Abstract
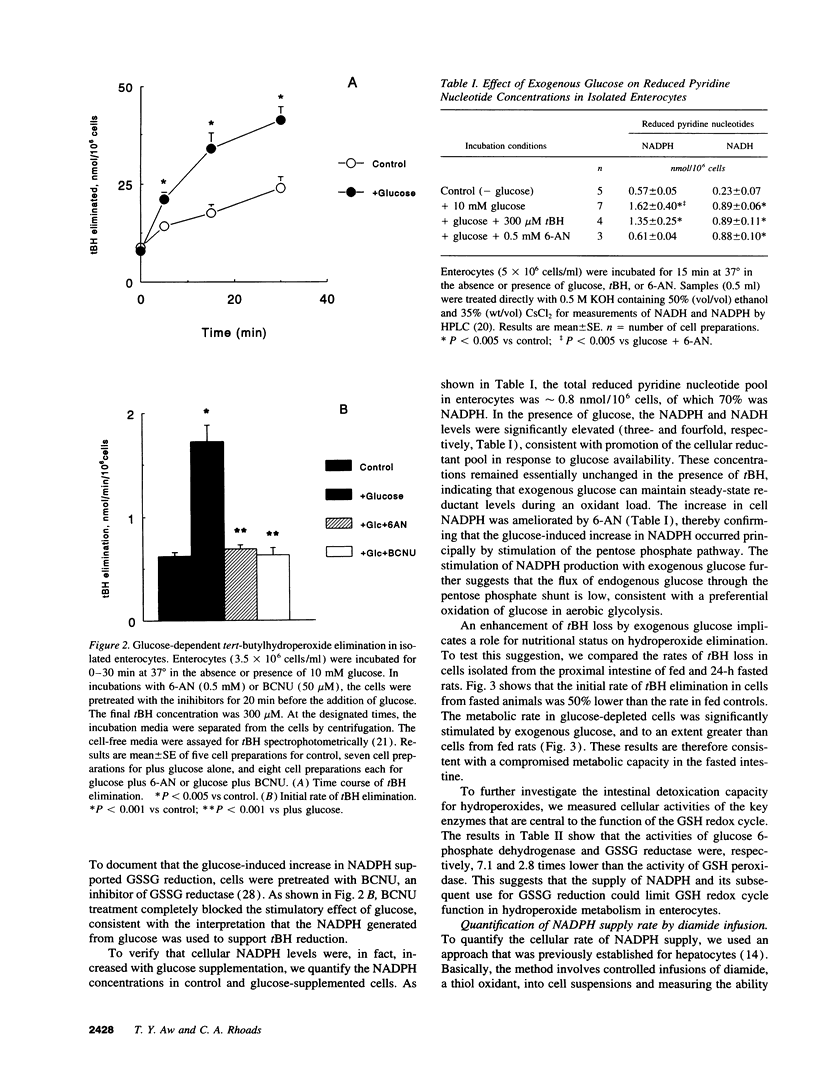
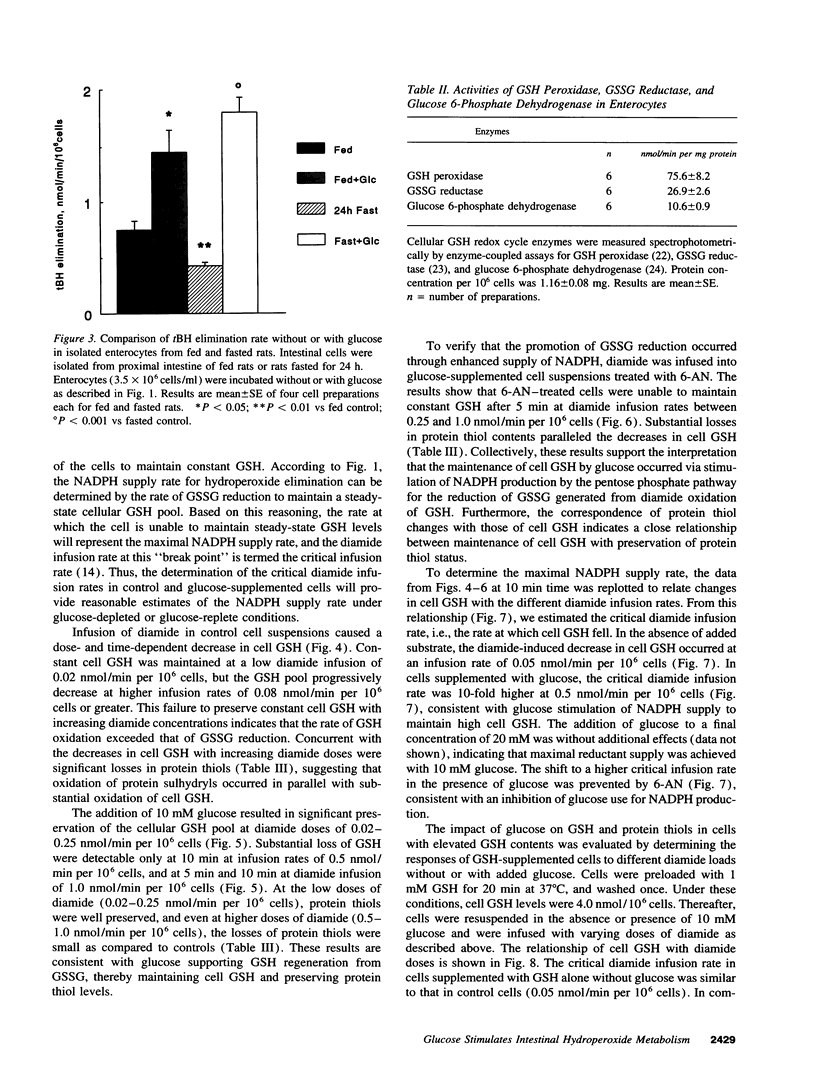
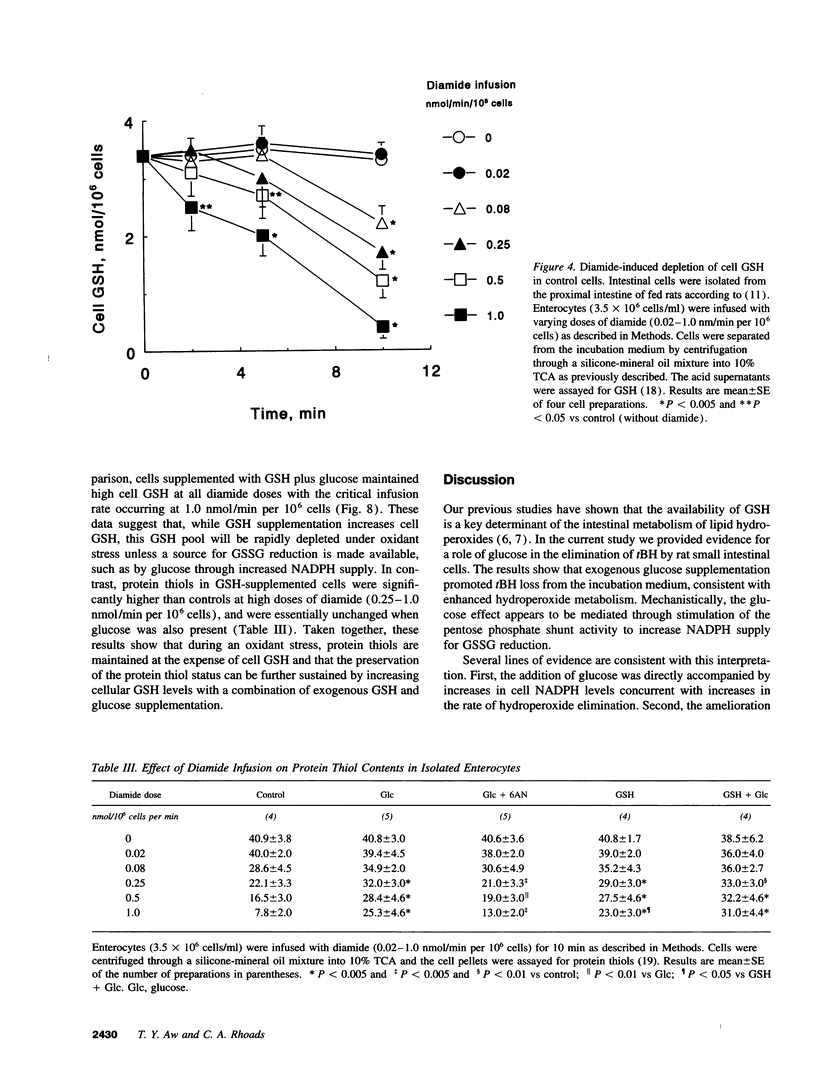
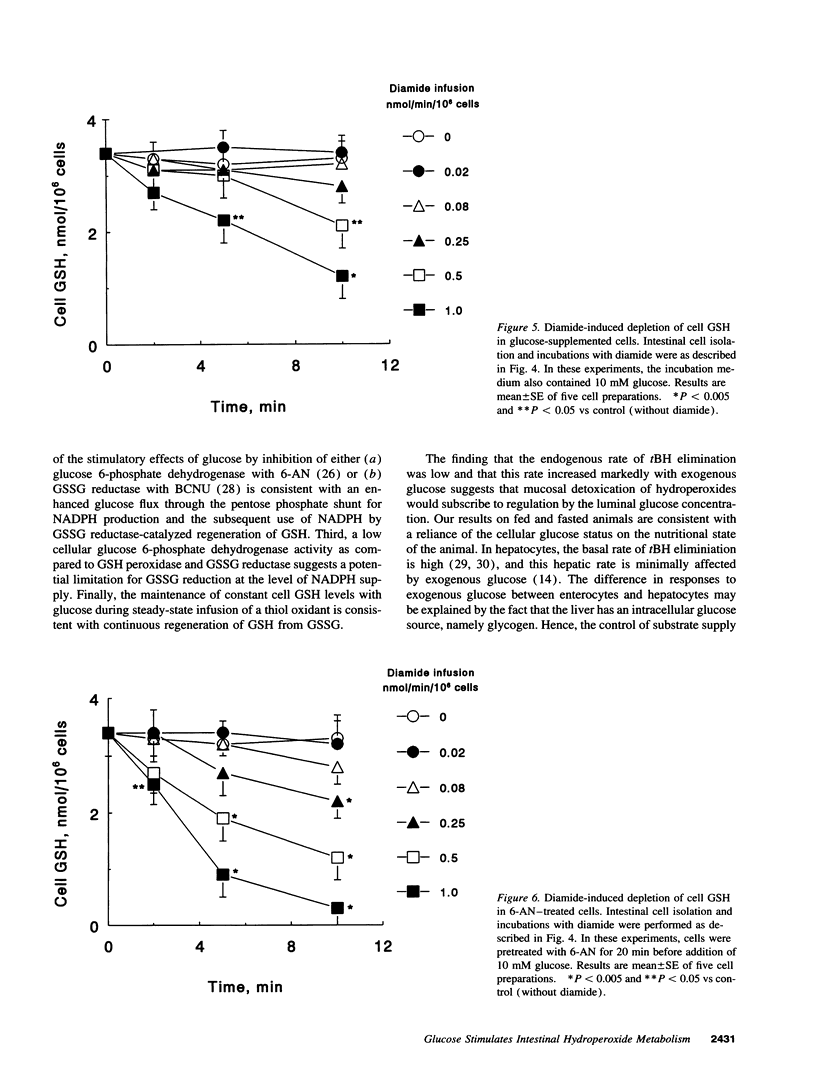
The regulation of intestinal metabolism of t-butylhydroperoxide by glucose was examined in isolated enterocytes from proximal rat intestine. The basal rate of hydroperoxide elimination in control cells was 0.57 +/- 0.05 nmol/min per 10(6) cells, and was increased threefold by 10 mM exogenous glucose (1.74 +/- 0.14 nmol/min per 10(6) cells). Concurrently, cellular NADPH levels increased threefold (1.62 +/- 0.40 nmol/10(6) cells vs 0.57 +/- 0.14 nmol/10(6) cells in controls). The glucose effect was blocked by 6-aminonicotinamide and by 1,3-bis-(2-chloroethyl) 1-nitrosourea, consistent with glucose stimulation of NADPH production by the pentose phosphate shunt, and of NADPH utilization for glutathione disulfide reduction. The NADPH supply rate was quantified by controlled infusions of diamide, a thiol oxidant. At diamide infusion of 0.05 nmol/min per 10(6) cells, GSH and protein thiols in control cells were decreased significantly, consistent with a limited capacity for glutathione disulfide reduction. With glucose, cell GSH and protein thiols were preserved at a 10-fold higher diamide infusion which was reversed by 6-aminonicotinamide, supporting the view that glucose promotes glutathione disulfide reduction by increased NADPH supply. Collectively, the results demonstrate that intestinal metabolism