Abstract
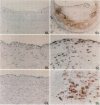
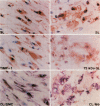
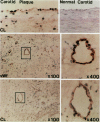
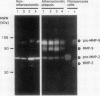
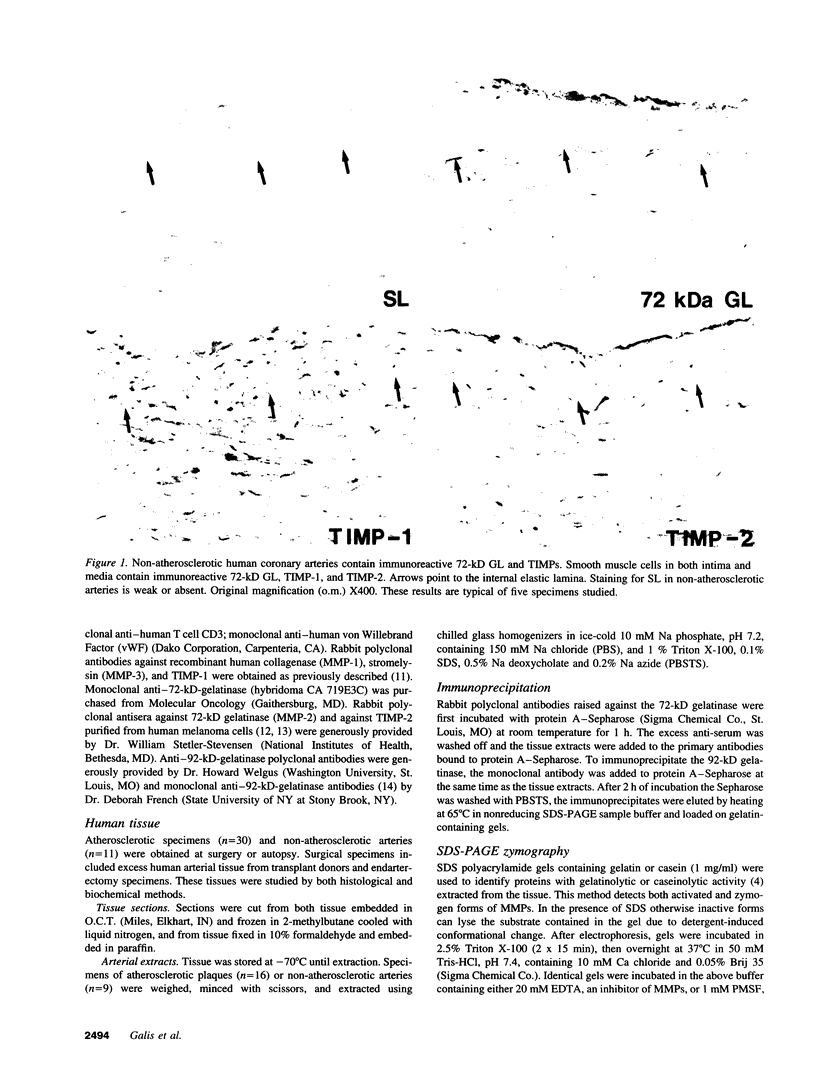
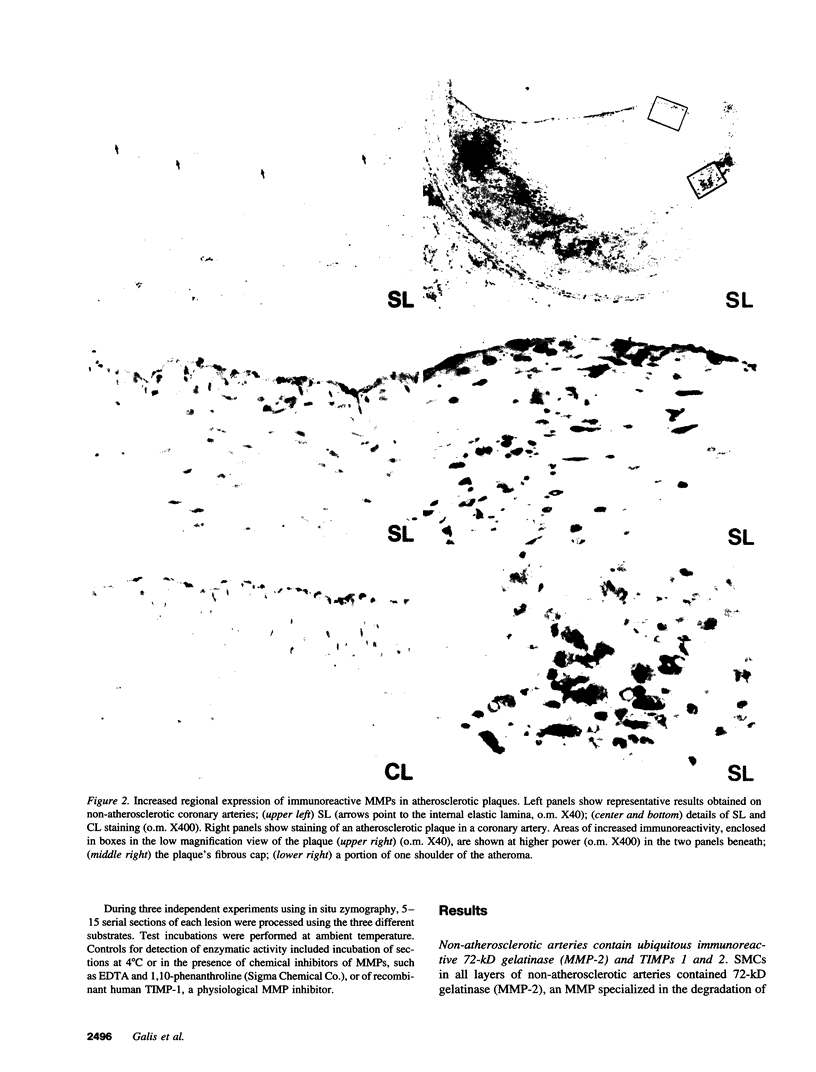
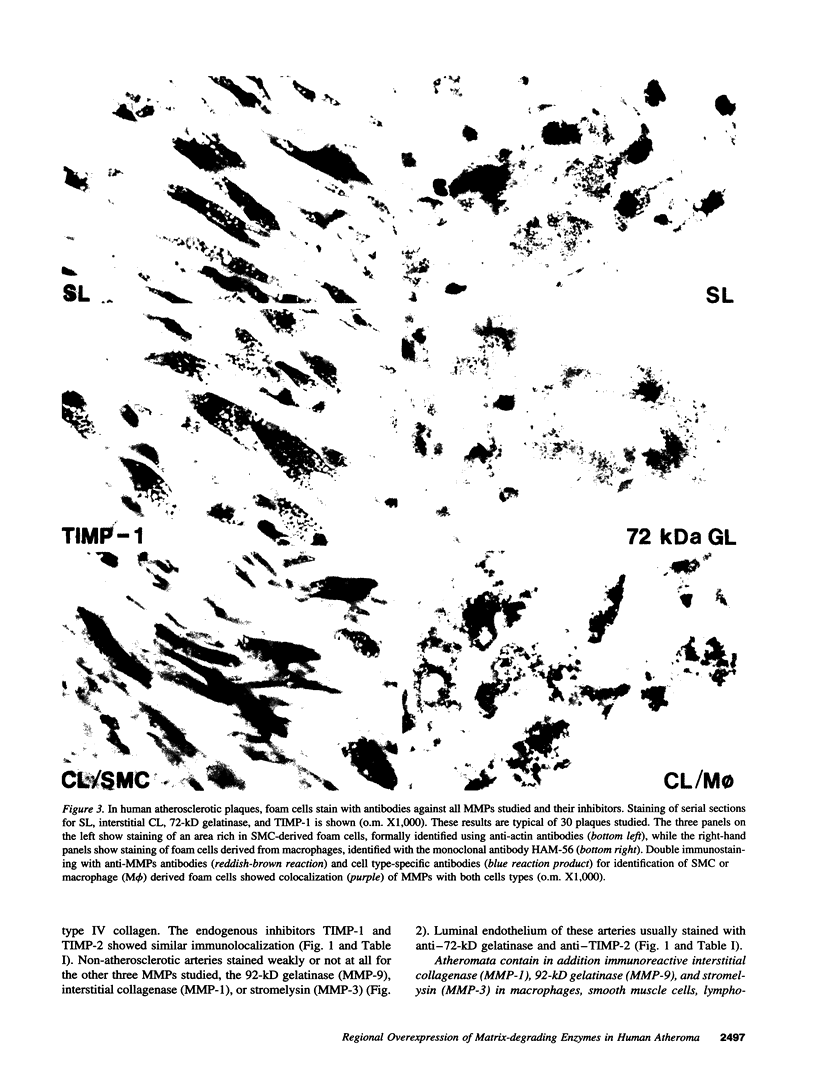
Dysregulated extracellular matrix (ECM) metabolism may contribute to vascular remodeling during the development and complication of human atherosclerotic lesions. We investigated the expression of matrix metalloproteinases (MMPs), a family of enzymes that degrade ECM components in human atherosclerotic plaques (n = 30) and in uninvolved arterial specimens (n = 11). We studied members of all three MMP classes (interstitial collagenase, MMP-1; gelatinases, MMP-2 and MMP-9; and stromelysin, MMP-3) and their endogenous inhibitors (TIMPs 1 and 2) by immunocytochemistry, zymography, and immunoprecipitation. Normal