Abstract
During the past four decades, a substantial progress has been made in the field of hematopoietic stem cell transplantation (HSCT). From July, 2007 to December, 2010, a transplant survey from 42 HSCT units indicates that the types of transplantation performed are related identical (43%), related mismatched/haploidentical (28%), unrelated donor matched (11%), unrelated donor mismatched (7%), umbilical cord blood (UCB, 2%) and autologous (9%). The distribution of disease entities being transplanted in allogeneic settings is acute myeloid leukemia (AML) (34%), acute lymphoblastic leukemia(ALL) (24%), chronic myeloid leukemia (CML) (20%), myelodysplastic syndrome (MDS) (8%), aplastic anemia (AA) (7%), Mediterranean anemia (MIA) (2%), non-Hodgkin's lymphoma (NHL) (3%), and other diseases (3%). Clinical data from Peking University Institute of Hematology and other transplant centers suggest that haploidentical transplantation has been a choice of the best alternative source of stem cells for individual patients without matched sibling donors. A modified donor lymphocyte infusion (DLI) approach can be safely used for prophylaxis and treatment of leukemia relapse in patients with advanced leukemia following mismatched transplant. The number of transplants from unrelated donor or related mismatched/haploidentical donor has increased significantly during recent years. Double UCBT is a promising strategy for the therapy of hematological disease. In addition, mesenchymal stem cell (MSC) transplantation may be a potential therapeutic approach for treating systemic lupus erythematosus (SLE).
Keywords: Hematopoietic, blood, stem cell, transplantation, HSCT, China, review
Introduction
It has been more than forty years since the first case of bone marrow transplantation (BMT) was successfully performed at Peking University People's Hospital in China. During the past four decades, a substantial progress has been made in the field of hematopoietic stem cell transplantation (HSCT) [1-11]: (1) a variety of stem cell sources, such as steady-state bone marrow, granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells (G-PB) [12], G-CSF-primed bone marrow (G-BM) [5, 8], umbilical cord blood (UCB) , and mixture grafts of G-PB and G-BM, are currently available [2, 6]; (2) haploidentical transplantation has been a choice of the best alternative source of stem cells for individual patients without matched sibling donors [1, 5, 6, 9]; (3) a modified donor lymphocyte infusion (DLI) approach can be safely used for prophylaxis and treatment of leukemia relapse in patients with advanced leukemia following mismatched transplantation [3, 13]; (4) double UCBT is a promising strategy for the therapy of hematological disease [14]; (5) mesenchymal stem cell (MSC) transplantation may be a potential therapeutic approach for treating systemic lupus erythematosus (SLE) [7].
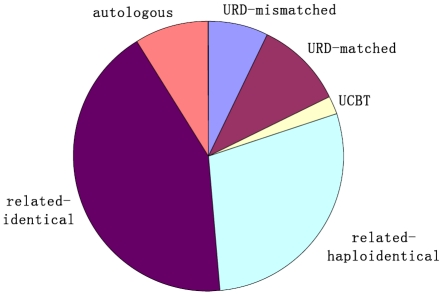
Currently, there are at least 60 active BMT units all over the country. From July, 2007 to December, 2010, a transplant survey from 42 HSCT units, including more than 4,300 patients, was carried out with a multiplication by 8.8% to 10.8% per year. The types of transplantation performed are related identical (43%), related mismatched/haploidentical (28%), unrelated donor matched (11%), unrelated donor mismatched (7%), umbilical cord blood (UCB, 2%) and autologous (9%), as shown in Figure 1. The low percentage of autologous transplantion may be related to that some autologous transplant centers are not included in this survey [7, 15-17]. The distribution of disease entities being transplanted in allogeneic settings is acute myeloid leukemia (AML) (34%), acute lymphoblastic leukemia (ALL) (24%), chronic myeloid leukemia (CML) (20%), myelodysplastic syndrome (MDS) (7%), aplastic anemia (AA) (7%), Mediterranean anemia (MIA) (2%), non-Hodgkin's lymphoma (NHL) (3%), and other diseases (2%), as shown in Figure 2. The distribution of prevalent diseases being transplanted in autologous settings is NHL (35%), mutiple myeloma (MM) (24%), AML (19%), ALL (9%), Hodgkin's disease (HD) (6%), autoimmune disease (AD) and other disease (5%), and POEMS syndrome (2%), as shown in Figure 3. The median age of patients who underwent transplantation at these transplant centers was 30 years, with a range from 2 years to 67 years. The number of transplants from unrelated donor or related mismatched/ haploidentical donor has increased significantly during recent years [1, 8-11, 18]. Therefore, the aim of this review was to identify current status and prospects of Chinese patients who treated with auto-HSCT and allo-HSCT.