of hydroperoxides subscribes to regulation by glucose availability. This responsiveness to glucose suggests that nutrient availability would be an important contributing factor in the detoxication of toxic hydroperoxides by the small intestine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Andersson B. S., Jones D. P. Use of digitonin fractionation to determine mitochondrial transmembrane ion distribution in cells during anoxia. Anal Biochem. 1985 Apr;146(1):164–172. doi: 10.1016/0003-2697(85)90411-7. [DOI] [PubMed] [Google Scholar]
- Andreoli S. P., Mallett C. P., Bergstein J. M. Role of glutathione in protecting endothelial cells against hydrogen peroxide oxidant injury. J Lab Clin Med. 1986 Sep;108(3):190–198. [PubMed] [Google Scholar]
- Aw T. Y., Andersson B. S., Jones D. P. Mitochondrial transmembrane ion distribution during anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C356–C361. doi: 10.1152/ajpcell.1987.252.4.C356. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Jones D. P. ATP concentration gradients in cytosol of liver cells during hypoxia. Am J Physiol. 1985 Nov;249(5 Pt 1):C385–C392. doi: 10.1152/ajpcell.1985.249.5.C385. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Jones D. P. Secondary bioenergetic hypoxia. Inhibition of sulfation and glucuronidation reactions in isolated hepatocytes at low O2 concentration. J Biol Chem. 1982 Aug 10;257(15):8997–9004. [PubMed] [Google Scholar]
