Abstract
Background: Anemia is an important cause of morbidity in MDS patients, principally through increased cardiovascular disease. Transfusion status has been seen to be a significant prognostic factor for disease progression and mortality, yet the relationship between anemia levels and cardiovascular disease is not well understood. Objective: This study aimed to review the published literature on the effect of anemia on cardiovascular outcomes in patients with MDS. Methods: A systematic literature review was undertaken to identify studies that investigated the relationship between anemia (as defined by hemoglobin levels) and cardiovascular outcomes in patients with MDS (all subtypes). Results: Three studies were identified that explicitly evaluated the relationship between anemia and cardiovascular outcomes in MDS, and another study reported the relationship between hemoglobin levels and survival. The four studies consistently showed a strong relationship between lower hemoglobin levels and worse cardiovascular outcomes, including cardiac remodeling, congestive heart failure, coronary artery disease, myocardial infarction, arrhythmia, heart valve disease, and cardiovascular mortality. Anemia was seen to be an independent predictor of cardiovascular disease outcomes in patients with MDS, beyond transfusion status and IPSS. Conclusion: Based upon a relatively small body of evidence, there appears to be a strong and clinically significant association between anemia and cardiovascular morbidity and mortality in MDS. While further research is needed, clinicians should seek to actively manage hemoglobin levels in MDS patients before the point of transfusion dependency is reached.
Keywords: Myelodysplastic syndromes, MDS; anemia, cardiovascular disease, mortality, quality of life, hemoglobin, transfusion
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous spectrum of myeloid neoplasms. According to the World Health Organization (WHO) 2008 classification, at least 10% of the cells of at least one myeloid bone marrow lineage must show unequivocal dysplasia in order that a diagnosis of MDS may be made, although the presence of recurring chromosomal abnormalities (e.g del[5q], del[7q]), alongside cytopenias of undefined origin, is also indicative of MDS [1]. The disease can occur de novo, or secondary, such as when due to treatment with chemotherapy or radiation for other diseases.
The incidence of MDS is approximately 2-13 per 100,000 people per year, with higher rates among older populations, where the incidence rate is 15-50 cases per 100,000 [2]. The median age at which patients present with MDS is between 65 and 70 years, and the disease is more common in men [3].
The burden of MDS is significant and life expectancy is curtailed. Mortality rate and risk of progression to acute myeloid leukemia (AML) at 4 years has been seen to be 18% and 9% in low risk patients, and 62% and 61% in high risk patients [4].
Anemia is a major cause of morbidity in MDS. The WHO defines anemia as hemoglobin levels <13 g/dL for men and <12 g/dL for women [5]. Up to 90% of MDS patients are anemic at the time of presentation, and approximately 60% have severe anemia (Hb <9g/dl) [6].
Anemia may be due to high - but ineffective -erythropoietic activity in lower risk patients, while patients with multilineage dysplasia or with excess blasts show relative erythroid marrow failure [7]. Accordingly, in MDS, patients' anemia is refractory and is often observed with normal vitamin B12 and folate levels [8]. Conventional treatments for anemia are often ineffective and therefore over 40% of MDS patients require regular red blood cell (RBC) transfusions at some stage of their disease [9].
Anemia is well recognized as leading to symptomatic impairment in older patients. Physical impairment is the primary symptom, which in turn affects patients' mobility and ability to perform activities of daily living [10]. Anemia also exacerbates the symptoms of existing comorbidities.
It is also recognized that anemia has an important effect on mortality. In conditions such as chronic kidney disease and congestive heart failure, the presence of anemia is strongly correlated with premature mortality [11, 12]. Equally in MDS, anemic patients have been seen to have a higher mortality rate than non-anemic patients [13].
This mortality burden is primarily mediated through a higher incidence - and exacerbation of pre-existing - cardiovascular disease (CVD). Anemia induces lower after-load and higher preload and can lead to cardiac remodeling, cardiac enlargement and left ventricular hypertrophy [14]. Furthermore, anemia can also reduce renal blood flow causing fluid retention, which may contribute to CVD [15]. In the presence of heart disease, anemia intensifies ischemia and angina, further contributing to an increased risk of cardiovascular complications [16].