Figure 1.
Types of HSCT (data from 40 HSCT units for the period July, 2007 to December, 2010).
Figure 2.
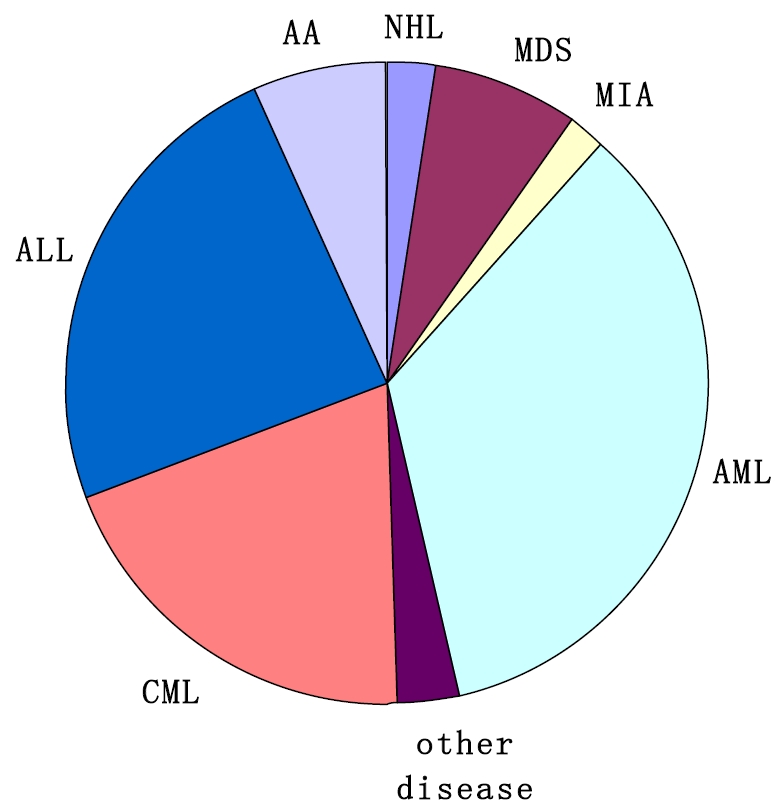
Distribution of disease entities being transplanted in allogeneic settings (data from 40 HSCT units for the period July, 2007 to December, 2010). Abbreviations: AML=acute myeloid leukemia; ALL=acute lymphoblastic leukemia; CML=chronic myeloid leukemia; MDS=myelodysplastic syndrome (MDS); AA=aplastic anemia; MIA=Mediterranean anemia; NHL=non-Hodgkin's lymphoma
Figure 3.
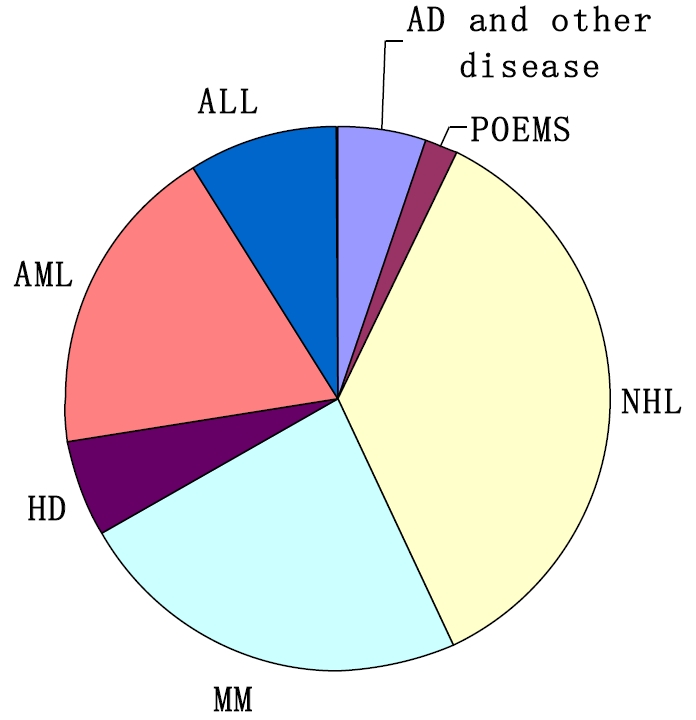
Distribution of disease entities being transplanted in autologous settings (data from 40 HSCT units for the period July, 2007 to December, 2010). NHL = non-Hodgkin's lymphoma; MM = multiple myeloma; AML = acute myeloid leukemia; ALL = acute lymphoblastic leukemia; HD = Hodgkin's disease; AD = autoimmune disease.