- Aw T. Y., Williams M. W. Intestinal absorption and lymphatic transport of peroxidized lipids in rats: effect of exogenous GSH. Am J Physiol. 1992 Nov;263(5 Pt 1):G665–G672. doi: 10.1152/ajpgi.1992.263.5.G665. [DOI] [PubMed] [Google Scholar]
- Babson J. R., Reed D. J. Inactivation of glutathione reductase by 2-chloroethyl nitrosourea-derived isocyanates. Biochem Biophys Res Commun. 1978 Jul 28;83(2):754–762. doi: 10.1016/0006-291x(78)91053-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coles B., Ketterer B. The role of glutathione and glutathione transferases in chemical carcinogenesis. Crit Rev Biochem Mol Biol. 1990;25(1):47–70. doi: 10.3109/10409239009090605. [DOI] [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Regulation of the pentose phosphate cycle. Biochem J. 1974 Mar;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G., Lysko H. A precise method for the determination of whole blood and plasma sulfhydryl groups. Anal Biochem. 1979 Feb;93(1):98–102. [PubMed] [Google Scholar]
- Erickson R. R., Holtzman J. L. Kinetic studies on the metabolism of ethylmorphine by isolated hepatocytes from adult rats. Biochem Pharmacol. 1976 Jul 1;25(13):1501–1506. doi: 10.1016/0006-2952(76)90068-x. [DOI] [PubMed] [Google Scholar]
- Geiger P. G., Thomas J. P., Girotti A. W. Lethal damage to murine L1210 cells by exogenous lipid hydroperoxides: protective role of glutathione-dependent selenoperoxidases. Arch Biochem Biophys. 1991 Aug 1;288(2):671–680. doi: 10.1016/0003-9861(91)90250-m. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Aw T. Y., Jones D. P. Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 1988 Jul;34(1):74–81. doi: 10.1038/ki.1988.147. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Brown L. A., Jones D. P. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986 Dec 15;35(24):4537–4542. doi: 10.1016/0006-2952(86)90776-8. [DOI] [PubMed] [Google Scholar]
- Heath R. L., Tappel A. L. A new sensitive assay for the measurement of hydroperoxides. Anal Biochem. 1976 Nov;76(50):184–191. doi: 10.1016/0003-2697(76)90277-3. [DOI] [PubMed] [Google Scholar]
- Jones D. P. Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J Chromatogr. 1981 Oct 9;225(2):446–449. doi: 10.1016/s0378-4347(00)80293-5. [DOI] [PubMed] [Google Scholar]
- Kowalski D. P., Aw T. Y., Park Y., Jones D. P. Postanoxic oxidative injury in rat hepatocytes: lactate-dependent protection against tert-butylhydroperoxide. Free Radic Biol Med. 1992;12(3):205–212. doi: 10.1016/0891-5849(92)90028-f. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Hagen T. M., Jones D. P. Exogenous glutathione protects intestinal epithelial cells from oxidative injury. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4641–4645. doi: 10.1073/pnas.83.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masola B., Evered D. F. Preparation of rat enterocyte mitochondria. Biochem J. 1984 Mar 1;218(2):441–447. doi: 10.1042/bj2180441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Rush G. F., Alberts D. tert.