CVD accounts for up to half of all mortality in MDS and is closely associated with the prevalence and severity of anemia [17]. Chronic transfusion dependent MDS patients have been seen to have twice the rate of mortality of transfusion independent patients [6]. Indeed, RBC transfusions aimed at correcting anemia may further impair a patient's prognosis because of the risk of associated iron-overload [18]. It has been shown that heart failure is significantly more frequent in transfusion-dependent MDS patients than those who do not require transfusions. In a study investigating the natural history of 37 patients with idiopathic refractory sidero-blastic anemia, complications of iron overload (primarily heart failure) were found to be the most frequent cause of death, accounting for 47% of mortality [19].
Beyond RBC transfusions, the management of anemia in MDS patients is not well established [20]. Erythropoietic stimulating agents have shown some efficacy [21, 22], yet not all patients are appropriate and the decision to prescribe needs to be considered in the context of the overall benefit/risk balance [23, 20]. Newer agents, such as lenalidomide also offer the potential to manage anemia and delay the requirement for transfusions [24].
Although the association between patient transfusion status and heart disease in MDS is established [6, 14], literature evaluating the specific relationship between anemia and cardiovascular outcomes is limited and has not been comprehensively summarized. The aim of this study is therefore to review the published literature on the effect of anemia on cardiovascular outcomes in patients with MDS (all subtypes).
Materials and methods
A systematic literature review was undertaken to identify studies that investigated the relationship between anemia (as defined by hemoglobin levels) and cardiovascular outcomes in patients with MDS (all subtypes). A broad and sensitive search strategy was devised in recognition of the small number of relevant studies expected to be published.
MEDLINE, EMBASE, Cochrane Library, CANCER-LIT, Web of Science, TRIP and DARE databases were searched for studies published between 1980 and May 2011. Additional searches were conducted in the online abstract catalogues from the American Society of Hematology and European Hematology Association annual meetings.
Abstracts of all the identified studies were downloaded into Refman software and reviewed against predefined inclusion criteria: MDS patient population (all subtypes); both hemoglobin data and cardiovascular outcome data reported (outcomes included proxy measures of CVD such as echocardiographic measurements, as well as myocardial infarction, cardiac failure and cardiovascular death). All experimental study designs were included, except case studies.
Based upon the abstract review, complete articles were reviewed fully to identify the studies to be summarized. For those studies that met the inclusion criteria, further citation reviews were conducted as well as ‘forward’ searches for papers that had since cited them, and a ‘related’ search was conducted in MEDLINE to triangulate from the selected studies to any other papers that had not been identified in the search so far.
Results
The primary literature search yielded 3,952 abstracts, of which 18 full papers were identified as suitable for further evaluation, plus a further five potentially relevant papers were found through cross-referencing. Following appraisal against inclusion/exclusion criteria, five papers were found that reported original data relevant to informing the relationship between anemia and cardiovascular outcomes. Of these, four papers [14, 21, 25, 26] reported on three studies that explicitly evaluated the relationship between anemia (as measured by hemoglobin levels) and cardiovascular endpoints. In addition, a further study [13] reported on the relationship between hemoglobin levels and survival, but did not describe the effect on cardiovascular death specifically. The findings from these studies are discussed below.
Malcovati et al [25, 26] conducted a retrospective, longitudinal analysis to study the relationship between degree of anemia and clinical outcome in MDS patients, with a view to incorporating severe anemia as a risk factor in an updated WHO classification-based Prognostic Scoring System (WPSS). Severe anemia was defined as hemoglobin levels lower than 9 g/dL in males and 8 g/dL in females.
Two cohorts were analyzed, a learning' group (840) from Pavia, Italy, and a ‘validation’ group (504) from Düsseldorf, Germany. Data were collected between 1992 and 2007 for the former, and 1982 and 2006 for the latter.
Variations in hemoglobin (in 1g/dl units) were monitored and regressed against the incidence of CVD, the definition of which included coronary artery disease, congestive heart failure, myocardial infarction, and ejection fraction lower than or equal to 50%. The correlation between hemoglobin and overall survival (OS) was also investigated.
At diagnosis, CVD was seen to be prevalent amongst 211 of 840 patients (25%) in the learning' cohort, and a further 79 patients (9%) developed CVD during follow-up. This included congestive heart failure (19%), coronary artery disease or myocardial infarction (8%), arrhythmia (7%), and heart valve disease (2%). CVD accounted for 63% of non-leukemic mortality in the cohort during follow-up.
