Abstract
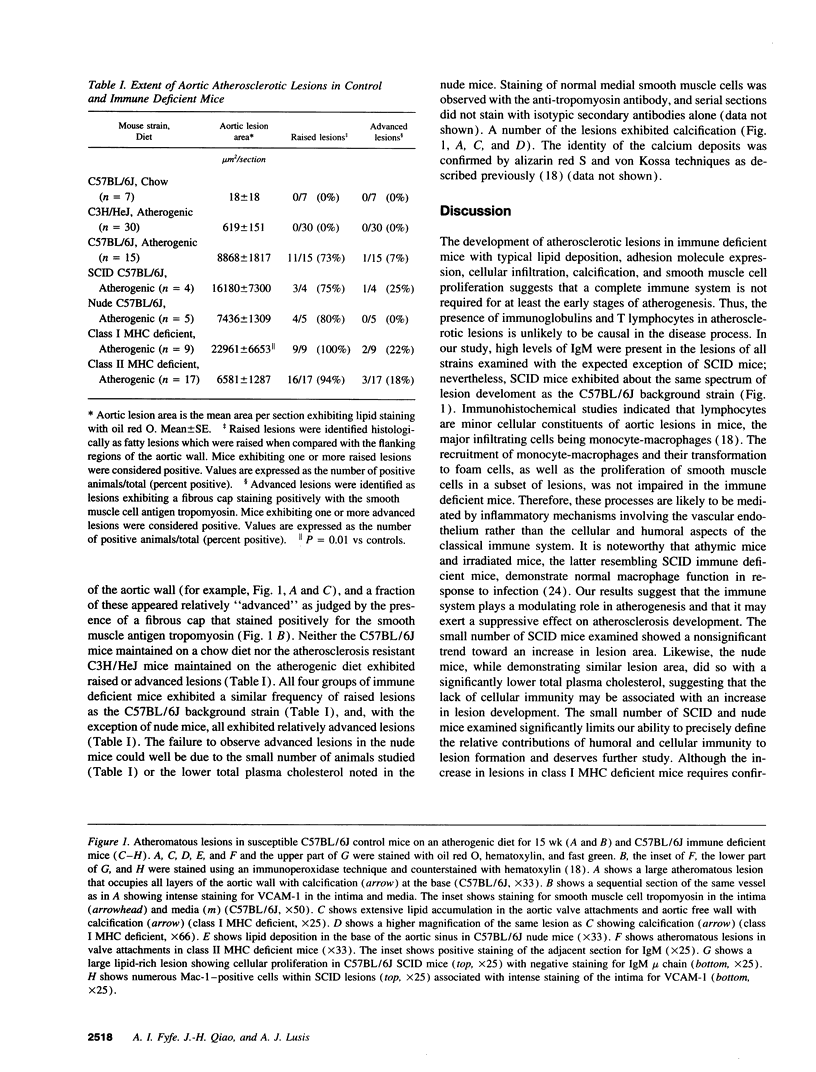
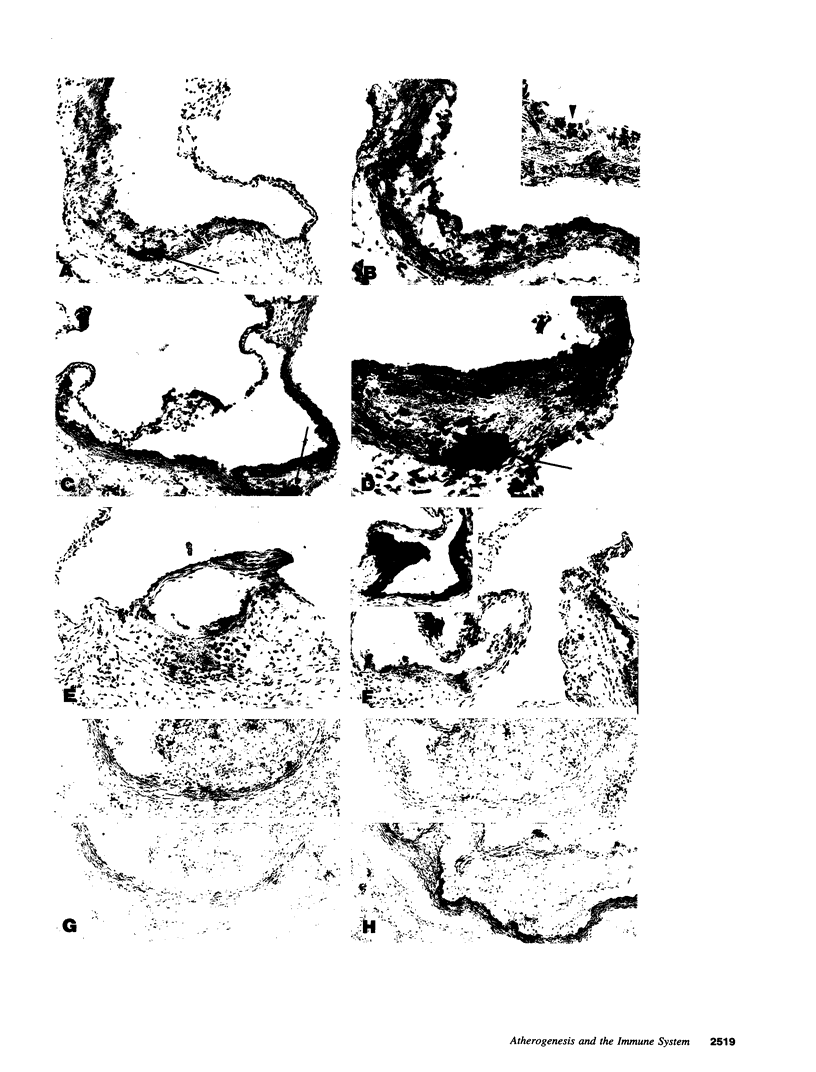
Inbred strain C57BL/6J mice develop typical atherosclerotic fatty streaks in the aorta after 15 wk on a high fat, high cholesterol diet. To investigate the effects of the immune system on the development of fatty streaks in this model, C57BL/6J mice with a normal immune system were compared with C57BL/6J mice carrying mutations resulting in various immune deficiencies. These included mice with severe combined immune deficiency, athymic "nude" mice, class I MHC deficient mice, and class II MHC deficient mice. Despite similar lipoprotein profiles, lesion development in the immune compromised strains was similar to or increased compared with normal C57BL/6J mice. Class I MHC deficient mice demonstrated a threefold increase in lesion area (22,961 +/- 6,653 vs 8,868 +/- 1,817 microns2, P = 0.01). Immunohistochemical analysis of lesions showed characteristic features of atherosclerosis with vascular cell adhesion molecule-1 expression, immunoglobulin deposition, monocyte infiltration, and smooth muscle cell proliferation. These data indicate that the classical immune system, while not essential for atherosclerotic fatty streak development, may act to suppress the development of lesions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Emeson E. E., Shen M. L. Accelerated atherosclerosis in hyperlipidemic C57BL/6 mice treated with cyclosporin A. Am J Pathol. 1993 Jun;142(6):1906–1915. [PMC free article] [PubMed] [Google Scholar]
- Fyfe A. I. Transplant atherosclerosis: the clinical syndrome, pathogenesis and possible model of spontaneous atherosclerosis. Can J Cardiol. 1992 Jun;8(5):509–519. [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Holm S., Fotev Z., Hedrich H. J., Fingerle J. T lymphocytes inhibit the vascular response to injury. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10530–10534. doi: 10.1073/pnas.88.23.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hedrick C. C., Castellani L. W., Warden C. H., Puppione D. L., Lusis A. J. Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. J Biol Chem. 1993 Sep 25;268(27):20676–20682. [PubMed] [Google Scholar]
