Abstract
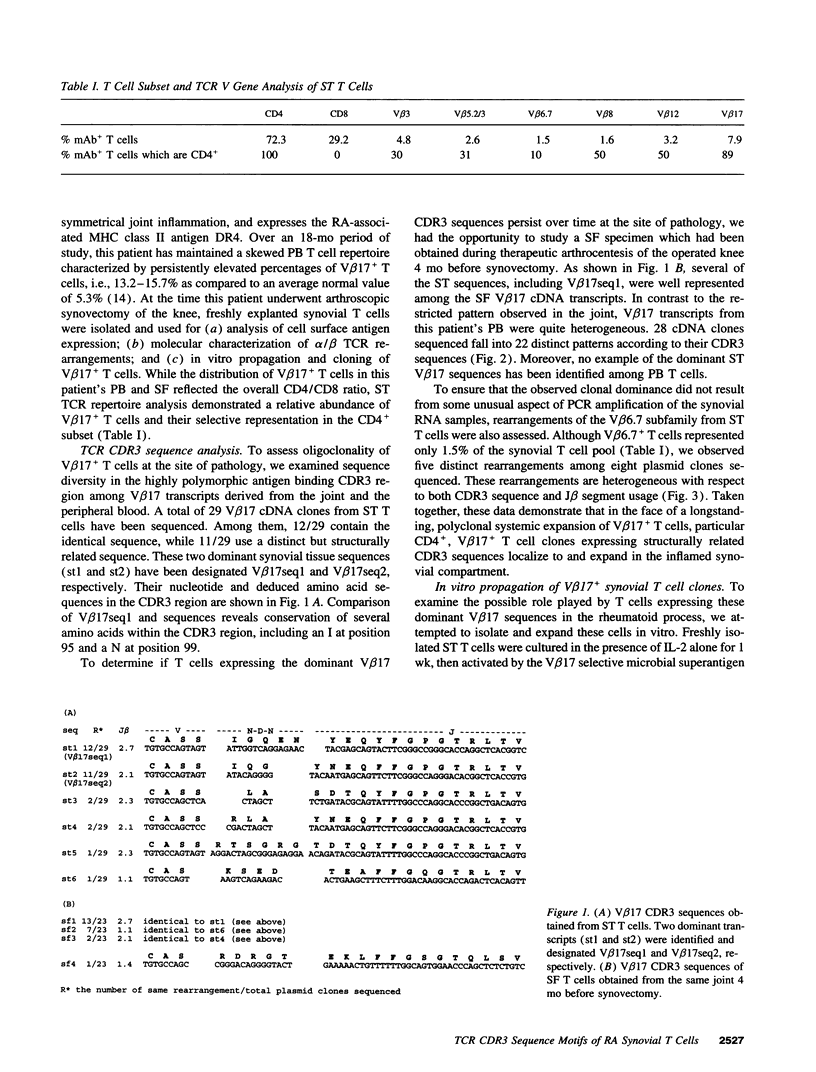
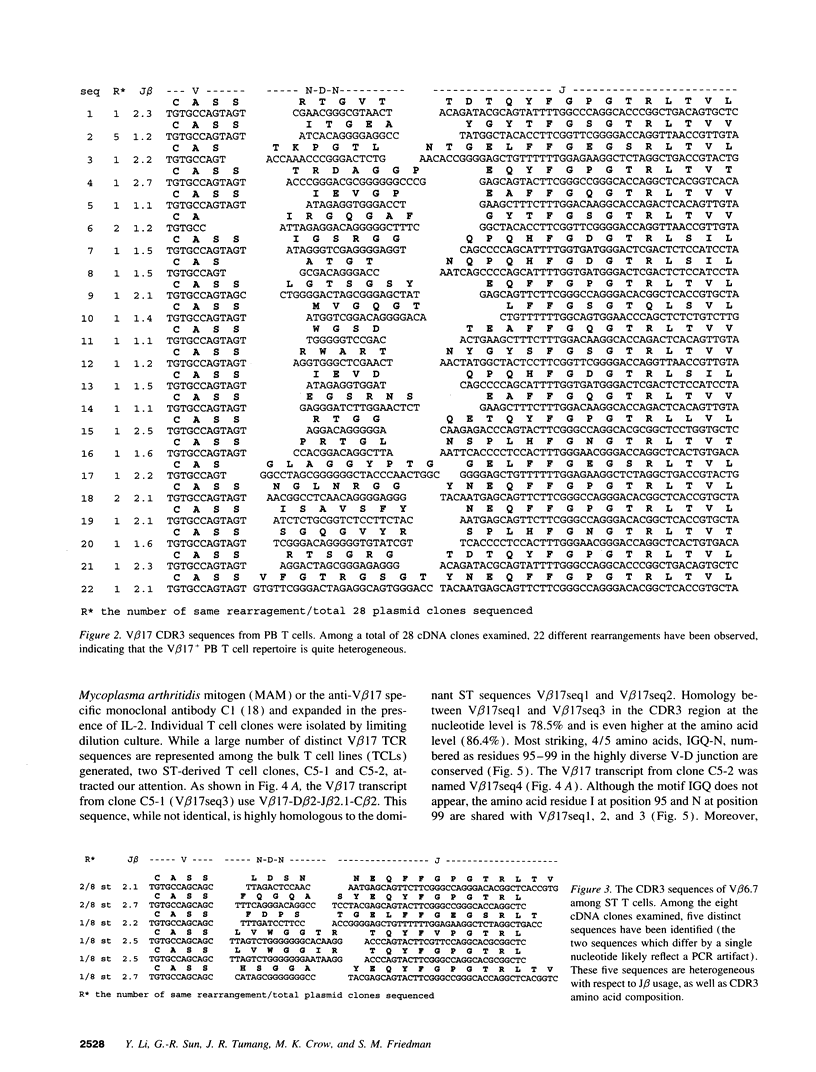
T lymphocytes reactive with as yet undefined joint-localized foreign or autoantigens may be important in the pathogenesis of RA. Molecular studies demonstrating skewed T cell antigen receptor (TCR) variable gene usage and selective expansion of particular T cell clones within the synovial compartment support this view. Based on our recent study documenting selective expansion of V beta 17+ T cells in RA, we have pursued the identification of T cells relevant to the disease process, in an informative patient, by combining molecular analysis of freshly explanted RA synovial tissue V beta 17 TCR transcripts with in vitro expansion of V beta 17+ synovial tissue T cell clones. Peripheral blood V beta 17 cDNA transcripts proved heterogeneous. In contrast, two closely related sequences, not found in the peripheral blood, dominated synovial tissue V beta 17 transcripts, suggesting selective localization and oligoclonal expansion at the site of pathology. CD4+, V beta 17+ synovial tissue-derived T cell clones, isolated and grown in vitro, were found to express TCR beta chain transcripts homologous to the dominant V beta 17 synovial tissue sequences. One clone shares with a dominant synovial tissue sequence a conserved cluster of 4/5 amino acids (IGQ-N) in the highly diverse antigen binding CDR3 region, suggesting that the T cells from which these transcripts derive may recognize the same antigen. These findings have permitted a complete characterization of the alpha/beta TCR expressed by putatively pathogenic T cell clones in RA. Functional analysis suggests that the conserved CDR3 sequence may confer specificity for, or restriction by, the MHC class II antigen, DR4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bröker B. M., Korthäuer U., Heppt P., Weseloh G., de la Camp R., Kroczek R. A., Emmrich F. Biased T cell receptor V gene usage in rheumatoid arthritis. Oligoclonal expansion of T cells expressing V alpha 2 genes in synovial fluid but not in peripheral blood. Arthritis Rheum. 1993 Sep;36(9):1234–1243. doi: 10.1002/art.1780360908. [DOI] [PubMed] [Google Scholar]