Related unmanipulated haploidentical stem cell transplantation
Haploidentical HSCT can be performed timely without waiting for a long time [19]. Related matched/mismatched donor has remarkable advantages in management of high-risk leukemia, because re-obtaining lymphocytes for Modified donor lymphocyte infusion (DLI/mDLI) is convenient [3, 4]. Almost every patient can find a Haploidentical donor. With the shrinking family size and reduced number of sibling donors in China, haploidentical transplantation brings hope for nearly all patients who need transplantation. Thus, the successful investigation of mismatched/haploidentical transplantation is an important contribution of Chinese doctors to the transplantation field [1, 2, 6, 8-10, 13].
Peking University researchers developed a novel approach to HLA-mismatched/ haploidentical blood and marrow transplantation without in vitro T cell depletion (the GIAC protocol) [2, 6, 9]. The protocol entails the following: treating donors with granulocyte colony-stimulating factor (G-CSF) to induce donor immune tolerance; intensified immunological suppression to both promote engraftment and to prevent GVHD; antithymocyte globulin (ATG) was included for the prophylaxis of GVHD and graft reject; and combination of G-CSF-primed bone marrow harvest (G-BM) and G-CSF-mobilized peripheral blood stem cell harvest (G-PB) as the source of stem cell grafts. In the GIAC protocol, the median time for myeloid engraftment was 12 days (range: 9-26 days) and for platelet 15 days (range: 8-151 days) [11]. In this unmanipulated haploidentical transplant settings, the incidence of grade III-IV aGVHD (23.1%) and extensive chronic GVHD (21.3%) were acceptable, although the T cell dose in grafts was more than 100×106/kg. The 2-year or 3-year probability of LFS for patients with hematological malignancies ranged from 24.8% to 74.5%. Similar LFS were achieved using the GIAC protocol compared with HLA-matched sibling transplantation or unrelated donor transplantation [1, 6, 9]. Above-mentioned studies suggest that haploidentical transplantation may provide better donor choice at experienced transplant centers especially under certain specialized circumstances.
In the First Affiliated Hospital of Xin Jiang Medical University, Yuan et al. [12] report on 42 patients with malignant hematological diseases, including 29 standard-risk patients and 13 high-risk patients, who underwent received PBSC from a haploidentical family donor with 1-3 mismatched loci of HLA antigens. In this study, all patients achieved complete and sustained donor-type engraftment. The 2-year cumulative incidences of acute GVHD were 50.8%, and the 2-year cumulative incidences of chronic GVHD were 57.1%. It shows that haploidentical PBSCT is also feasible.
Modified haploidentical nonmyeloablative transplantation without T cell depletion for high-risk acute leukemia was carried out in Chinese PLA Hospital 307. Guo et al. [10] report a strategy that included a haploidentical PBSCT combined with mesenchymal stem cells (MSCs), modified nonmyeloablative (NMA) conditioning, and GVHD prophylaxis. The modified conditioning approach consisted of Fludaraine, low-dose total body irradiation (TBI), cyclophosphamide (Cy), cytarabine, and ATG, whereas the GVHD prophylaxis consisted of cyclosporine (CsA), mycophenolate mofetil (MMF), Anti-CD25 monoclonal antibody (basiliximab) and intra-bone marrow injection of MSCs. Thirty-three patients with high-risk acute leukemia underwent transplantation with PBSC from HLA-haploidentical donors without T cell depletion. All of the patients achieved full donor chimerism. Neutrophils engraftment was done on Day 11, and platelets, on Day 14. Of these patients, 45.5% developed grade I-IV acute GVHD, and 6.1% developed grades III -IV acute GVHD. The probability of 3-year survival was 57.2%. The results suggest that this strategy is effective in improving donor engraftment and preventing severe GVHD.