-Butyl hydroperoxide metabolism and stimulation of the pentose phosphate pathway in isolated rat hepatocytes. Toxicol Appl Pharmacol. 1986 Sep 30;85(3):324–331. doi: 10.1016/0041-008x(86)90339-x. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Scholz R. Interaction of mixed-function oxidation with biosynthetic processes. 2. Inhibition of lipogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):73–78. doi: 10.1111/j.1432-1033.1973.tb03035.x. [DOI] [PubMed] [Google Scholar]
- Tribble D. L., Jones D. P., Edmondson D. E. Effect of hypoxia on tert-butylhydroperoxide-induced oxidative injury in hepatocytes. Mol Pharmacol. 1988 Sep;34(3):413–420. [PubMed] [Google Scholar]
- Tribble D. L., Jones D. P. Oxygen dependence of oxidative stress. Rate of NADPH supply for maintaining the GSH pool during hypoxia. Biochem Pharmacol. 1990 Feb 15;39(4):729–736. doi: 10.1016/0006-2952(90)90152-b. [DOI] [PubMed] [Google Scholar]
- Waydhas C., Weigl K., Sies H. The disposition of formaldehyde and formate arising from drug N-demethylations dependent on cytochrome P-450 in hepatocytes and in perfused rat liver. Eur J Biochem. 1978 Aug 15;89(1):143–150. doi: 10.1111/j.1432-1033.1978.tb20906.x. [DOI] [PubMed] [Google Scholar]
- Weigl K., Sies H. Drug oxidations dependent on cytochrome P-450 in isolated hepatocytes. The role of the tricarboxylates and the aminotransferases in NADPH supply. Eur J Biochem. 1977 Jul 15;77(2):401–408. doi: 10.1111/j.1432-1033.1977.tb11680.x. [DOI] [PubMed] [Google Scholar]