Results from the learning' cohort showed a strong correlation between hemoglobin levels and CVD. Both CVD and cardiovascular death were significantly more frequent in patients with severe anemia, compared to those with higher hemoglobin levels (IRR 1.70, P<0.001 and IRR 4.22, P<0.001, respectively). Patients who developed severe anemia at any time during the follow-up had a significantly higher risk of cardiovascular death (HR 4.35, P<0.001). This result held when the model was adjusted for sex, age and cardiovascular comorbidity (HR 3.62, P<0.001).
A linear relationship between hemoglobin and overall survival was noted, with a statistically significant difference being observed at hemoglobin levels lower than 11 g/dl in males (HR from 5.23 to 15.56) and lower than 9 g/dl in females (HR from 6.26 to 11.82).
This relationship was seen to stand even among those patients who did not have CVD at diagnosis. In those patients, severe anemia increased the risk of cardiac complications compared to patients with higher hemoglobin levels (HR 3.85, P<0.001) (Figure 1).
Figure 1.
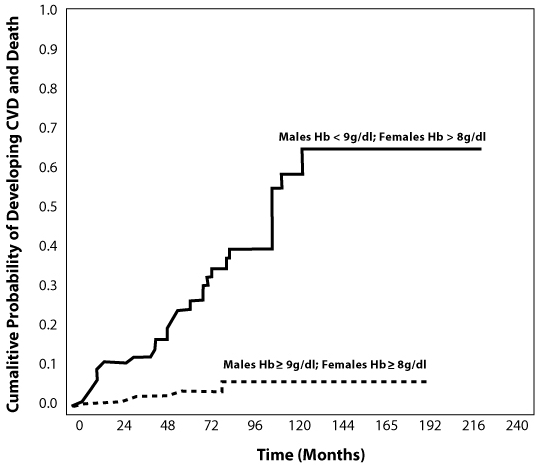
Cumulative probability of cardiac complications in MDS patients without cardiac disease at presentation, according to the presence or absence of severe anemia (HR 3.85, P<0.001) [25].
As well as supporting the relationship between anemia and cardiovascular outcomes, the results from this study reinforced the well-established relationship between transfusion dependency and negative cardiovascular outcomes. Of note, the authors concluded that anemia had been shown to be as important a prognostic factor for predicting cardiovascular outcomes and mortality as transfusion status - a novel finding.
Oliva et al [14] sought to investigate the relationship between anemia and cardiovascular outcomes (and related quality of life) in a cohort of 39 MDS patients recruited from a clinic in Reggio Calabria, Italy. The study utilized a cross-sectional regression analysis design and compared two cohorts of patients according to transfusion status. For each cohort evaluations were conducted to measure hemoglobin levels and cardiac remodeling, as estimated by echocardiographic evaluation of left ventricular hypertrophy (LVH). Health-related quality of life (HRQoL) was measured using the disease-specific QOL-E© instrument.
Results from the study demonstrated a statistically significant relationship between hemoglobin levels and cardiac remodeling (Table 1). After adjusting for age and transfusion status, the logistic regression model predicted that each unit increase of hemoglobin could lead to a 49% (Cl 69-20%) decrease in the risk of occurrence of remodeling (P = 0.004), with a model accuracy of 84.6%. HRQoL was seen to be correlated with anemia: functional well-being was observed to be poorer in patients with hemoglobin values below 10.7 g/dL (P = 0.07).
Table 1.
Patient demographics, hemoglobin levels and cardiac remodeling by transfusion status [14]
| TD | TI | |
|---|---|---|
| MDS Patient N | 12 | 27 |
| Female % | 51% | |
| Incidence of cardiac remodeling Likelihood ratio (95% CI; P-value) | 92% | 48% |
| 1.8 (1.2-2.6; P = 0.01) | ||
| Mean Hb level of patients without CRM g/dl | 11.3 ± 2.4 | |
| Mean Hb level of patients with CRM g/dl | 8.7 ± 1.4 |
TD, Transfusion Dependent; TI, Transfusion Independent; Hb, Hemoglobin; CRM, Cardiac Re-Modeling
Based upon these results, the authors concluded that there was a clear relationship between hemoglobin levels, cardiac remodeling and HRQoL, and that the target of therapeutic intervention ought to be to maintain hemoglobin at near-normal levels.