A certain number of patients underwent mis-matched/haploidentical transplantation has reported by the Air Force General Hospital [5, 8]. Their preliminary data showed that G-CSF-primed BMT is also feasible in haploidentical setting [5, 8]. More recently, more and more patients in this center were transplanted with G-CSF-primed bone marrow and G-CSF-mobilized peripheral stem cell grafts. A control study conducted by this group in 2009 showed that bone marrow combined with peripheral blood stem cell transplantation was better than single G-CSF-primed bone marrow transplantation. The former was well tolerated to the donors, the engraftment was fast, and the recurrence rate was low; the degree of aGVHD II-IV increased, but no difference was observed at grades III-IV, cGVHD and extensive cGVHD, OS, as well as LFS [20]. Data from these transplant centers warrant further randomized clinical trials to elucidate which stem cell source is best in haploidentical transplant settings, G-CSF-primed bone marrow, G-CSF-mobilized peripheral blood grafts, or both of which ?
Modified DLI for prophylaxis and treatment of leukemia relapse following haploidentical transplantation
Leukemia recurrence after HSCT is an important obstacle to cure disease. DLI is helpful to protect against relapse of AL. However, conventional DLI may induce mye suppression and severe GVHD with GVL. The preliminary results of Peking University Institute of Hematology suggest that using immunosuppressive agents for 2 -4 weeks may reduce DLI-associated aGVHD without influencing relapse and survival after G-CSF-primed DLI in HLA-matched sibling HSCT [4].
With respect to the safety and efficiency of modified DLI in HLA-identical transplant setting, modified DLI was used to treat relapse of patients after unmanipulated haploidentical transplantation [3, 4, 13]. Twenty patients who underwent haploidentical T-cell-deplete HSCT between April 1, 2002 and May 1, 2005 and then relapsed, were included in this study [13]. These patients were diagnosed with relapse of leukemia at a median of 4.5 (1.5-35) months after HLA-mismatched/haploidentical transplantation without in vitro T-cell depletion. Nine patients received chemotherapies before DLI. Two patients, one with Ph+ ALL, the other with CML in blastic phase, were given imatinib (300-400 mg/day) for 22 and 89 days, respectively. Finally the patient with CML achieved complete remission. Nine patients received DLI without any prior intervention. After DLI, eleven patients received CsA (blood concentration of 150-250 ng/mL for 2-4 weeks) or a low dose of MTX (10mg once per week for 2-4 weeks) for preventing GVHD, and nine patients received no GVHD prophylaxis. The incidence of grade III-IV aGVHD was significantly lower in patients with GVHD prophylaxis than those without (55.56% vs 9.09%, P=0.013). Fifteen patients achieved CR at a median of 289 (40-1388) days after DLI, rarely accompanied by pancytopenia. Eight of 20 patients survived in CR for a median of 1118 (range, 754-1468) days after HSCT and 808 days (range, 627-1388) days after modified DLI. The 1-year and 2-year LFS were 60% and 40%. These results suggest that G-CSF-primed DLI was a potentially effective therapeutic option for patients who relapsed after HLA-mismatched/haploidentical HSCT. At the same year, Hang et al [4]. first reported that a modified DLI approach can be safely used for prophylaxis of leukemia relapse in patients with advanced leukemia receiving mismatched transplant [4]. Moreover, administering short-term immunosuppressive agent, such as CsA and MTX, may decrease the incidence of GVHD following DLI [13].
Unrelated volunteer donor and URD HSCT
The Chinese Stem Cell Donor Database Management Center was established in 2001. As of August 31, 2010, there are 1,149,189 people in the China Marrow Donor Program (CMDP) with 1807 blood stem cell cases [21]. The main suppliers of unrelated hematopoietic stem cells are peripheral blood in mainland China, and peripheral blood or BM at the Tzu Chi Stem Cell Center. The top four diseases were CML, ALL, AML and MDS. The median time for neutrophil and platelet engraftment was 13, and 14 days, respectively. The incidence of acute GVHD was 43.4% for grades II-IV and 14.3% for grades III-IV, the incidence of extensive chronic GVHD was 18.4%. Five-year overall survival (OS) was 54.3%. Better transplant outcomes of patients who underwent unrelated donor transplantation were observed after 2006 [22].