arteries stained uniformly for 72-kD gelatinase and TIMPs. In contrast, plaques' shoulders and regions of foam cell accumulation displayed locally increased expression of 92-kD gelatinase, stromelysin, and interstitial collagenase. However, the mere presence of MMP does not establish their catalytic capacity, as the zymogens lack activity, and TIMPs may block activated MMPs. All plaque extracts contained activated forms of gelatinases determined zymographically and by degradation of 3H-collagen type IV. To test directly whether atheromata actually contain active matrix-degrading enzymes in situ, we devised a method which allows the detection and microscopic localization of MMP enzymatic activity directly in tissue sections. In situ zymography revealed gelatinolytic and caseinolytic activity in frozen sections of atherosclerotic but not of uninvolved arterial tissues. The MMP inhibitors, EDTA and 1,10-phenanthroline, as well as recombinant TIMP-1, reduced these activities which colocalized with regions of increased immunoreactive MMP expression, i.e., the shoulders, core, and microvasculature of the plaques. Focal overexpression of activated MMP may promote destabilization and complication of atherosclerotic plaques and provide novel targets for therapeutic intervention.
Full text
PDF
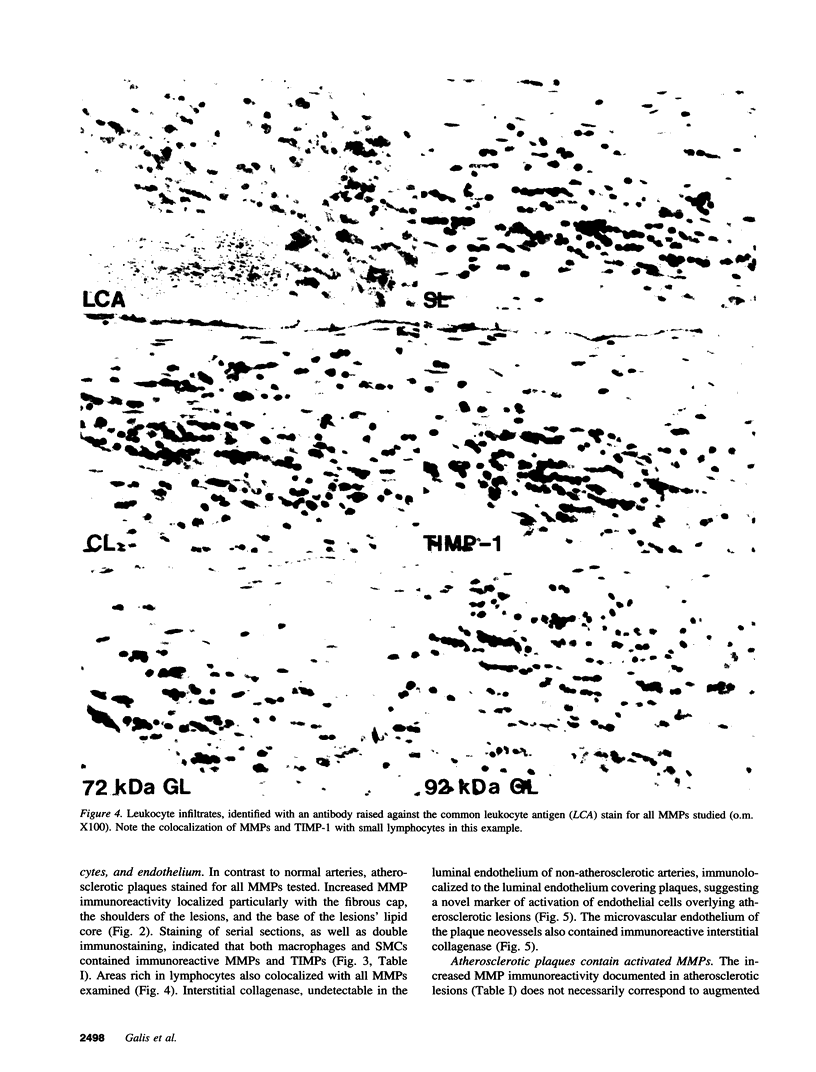
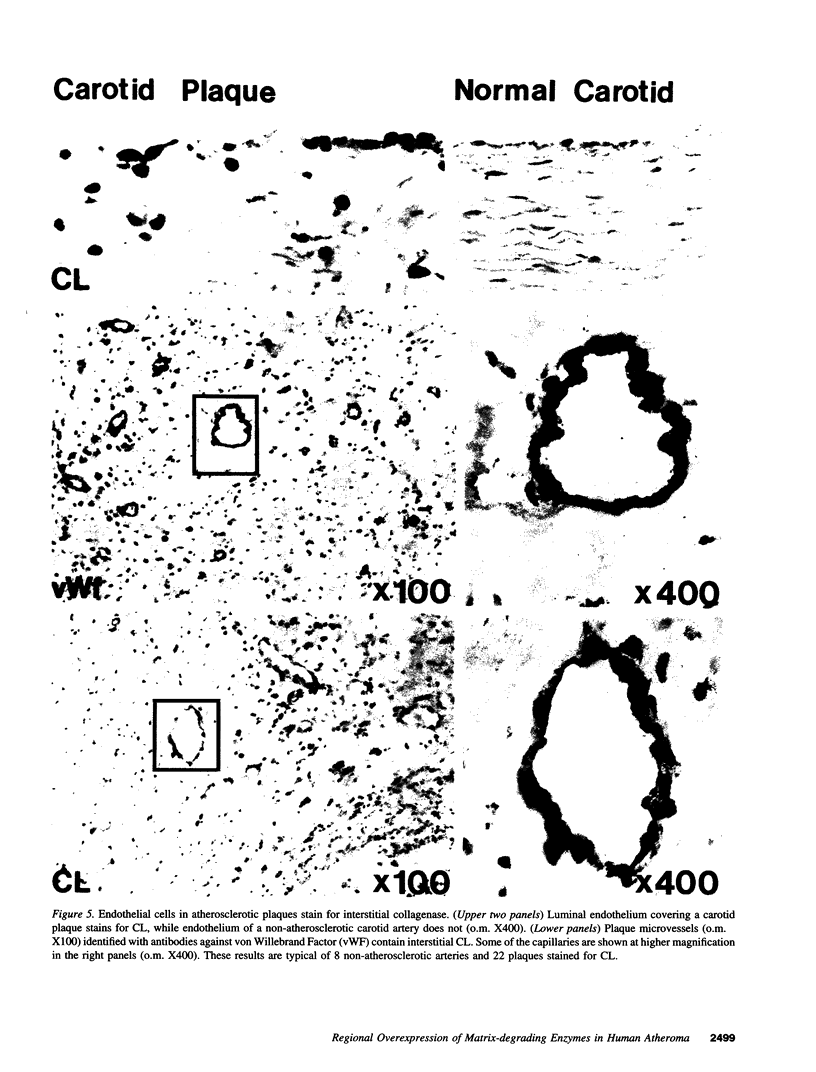
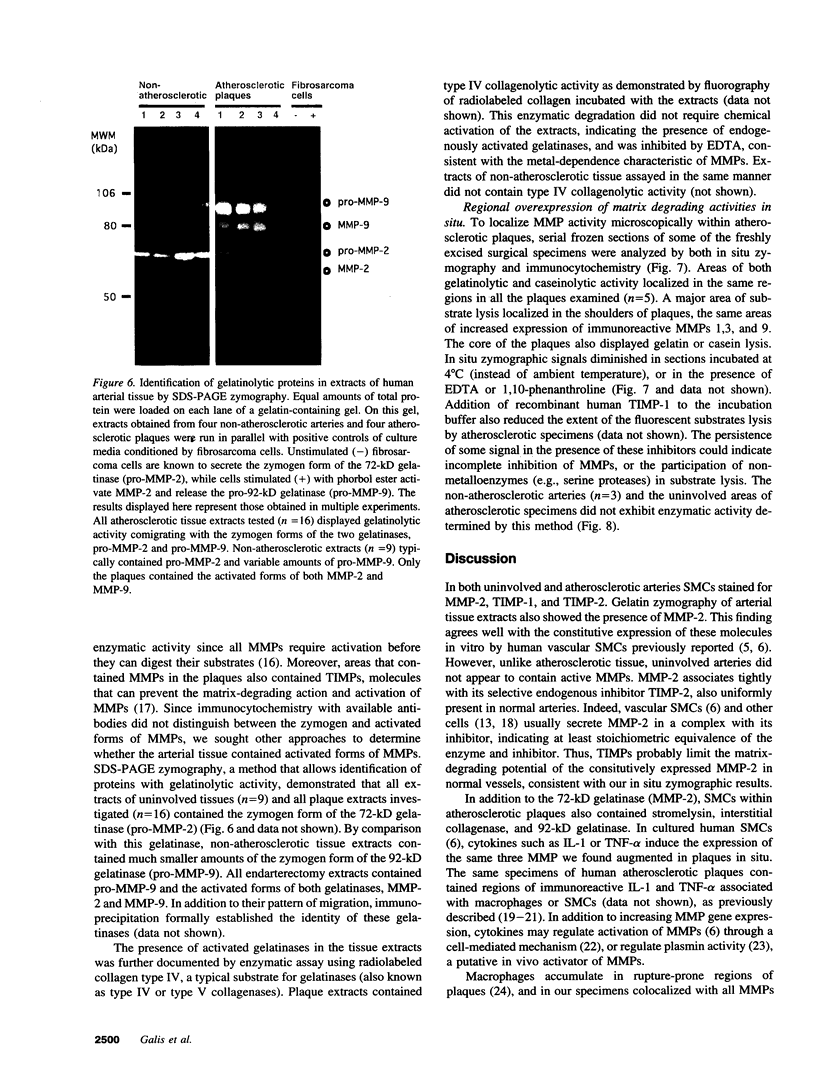
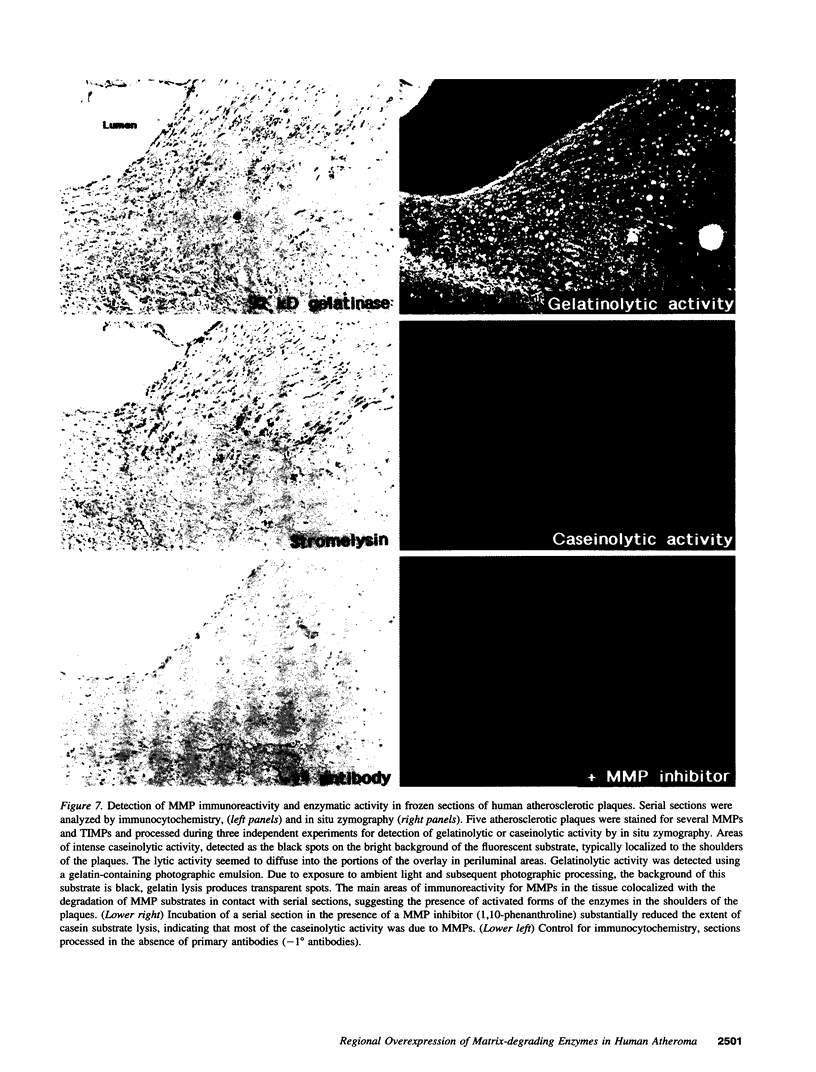

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990 Feb 1;65(5):297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Schleef R. R., Gimbrone M. A., Jr, Loskutoff D. J. Regulation of the fibrinolytic system of cultured human vascular endothelium by interleukin 1. J Clin Invest. 