A second study by Oliva et al [21] investigated the improvement in cardiac remodeling associated with effective treatment of anemia in MDS patients. Data on hemoglobin levels and cardiac remodeling was collected during a 24 week, Phase II, non-controlled, longitudinal study of darbepoietin alfa 150 mcg s.c weekly (to be increased to 300 mcg weekly in non-responders) in 20 patients with low and intermediate-1 risk MDS and hemoglobin levels less than 11 g/dl at baseline.
Hemoglobin levels and cardiac geometry were measured at baseline, 24 weeks, and one year. The latter was evaluated according to left ventricular mass index (LVMI) quantified by echo-cardiogram.
Amongst the 14 patients who received at least 24 weeks of treatment, there was a positive hemoglobin response of at least 1g/dl in 9 out of 11 patients who were not transfusion-dependent. Of the 3 patients who were receiving transfusions, one patient responded with a decrease in transfusion requirement.
Cardiac geometry was also seen to improve at 24 weeks and was significant amongst patients who had seen a gain in hemoglobin (p=0.033). The median reduction in LVMI was -3.5 (range -53.37 to 51.43) and 2 patients with baseline hypertrophy saw partial and complete responses, respectively.
No regression analysis was reported on the strength of the relationship between hemoglobin and LVMI, but univariate ANOVA analysis showed that improvements in hemoglobin was associated with improvements in QOL-E© treatment outcome index (p=0.021), specific (p=0.001), fatigue (p=0.02), physical (p=0.028), functional (p=0.022), and general (p=0.048) scores.
Despite the relatively small patient numbers in this study, the results were considered important as they demonstrated a causative effect between changing anemia levels and cardiovascular outcomes, whereas previous studies described in this review only demonstrated an association between these factors.
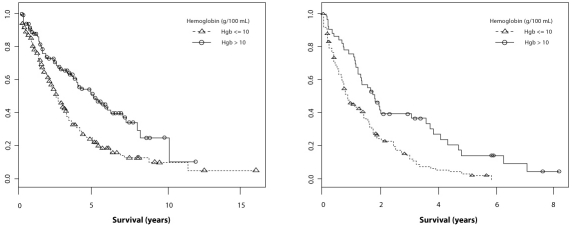
The final study identified through the systematic review was a retrospective analysis of data relating to 815 MDS patients held in the International MDS Risk Analysis Workshop (IMRAW) Database [13]. The aim of the study was to improve the International Prognostic Scoring System (IPSS) by including additional risk factors, particularly the role of anemia per se, as opposed to the proxy of transfusion status. Accordingly, the effect of hemoglobin level was assessed against long-term patient outcomes using multivariate regression.
Although the relationship to cardiovascular outcomes was not reported specifically, hemoglobin level was observed as being highly statistically significantly associated with overall survival (P<0.0001). This relationship was particularly pronounced in the lntermediate-1 and Intermediate-2 subgroups of the IPSS (Figure 2) and remained significant after adjustment for gender and IPSS.
Figure 2.
Overall survival (OS) of patients with MDS in the Intermediate IPSS categories (Int-1 and Int-2) relative to baseline levels of hemoglobin (Kaplan Meier curves) [13].
The authors suggested that the findings from the analysis not only implied that anemia levels should be used as a prognostic factor in MDS, but that patients with MDS in the intermediate IPSS risk groups who have hemoglobin levels below 10 g/dl should be considered for disease-altering treatment.
Discussion
This systematic review identified a small, but relatively homogenous, body of evidence investigating the role of anemia in affecting cardiovascular outcomes and overall survival in patients with MDS.
The four studies consistently showed a strong relationship between lower hemoglobin levels and worse cardiovascular outcomes, including cardiac remodeling, congestive heart failure, coronary artery disease, myocardial infarction, arrhythmia, heart valve disease, and cardiovascular mortality. Further, anemia has been shown to be an independent predictor of CVD outcomes in patients with MDS, beyond transfusion status and IPSS.