To investigate the effect of HLA mismatch on transplant outcomes following unrelated donor transplantation. Liang et al. [23] compared unrelated bone marrow transplantation using perfectly matched (HLA-M) or non-perfectly matched HLA donors (HLA-mis). Thirty-nine patients received HLA-M, and 21 received HLA with 1-2 non-matched loci URD-BMT for the treatment of acute leukemia, CML-CP1, and MDS. Successful engraftment was achieved in thirty-eight of the HLA-M group and 18 patients from the HLA-mismatched group. The 3-year probabilities of DFS for the HLA-Matched and HLA-mismatched groups were 79.2 % and 45.8 %, respectively (P <0.05). This result suggests that the outcome after unrelated donor (URD) HSCT can be optimized by matching the HLA-A, B and DR alleles.
At NaiFang University [24], seventy-one cases of UD-HSCT, including 37 cases with highly HLA-matched donors and 34 cases with HLA mismatched donors, the incidence of acute GVHD showed no significant difference (P=0.558), but the incidence of acute GVHD above grade II was significantly different (11.1% vs. 60.6%, P=0.000). No significant difference was observed for the 3-year DFS, relapse rate and TRM (all P>0.05). This study indicates that the HLA compatibility between unrelated donor and recipient did not significantly affect the transplantation outcome. Qu et al. [24] suggested that transplant for high-risk patients should be performed as soon as possible, not necessarily pursue the matched donor.
Either BM or PBSC can be donated for URD HSCT. Recently, Liu et al. [25] analyzed data from 89 patients with leukemia undergoing unrelated donor BMT (n=44) and PBSCT (n=45). They found that PBSCT resulted in faster hematopoietic engraftment (P<0.001). The 5-year relapse rate was 18.5% in BMT and 48.6% in PBSCT (P=0.041), the 5-year DFS was 50.8% and 38.9% (P=0.439); and OS was 55.3% and 48.5% (P=0.447), transplantation related mortality (TRM) was 40% and 29.5% (P=0.800). This study indicates that either BM or PBSC can be chosen if a unrelated donor is available. Presently, PBSC collection is preferred by volunteer unrelated donors in China, because it is easy to collect and more acceptable.
In term of efficacy, unrelated transplantation demonstrated comparable to matched sibling transplantation. Yang et al. [26] report on 115 patients who received allo-HSCT, of whom 68 received RD-HSCT and 47 received URD-HSCT. The incidence of II-IV acute GVHD, chronic GVHD was 45.5% and 52.3%, 45.3% and 63.2%, respectively, in RD-HSCT and URD-HSCT groups. The OS and the DFS at three-year follow-up were 67.8% and 61.6% (P=0.133), and 62.3% and 56.8% (P=0.177) in RD-HSCT and URD-HSCT groups. Thus, unrelated donors can be considered when matched sibling donor is unavailable.
Cord blood bank and CBT in China
The oldest cord blood bank in China is the Beijing Cord Blood Stem Cell Bank, the six cord blood banks were approved by Ministry of Health by the end of 2009. Thousands of cord blood donations reserved for searches in the public bank, and more cryopreserved copies were stored for private use. According to incomplete statistics, tens of cases of cord blood transplantation are performed each year, which only account for 2% of registered transplantation cases.
Due to the limited cells number of cord blood and growing haploidentical family donor, there are limited UCBT for pediatric patients and double UCBT for adult patients. In China, the first successful case of double UCBT for the treatment of high risk adult acute leukemia in Peking University People's Hospital was reported [27]. Ma et al. [14] from Suzhou University, reported 28 cases of hematological disease treatment using the reduced intensity conditioning (RIC) pre-treatment procedure and double UCBT. The median total number of nuclear cells was more than 3.5×107/kg. After transplantation, the hematopoietic reconstitution time was 42 days. All patients except for one achieved engraftment. The incidence of severe GVHD was 12%. The expected 5-year overall survival (OS) and disease free survival (DFS) was 56% and 48%, respectively. Several studies [28-30] suggests that, comparing to single UCBT, double UCBT is accompanied by: (1) similar treatment-related mortality or chronic GvHD, although higher incidence of grade II acute GvHD; (2) a potentially higher graft-versus-leukemia effect. Therefore, a nationwide multiple center trial on double UCBT for adult patients was going on. Moreover, the biology underlying double UCBT and the factors that determine unit dominance should be further investigated.