- Khoo J. C., Miller E., Pio F., Steinberg D., Witztum J. L. Monoclonal antibodies against LDL further enhance macrophage uptake of LDL aggregates. Arterioscler Thromb. 1992 Nov;12(11):1258–1266. doi: 10.1161/01.atv.12.11.1258. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Gown A. M., Benditt E. P., Grayston J. T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993 Oct;13(10):1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993 Dec;143(6):1551–1559. [PMC free article] [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Mondola P., Santillo M., Cammarota L., Santangelo F. Role of a calf thymus preparation in the degradation of native and reductively methylated low density lipoprotein. Int J Biochem. 1991;23(9):819–821. doi: 10.1016/0020-711x(91)90065-u. [DOI] [PubMed] [Google Scholar]
- O'Brien K. D., Allen M. D., McDonald T. O., Chait A., Harlan J. M., Fishbein D., McCarty J., Ferguson M., Hudkins K., Benjamin C. D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993 Aug;92(2):945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen B., Morrow A., Brandon C., Mitchell D., Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985 Oct;57(1):65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Libby P., Reiss C. S. Inducible expression of class II major histocompatibility complex antigens and the immunogenicity of vascular endothelium. Transplantation. 1986 Feb;41(2):141–146. doi: 10.1097/00007890-198602000-00001. [DOI] [PubMed] [Google Scholar]
- Qiao J. H., Castellani L. W., Fishbein M. C., Lusis A. J. Immune-complex-mediated vasculitis increases coronary artery lipid accumulation in autoimmune-prone MRL mice. Arterioscler Thromb. 1993 Jun;13(6):932–943. doi: 10.1161/01.atv.13.6.932. [DOI] [PubMed] [Google Scholar]
- Qiao J. H., Xie P. Z., Fishbein M. C., Kreuzer J., Drake T. A., Demer L. L., Lusis A. J. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994 Sep;14(9):1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Page C., Hengstenberg C., Yacoub M. H. Identification of antigen presenting cells in normal and transplanted human heart: importance of endothelial cells. Hum Immunol. 1990 Jun;28(2):179–185. doi: 10.1016/0198-8859(90)90017-j. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989 Jan 15;142(2):471–480. [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Hedrick C. C., Qiao J. H., Castellani L. W., Lusis A. J. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science. 1993 Jul 23;261(5120):469–472. doi: 10.1126/science.8332912. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Hruban R. H., Ambinder R. F., Pizzorno M., Cameron D. E., Baumgartner W. A., Reitz B. A., Hayward G. S., Hutchins G. M. Demonstration of cytomegalovirus nucleic acids in the coronary arteries of transplanted hearts. Am J Pathol. 1992 Mar;140(3):739–747. [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]