- Burns F. R., Li X. B., Shen N., Offner H., Chou Y. K., Vandenbark A. A., Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar V alpha and V beta chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989 Jan 1;169(1):27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. L., Cerottini J. C., Matthes M., Necker A., Gournier H., Barra C., Widmann C., MacDonald H. R., Lemonnier F., Malissen B. H-2-restricted cytolytic T lymphocytes specific for HLA display T cell receptors of limited diversity. J Exp Med. 1992 Aug 1;176(2):439–447. doi: 10.1084/jem.176.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. L., Romero P., Widmann C., Kourilsky P., Maryanski J. L. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991 Dec 1;174(6):1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. P., Vainiene M., Celnik B., Wiley S., Gibbs C., Hashim G. A., Vandenbark A. A., Offner H. Characterization of the immune response to a secondary encephalitogenic epitope of basic protein in Lewis rats. II. Biased T cell receptor V beta expression predominates in spinal cord infiltrating T cells. J Immunol. 1992 Mar 15;148(6):1712–1717. [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Grom A. A., Thompson S. D., Luyrink L., Passo M., Choi E., Glass D. N. Dominant T-cell-receptor beta chain variable region V beta 14+ clones in juvenile rheumatoid arthritis. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11104–11108. doi: 10.1073/pnas.90.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Engel I., McElligott D. L., Fink P. J., Hsu M. L., Hansburg D., Matis L. A. Selection of amino acid sequences in the beta chain of the T cell antigen receptor. Science. 1988 Mar 25;239(4847):1541–1544. doi: 10.1126/science.2832942. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Horneff G., Burmester G. R., Emmrich F., Kalden J. R. Treatment of rheumatoid arthritis with an anti-CD4 monoclonal antibody. Arthritis Rheum. 1991 Feb;34(2):129–140. doi: 10.1002/art.1780340202. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson C. H., Tehrani M. J., Mellstedt H., Wigzell H. Anti-idiotypic monoclonal antibody to a T-cell chronic lymphatic leukemia. Characterization of the antibody, in vitro effector functions and results of therapy. Cancer Immunol Immunother. 1989;28(3):225–232. doi: 10.1007/BF00204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Lymphoid V(D)J recombination: nucleotide insertion at signal joints as well as coding joints. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8588–8592. doi: 10.1073/pnas.85.22.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Howell M. D., Jaraquemada D., Flerlage M., Richert J., Brostoff S., Long E. O., McFarlin D. E., McFarland H. F. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991 Jan 1;173(1):19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Byers P., Seyfried C., Healey L. A., Wilske K. R., Stage D., Nepom B. S. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989 Jan;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Ricken G., Taube H., Kroninger S., Melchers I., Peter H. H., Eichmann K., Krawinkel U. Biased T cell receptor V alpha region repertoire in the synovial fluid of rheumatoid arthritis patients. Eur J Immunol. 1991 Nov;21(11):2749–2754. doi: 10.1002/eji.1830211115. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier J., Petersen J., Rhodes G. H., Luka J., Carson D. A. Susceptibility to rheumatoid arthritis maps to a T-cell epitope shared by the HLA-Dw4 DR beta-1 chain and the Epstein-Barr virus glycoprotein gp110. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5104–5108. doi: 10.1073/pnas.86.13.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Van Laar J. M., Miltenburg A. M., Verdonk M. J., Daha M. R., De Vries R. R., Van den Elsen P. J., Breedveld F. C. Lack of T cell oligoclonality in enzyme-digested synovial tissue and in synovial fluid in most patients with rheumatoid arthritis. Clin Exp Immunol. 1991 Mar;83(3):352–358. doi: 10.1111/j.1365-2249.1991.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Fang Q., Demarco D., VonFeldt J., Zurier R. B., Weiner D. B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992 Aug;90(2):326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon G., Tumang J. R., Li Y., Friedman S. M., Crow M. K. Increased frequency of V beta 17-positive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1994 Oct;37(10):1431–1440. doi: 10.1002/art.1780371005. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Lee N. E., Moore A. C., Waldor M. K., Sakai K., Rothbard J. B., McDevitt H. O., Steinman L., Acha-Orbea H. Predominant expression of a T cell receptor V beta gene subfamily in autoimmune encephalomyelitis. J Exp Med. 1988 May 1;167(5):1586–1596. doi: 10.1084/jem.167.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]