MSC transplantation for treating SLE
The rationale for using auto-SCT to treat AD is based on the principle of complete ablation of an aberrant immune system through high dose cyclophosphamide or TBI followed by reconstitution of a new immune system deriving from in vivo or in vitro T-cell depletion [16, 31, 32]. In China, more than 300 patients have received an autologous HSCT, most transplants have been performed for MS, RA, SLE [32]. Xu et al. [16] reported thirty-six secondary progressive MS patients who underwent APBSCT and were followed up for an average of 48.92 months (range, 10-91 months). The confirmed relapse-free survival rate was 62.9% and progression-free survival rate was 83.3% after 91 months of follow-up. In another study, seventeen patients with SLE were treated with auto-SCT. After a median follow-up of 89 (33-110) months, Song et al. [15] found that probabilities of 7-year OS and PFS were 82.4±9.2%% and 64.7±11.6%, respectively. The most important progress in the therapy of SLE is MSC transplantation [7, 17]. Currently, the Affiliated Drum Tower Hospital of Nanjing University Medical School is the only unit engaged in MSCT for the treatment of AD in Asia [7, 17, 32]. A total of 58 SLE patients have received MSCT in Drum Tower Hospital, of which 29 received BM-MSCT and 29 received umbilical cord -MSCT. The researchers did not observed adverse events during or immediately after infusions of MSCs in any of these patients. The cost of MSCT is much less than that of HSCT, such that most SLE patients in developing countries can afford this treatment (RMB 13,000 vs. RMB 100,000 in China). Sun et al. [7, 17, 32] believe that further follow-ups and additional patient enrollment are warranted to evaluate the long-term efficacy. Available data suggest that the outcome for AD patients can be improved through stem cell transplantation. In addition, an integrated approach with the participation of HSCT and MSCT for the treatment of SLE could be superior to each of these two protocols alone.
Future prospects
In China, recent progress in the field of transplantation provides new opportunities for more patients, especially those with hematological malignancies, to cure their disease. However, many questions still remain unanswered at this stage. Firstly, it is impossible to determine which transplantation should be preferentially chosen for patients without an HLA-matched sibling donor [33, 34], unrelated, haploidentical, or umbilical cord blood [35]. Therefore, the comparison of clinical outcomes between haploidentical transplantation, unrelated donor transplantation, and/or UCBT remains to be established by prospective, multicenter, randomized studies. Secondly, various strategies to improve clinical outcomes after haploidentical transplantation should be evaluated, such as infusions of cells (NK cells and cytotoxic T lymphocytes) with anti-infection and anti-leukemic specificities, co-transplantation with MSCs and/or regulatory T cells ex-vivo expansion with cytokines [18, 36]. Thirdly, the road for us to capture an optimal GVL effect without GVHD is still tortuous and long. Finally, imbalance distribution of the transplant centers and economic issues limit the application of HSCT for candidate patients who live in rural area or western region of China. In this regard, approaches that employ financial support and technological improvements, such as balancing the distribution of transplant centers, and performing multicenter, randomized clinical trials, may be particularly promising
Acknowledgments
This study was Supported by National Outstanding Yong Scientists’ Foundation of China (grant no.30725038), and the National Natural Science Foundation of China (grant no. 30971292), and HI-Tech Research Development Program of China 863 (2006AA02A405).
Conflict of interest.
The authors claim no potential conflicts of interest.