1986 Aug;78(2):587–591. doi: 10.1172/JCI112613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Cheng G. C., Loree H. M., Kamm R. D., Fishbein M. C., Lee R. T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993 Apr;87(4):1179–1187. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Davies M. J., Richardson P. D., Woolf N., Katz D. R., Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993 May;69(5):377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z. S., Muszynski M., Sukhova G. K., Simon-Morrissey E., Unemori E. N., Lark M. W., Amento E., Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994 Jul;75(1):181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- Glagov S., Weisenberg E., Zarins C. K., Stankunavicius R., Kolettis G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Henney A. M., Wakeley P. R., Davies M. J., Foster K., Hembry R., Murphy G., Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Herron G. S., Werb Z., Dwyer K., Banda M. J. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem. 1986 Feb 25;261(6):2810–2813. [PubMed] [Google Scholar]
- Höyhtyä M., Turpeenniemi-Hujanen T., Stetler-Stevenson W., Krutzsch H., Tryggvason K., Liotta L. A. Monoclonal antibodies to type IV collagenase recognize a protein with limited sequence homology to interstitial collagenase and stromelysin. FEBS Lett. 1988 Jun 6;233(1):109–113. doi: 10.1016/0014-5793(88)81365-6. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McPherson D. D., Sirna S. J., Hiratzka L. F., Thorpe L., Armstrong M. L., Marcus M. L., Kerber R. E. Coronary arterial remodeling studied by high-frequency epicardial echocardiography: an early compensatory mechanism in patients with obstructive coronary atherosclerosis. J Am Coll Cardiol. 1991 Jan;17(1):79–86. doi: 10.1016/0735-1097(91)90707-g. [DOI] [PubMed] [Google Scholar]
- Ramos-DeSimone N., Moll U. M., Quigley J. P., French D. L. Inhibition of matrix metalloproteinase 9 activation by a specific monoclonal antibody. Hybridoma. 1993 Aug;12(4):349–363. doi: 10.1089/hyb.1993.12.349. [DOI] [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Liotta L. A., Kleiner D. E., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- Walakovits L. A., Moore V. L., Bhardwaj N., Gallick G. S., Lark M. W. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992 Jan;35(1):35–42. doi: 10.1002/art.1780350106. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989 Jan 1;142(1):100–109. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yanagi H., Sasaguri Y., Sugama K., Morimatsu M., Nagase H. Production of tissue collagenase (matrix metalloproteinase 1) by human aortic smooth muscle cells in response to platelet-derived growth factor. Atherosclerosis. 1991 Dec;91(3):207–216. doi: 10.1016/0021-9150(91)90168-3. [DOI] [PubMed] [Google Scholar]