Google Scholar]
- Rapp J. P. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982 Nov-Dec;4(6):753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- Rapp J. P., Dahl L. K. Mutant forms of cytochrome P-450 controlling both 18- and 11beta-steroid hydroxylation in the rat. Biochemistry. 1976 Mar 23;15(6):1235–1242. doi: 10.1021/bi00651a010. [DOI] [PubMed] [Google Scholar]
- Rapp J. P., Wang S. M., Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science. 1989 Jan 27;243(4890):542–544. doi: 10.1126/science.2563177. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman R. J., Ma Y. H., Frohlich B., Markham B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension. 1993 Jun;21(6 Pt 2):985–988. doi: 10.1161/01.hyp.21.6.985. [DOI] [PubMed] [Google Scholar]
- Satoh T., Cohen H. T., Katz A. I. Different mechanisms of renal Na-K-ATPase regulation by protein kinases in proximal and distal nephron. Am J Physiol. 1993 Sep;265(3 Pt 2):F399–F405. doi: 10.1152/ajprenal.1993.265.3.F399. [DOI] [PubMed] [Google Scholar]
- Satoh T., Cohen H. T., Katz A. I. Intracellular signaling in the regulation of renal Na-K-ATPase. II. Role of eicosanoids. J Clin Invest. 1993 Feb;91(2):409–415. doi: 10.1172/JCI116215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman J. B., Thummel K. E., Favreau L. V. Physiological and pathophysiological alterations in rat hepatic cytochrome P-450. Drug Metab Rev. 1989;20(2-4):557–584. doi: 10.3109/03602538909103562. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., Petty E., Oates J. A., Jacoby M., Levine S. D. Epoxygenase metabolites of arachidonic acid inhibit vasopressin response in toad bladder. Am J Physiol. 1987 Sep;253(3 Pt 2):F464–F470. doi: 10.1152/ajprenal.1987.253.3.F464. [DOI] [PubMed] [Google Scholar]
- Simonet L., St Lezin E., Kurtz T. W. Sequence analysis of the alpha 1 Na+,K(+)-ATPase gene in the Dahl salt-sensitive rat. Hypertension. 1991 Nov;18(5):689–693. doi: 10.1161/01.hyp.18.5.689. [DOI] [PubMed] [Google Scholar]
- Wada A., Ohnishi T., Nonaka Y., Okamoto M. Inhibition of bovine adrenocortical mitochondrial cytochrome P-450(11)beta-mediated reactions by imidazole derivatives and mineralocorticoid analogs. J Steroid Biochem. 1988 Nov;31(5):803–808. doi: 10.1016/0022-4731(88)90289-0. [DOI] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]