References
- 1.Chen XH, Zhang C, Zhang X, Gao L, Kong PY, Peng XG, Qi DG, Sun AH, Zeng DF, Liu H, Gong Y, Wang QY. Role of antithymocyte globulin and granulocyte-colony stimulating factor-mobilized bone marrow in allogeneic transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplant. 2009;15:266–73. doi: 10.1016/j.bbmt.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, Chen YH, Wang JZ, Gao ZY, Zhang YC, Jiang Q, Shi HX, Lu DP. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38:291–7. doi: 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 3.Huang XJ, Liu DH, Liu KY, Xu LP, Chen YH, Wang Y, Han W, Chen H. Modified donor lymphocyte infusion after HLA-mismatched/ haploidentical T cell-replete hematopoietic stem cell transplantation for prophylaxis of relapse of leukemia in patients with advanced leukemia. J Clin Immunol. 2008;28:276–83. doi: 10.1007/s10875-007-9166-z. [DOI] [PubMed] [Google Scholar]
- 4.Huang XJ, Wang Y, Liu DH, Xu LP, Chen H, Chen YH, Han W, Shi HX, Liu KY. Modified donor lymphocyte infusion (DLI) for the prophylaxis of leukemia relapse after hematopoietic stem cell transplantation in patients with advanced leukemia–feasibility and safety study. J Clin Immunol. 2008;28:390–7. doi: 10.1007/s10875-008-9193-4. [DOI] [PubMed] [Google Scholar]
- 5.Ji SQ, Chen HR, Wang HX, Yan HM, Zhu L, Liu J, Xue M, Xun CQ. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: an excellent alternative for high-risk leukemia. Bone Marrow Transplant. 2002;30:861–6. doi: 10.1038/sj.bmt.1703769. [DOI] [PubMed] [Google Scholar]
- 6.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, Chen H, Liu DH, Gao ZY, Chen YH, Xu LP, Zhang YC, Ren HY, Li D, Liu KY. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–75. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 8.Wang HX, Yan HM, Duan LN, Wang ZD, Zhu L, Xue M, Liu J, Hu LD, Guo ZK. Haploidentical hematopoietic stem cell transplantation in child hematologic malignancies with G-CSF-mobilized marrow grafts without T-cell depletion: a single-center report of 45 cases. Pediatr Hematol Oncol. 2009;26:119–28. doi: 10.1080/08880010902772208. [DOI] [PubMed] [Google Scholar]
- 9.Xiao-Jun H, Lan-Ping X, Kai-Yan L, Dai-Hong L, Yu W, Huan C, Yu-Hong C, Wei H, Jing-Zhi W, Yao C, Xiao-Hui Z, Hong-Xia S, Feng-Rong W, Fei-Fei T. Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res. 2009;15:4777–4783. doi: 10.1158/1078-0432.CCR-09-0691. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Sun Z, Sun QY, Han Q, Yu CL, Wang DH, Qiao JH, Chen B, Sun WJ, Hu KX, Liu GX, Liu B, Zhao RC, Ai H. A modified haploidentical non-myeloablative transplantation without T cell depletion for high-risk acute leukemia: successful engraftment and mild GVHD. Biol Blood Marrow Transplant. 2009;15:930–37. doi: 10.1016/j.bbmt.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Huang XJ, Chang YJ. Unmanipulated HLA-Mismatched/Haploidentical Blood and Marrow Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2011;17:197–204. doi: 10.1016/j.bbmt.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Yuan HL, Wen BZ, Qu JH, Li L, Jiang M, Hao JP, Chen R, Guo XH. Haploidentical peripheral blood stem cell transplantation without in vitro T-cell depletion for the treatment of malignant hematological diseases. J Clin Reh Tissue Eng Res. 2009;13:1185–90. [Google Scholar]
- 13.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/ haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007;92:414–7. doi: 10.3324/haematol.10570. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Wu DP, Sun AN, Qiu HY, Fu ZZ, Tang XW, Liu YJ, Han Y. A clinical study on double cord blood transplantation for adults patients with hematological diseases. Suzhou Univ J Med Sci. 2009;29:1178–81. [Google Scholar]
- 15.Song XN, Li HY, Sun LX, Meng JB, Wang JK, Zhang JQ, Chang YJ. Autologous stem cell transplantation for systemic lupus erythematosus: report of efficacy and safety at seven year of follow up in 17 patients. Transplant Proc. 2011 doi: 10.1016/j.transproceed.2011.03.039. in press. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Ji BX, Su L, Dong HQ, Sun WL, Wan SG, Liu YO, Zhang P, Liu CY. Clinical outcome of autologous peripheral blood stem cell transplantation in opticospinal and conventional forms of secondary progressive multiple sclerosis in a Chinese population. Ann Hematol. 2011;90:343–8. doi: 10.1007/s00277-010-1071-5. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Xu S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–32. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K, Chen Y, Zeng Y, Xu L, Liu D, Chen H, Zhang X, Han W, Wang Y, Zhao T, Wang J, Han Q, Zhao C, Huang X. Coinfusion of Mesenchymal Stromal Cells Facilitates Platelet Recovery Without Increasing Leukemia Recurrence in Haploidentical Hematopoietic Stem Cell Transplantation: A Randomized, Controlled Clinical Study. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0447. [DOI] [PubMed] [Google Scholar]
- 19.Aversa F. Haploidentical haematopoietic stem cell transplantation for acute leukaemia in adults: experience in Europe and the United States. Bone Marrow Transplant. 2008;41:473–81. doi: 10.1038/sj.bmt.1705966. [DOI] [PubMed] [Google Scholar]
- 20.Yan HM, Xue M, Wang ZD, Zhu L, Liu J, Ding L, Pan SP, Duan LN, Wang HX. haploidentical Allogeneic Bone Marrow Stem Cell Transplantation Combined with Peripheral Blood Stem Cells for therapy of Leukemia. J Exp Hematol. 2009;17:1330–4. [PubMed] [Google Scholar]
- 21. www.cmdp.org.cn..