The magnitude of the relationship appears to be clinically significant, as well as statistically so. In the Oliva et al (2005) study [21], a 1 g/dl improvement in hemoglobin was associated with a 49% reduction in risk in cardiac remodeling. And in the largest study conducted to date [25] non-leukemic death was seen to be 4 times higher in patients with Hb levels <9g/dl versus patients with Hb levels>11g/dl. Anemia was also observed to affect patient HRQoL [21].
Not only were results consistent between studies, but also between study designs. Three retrospective regression analyses supported the importance of anemia as a cardiovascular risk-factor, and this was validated by the single prospective, interventional study in which a change in hemoglobin level led to improved cardiac geometry.
Investigator conclusions drawn from these studies have also been consistent. Given that anemia has been shown to be as good a predictor of outcome as transfusion status [25], all authors advocated the increased use of this measure as a prognostic marker in clinical practice. Similarly, given the size of the burden associated with CVD in MDS, many of the published papers have recommended addressing anemia early after diagnosis so as to avoid transfusion dependency and related morbidity. For example, Oliva et al (2005) [21] suggested that clinicians should seek to maintain near-normal hemoglobin levels to prevent cardiac remodeling.
Yet despite the uniform conclusions from these analyses, there is still a need for further research to inform clinical practice in this area. The high quality data necessary to justify a global change in the management of MDS are not currently available. There is a shortage of studies looking specifically at the relationship between anemia and cardiovascular outcomes in MDS patients, while the studies that have been conducted have mostly been retrospective or cross-sectional analyses that are subject to confounding. The only prospective study to date has been very small and requires validation in a larger cohort. More studies are therefore needed to address these issues, particularly MRI studies and controlled studies of therapeutic interventions.
Conclusion
Based upon a relatively small body of evidence, there appears to be a strong and clinically significant association between anemia and cardiovascular morbidity and mortality in MDS. While further research is needed, clinicians should seek to actively manage hemoglobin levels in MDS patients before the point of transfusion dependency is reached.
Acknowledgments
The authors should like to thank Reinhard Wentz for his assistance in conducting the systematic literature review.
Declarations
This study was funded by Celgene Corporation. AH and CS are employed by GMAS, an organization that provides scientific consultancy services to pharmaceutical companies, including Celgene. ENO has provided consultancy services to pharmaceutical companies, including Celgene.
References
- 1.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, CD Bloomfield. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Aul C, Giagounidis A, Germing U. Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital-based statistics. Int J Hematol. 2001;73(4):405–10. doi: 10.1007/BF02994001. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 4.Malcovati L, Germing U, Kuendgen A, Delia Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B, Bernasconi P, Knipp S, Strupp C, Lazzarino M, Aul C, Cazzola M. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Worldwide prevalence of anaemia 1993-2005 : WHO global database on anaemia. In: de Benoist B, editor. Geneva: World Health Organization; 2008. p. 4. [Google Scholar]
- 6.Malcovati L, Della Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino M, Passamonti F, Arcaini L, Maffioli M, Bernasconi P, Lazzarino M, Cazzola M. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;30:7594–7603. doi: 10.1200/JCO.2005.01.7038. 23. [DOI] [PubMed] [Google Scholar]
- 7.Cazzola M, Della Porta MG, Malcovati L. Clinical Relevance of Anemia and Transfusion Iron Overload in Myelodysplastic Syndromes. Hematology Am Soc Hematol Educ Program. 2008:166–75. doi: 10.1182/asheducation-2008.1.166. [DOI] [PubMed] [Google Scholar]