- 22.Chen H. A report on clinical analysis of 721 unrelated donor hematopoietic stem cell transplantation cases. In the symposium on development of HLA mismatched HSCT. 2010 Beijing 2010-09-24. [Google Scholar]
- 23.Liang B, Huang H, Cai Z, Xie WZ, Li L, He JS, Luo Y, Meng XJ, Zheng WY, Zhang J, Ye XJ, Hu XR, Chen SY, Jin AY, Lin MF. [A comparison of clinical outcomes between HLA allele matched and 1 - 2 alleles mismatched unrelated allogeneic bone marrow transplantations] Zhonghua Xue Ye Xue Za Zhi. 2004;25:74–7. [PubMed] [Google Scholar]
- 24.Qu H, Sun J, Liu QF, Meng FY, Xu D, Zhang Y, Jiang QL, Xu XJ. The impact of HLA incompatibility between Unrelated donor and recipient on outcomes. J Chi Prac Inter Med. 2008;24:4076–78. [Google Scholar]
- 25.Liu QF, Liu C, Zhang Y, Sun J, Yi ZS, Fan ZP, Xu D, Jiang QL, Xu XJ. Peripheral blood stem cell transplantation compared with bone marrow transplantation from unrelated donors in patients with leukemia: a single institutional experience. Blood Cells Mol Dis. 2010;45:75–81. doi: 10.1016/j.bcmd.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Yang K, Liu QF, Fan ZP, Sun J, Xu D, Wei YQ, Zhang W, Meng FY. A comparison of the therapeutic effects between related donor and unrelated donor allogeneic hematopoietic stem cell transplantation in treatment of leukemia. Chin J Inter Med. 2007;46:135–9. [PubMed] [Google Scholar]
- 27.Wang FR, Zhang YC, Lu DP. Successful transplantation of double unit cord blood from unrelated donors in high risk leukemia. Chin J Organ Transplant. 2003;24:217–9. [PubMed] [Google Scholar]
- 28.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, Blazar BR, Wagner JE. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–5. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, Burke MJ, Blazar BR, Miller JS, McGlave PB, Weisdorf DJ, Wagner JE. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–9. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, de Lima M, Cairo MS, Furst S, Rio B, Dalley C, Carreras E, Harousseau JL, Mohty M, Taveira D, Dreger P, Sureda A, Gluckman E, Rocha V. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–63. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 31.Sykes M, Nikolic B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature. 2005;435:620–7. doi: 10.1038/nature03728. [DOI] [PubMed] [Google Scholar]
- 32.Sun L. Stem cell transplantation: progress in Asia. Lupus. 2010;19:1468–73. doi: 10.1177/0961203310370051. [DOI] [PubMed] [Google Scholar]
- 33.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, Aloisi T, Saab JP, Santucci A, Perruccio K, Martelli MP, Mecucci C, Reisner Y, Martelli MF. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–54. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 34.Bethge WA, Faul C, Bornhauser M, Stuhler G, Beelen DW, Lang P, Stelljes M, Vogel W, Hagele M, Handgretinger R, Kanz L. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008;40:13–9. doi: 10.1016/j.bcmd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Ballen KK, Spitzer TR. The great debate: haploidentical or cord blood transplant. Bone Marrow Transplant. 2011;46:323–9. doi: 10.1038/bmt.2010.260. [DOI] [PubMed] [Google Scholar]
- 36.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F, Martelli MF. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]