- 8.Catenacci DV, Schiller GJ. Myelodysplasic syndromes: a comprehensive review. Blood Rev. 2005;19(6):301–19. doi: 10.1016/j.blre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Brechignac S, Hellstrom-Lindberg E, Bowen DT, DeWitte TM, Cazzola M, Fenaux P. Quality of Life and Economic Impact of Red Blood Cell (RBC) Transfusions on Patients with Myelodysplastic Syndromes (MDS) Blood. 2004;104:4716. [Google Scholar]
- 10.Balducci L. Anemia, fatigue and aging. Transfusion Clinique et Biologique. 2010;17:375–381. doi: 10.1016/j.tracli.2010.09.169. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107(2):223–5. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 12.Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations, and end-stage renal disease among patients with chronic kidney disease. Nephrology. 2009;14(2):240–46. doi: 10.1111/j.1440-1797.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 13.Kao JM, McMillan A, Greenberg PL. International MDS risk analysis workshop (IMRAW)/ IPSS reanalyzed: impact of cytopenias on clinical outcomes in myelodysplastic syndromes. American journal of hematology. 2008;83:765–770. doi: 10.1002/ajh.21249. [DOI] [PubMed] [Google Scholar]
- 14.Oliva EN, Dimitrov BD, Benedetto F, D'Angelo A, Nobile F. Hemoglobin level threshold for cardiac remodeling and quality of life in myelodysplastic syndrome. Leuk Res. 2005;29:1217–1219. doi: 10.1016/j.leukres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg DS, Wexler D, Iaina A. The role of anemia in the progression of congestive heart failure. Is there a place for erythropoietin and intravenous iron? J Nephrol. 2004;17:749–761. [PubMed] [Google Scholar]
- 16.Goodnough LT, Bach RG. Anemia, transfusion and mortality. N Engl J Med. 2001;345:1272–4. doi: 10.1056/NEJM200110253451711. [DOI] [PubMed] [Google Scholar]
- 17.Patnaik MM, Lasho TL, Finke CM, Gangat N, Caramazza D, Holtan SG, Pardanani A, Knudson RA, Ketterling RP, Chen D, Hoyer JD, Hanson CA, Tefferi A. WHO-defined ‘myelodysplastic syndrome with isolated del (5q)’ in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia. 2010;24:1283–1289. doi: 10.1038/leu.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balducci L. Transfusion independence in patients with myelodysplastic syndromes: impact on outcomes and quality of life. Cancer. 2006;106:2087–2094. doi: 10.1002/cncr.21860. [DOI] [PubMed] [Google Scholar]
- 19.Cazzola M, Barosi G, Gobbi PG, Invernizzi R, Riccardi A, Ascari E. Natural history of idiopathic refractory sideroblastic anemia. Blood. 1988;71(2):305–12. [PubMed] [Google Scholar]
- 20.Hamblin TJ. The management of anemia in the myelodysplastic syndrome. Leukemia Research. 2005;29:1101–1102. doi: 10.1016/j.leukres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Oliva EN, Benedetto F, Specchia G, Vincelli I, Colonna P, Liso V, Fortugno F, Nobile F. Treatment of Anemia of Myelodysplastic Syndromes and Improvements in Cardiac Geometry. Blood. 2006;108 [Google Scholar]
- 22.Spiriti MA, Latagliata R, Niscola P, Cortelezzi A, Francesconi M, Ferrari D, Volpe E, Clavio M, Grossi A, Reyes MT, Musto P, Mitra ME, Azzará A, Pagnini D, D'Arena G, Spadano A, Balleari E, Pecorari P, Capochiani E, De Biasi E, Perego D, Monarca B, Pisani F, Scaramella G, Petti MC. Impact of a new dosing regimen of epoetin alfa on quality of life and anemia in patients with low-risk myelodysplastic syndrome. Ann Hematol. 2005;84:167–176. doi: 10.1007/s00277-004-0961-9. [DOI] [PubMed] [Google Scholar]
- 23.Bowen D, Culligan D, Jowitt S, On behalf of the UK MDS Guidelines Group Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 24.Fenaux P, Giagounidis A, Selleslag DL, Beyne-Rauzy O, Mittelman M, Muus P, Knight RD, Fu T, Hellstrom-Lindberg E, The MDS-004 Len del (5q) Study Group Safety of lenalidomide (LEN) from a randomized phase III trial (MDS-004) in low-/int-1-risk myelodysplastic syndromes (MDS) with a del(5q) abnormality. J Clin Oncol. 2010;28(15):6598. [Google Scholar]
- 25.Malcovati L, Della Porta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K, Travaglino E, Invernizzi R, Pascutto C, Lazzarino M, Germing U, Cazzola M. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS) Haematologica. doi: 10.3324/haematol.2011.044602. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambaglio I, Travaglino E, Della Porta MG, Pascutto C, Ubezio M, Invernizzi R, Malcovati L, Cazzola M. The impact of the degree of anemia on survival of patients with myelodysplastic syndrome. A basis for prognostic assessment and clinical decision making. Haematologica. 2010;95(2):124. [Google Scholar]