Abstract
Bim, a BH3-only Bcl-2-family protein, is essential for T-cell negative selection in the thymus as well as for the death of activated T cells in the periphery. The role of Bim has been extensively studied in T-cell responses to self-antigens and viral infections. Recent findings on Bim in autoimmunity triggered our interest in investigating whether Bim may play a role in another disease with inflammatory symptoms as graft-versus-host disease (GVHD). Here we report that Bim is required for optimal T-cell responses to alloantigens in vivo and for the development of GVHD. Using murine models of allogeneic bone marrow transplantation (BMT), we found that donor T cells deficient for Bim are impaired in the induction of GVHD primarily due to a significant defect in T cell activation and expansion in vivo. Upon TCR engagement, Bim-/- T cells exhibited selective defects in CD69 expression and phosphorylation of PLCγ1. Our studies uncover a novel aspect of Bim function in T-cell activation with important implications in understanding the mechanisms of T-cell activation and tolerance under allogeneic transplantation.
Keywords: Bim, T cells, proliferation, apoptosis, alloantigen, GVHD, GVL, and BMT
Introduction
Antigen-driven T-cell activation and expansion is an essential player during adaptive immune response [1,2]. T-cell responses to antigens are characterized by rapid expansion in numbers of specific cells [3,4]. After the peak response, the majority of activated T cells die through apoptosis while those that remain become memory T cells. The decision between death and survival is crucial for avoiding autoimmunity and for promoting the development of immunological memory and protective immunity. Bim, a proapoptotic Bcl-2 family member, plays a critical role in the apoptosis of activated T cells in vivo [5-8]. Proapoptotic activity of Bim is determined by the level of antiapoptotic molecule Bcl -2 before T cells undergo apoptosis in vivo [9,10]: while Bcl-2 levels significantly decrease, Bim initiates the apoptotic process [11-13]. Bim signaling is also involved in various cell fate decisions during the development of multicellular organisms, including survival, proliferation, lineage commitment and tissue architecture [14-18]. Bim also plays a critical role in regulating lymphocytes’ homeostasis in the lymphoid and myeloid compartments [19]. Moreover, deficiency in Bim leads to defective negative selection for the autoreactive T and B cells followed by the subsequent expansion of lymphocytes and the development of autoimmunity in mice [19]. However, it remains unclear whether the T cells that would normally be eliminated during an immune response can, in the absence of apoptotic signals, result in increased T-cell memory and protective immunity.
In this study, we investigated the role of Bim in the control of T-cell responses under homeostasis and upon alloantigen stimulation using well-defined models of bone marrow transplantation (BMT). Given that Bim is an essential mediator in the elimination of activated T cells in vivo, we hypothesized that activated T cells would accumulate after being transplanted in allogeneic recipient and induce exacerbated GVHD under BMT settings. However, we found that donor T cells deficient for Bim are impaired in the induction of GVHD in allogeneic recipient, primarily due to the significant reduction of expansion and activation of alloreactive T cells in vivo. This finding provides additional insight to understand how Bim regulates T-cell activation.
Material and methods
Mice
C57BL/6 (B6, H-2b) and BALB/c (H-2d) mice were purchased from the National Cancer Institute, B6 mice that express congenic Ly5.1 or Thy1.1 strains, and B6.bm12 mice were from the Jackson Laboratory. The founders of B6 Bim-/- mice were kindly provided by P. Marrack (National Jewish Medical and Research Center, Denver, CO). All the mice were housed in a pathogen-free condition. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
T-cell purification and proliferation in vitro
Our protocol for T-cell purification and transplantation has been described in detail elsewhere [20,21]. Briefly, CD4+ or total T cells were purified by negative selection with a magnetic cell separation system (Miltenyi Biotec, Auburn, CA). The purity of T cells used for transplantation ranged from 91-97%. Cells were cultured in RPMI 1640 medium containing 10% FBS, 2 mM glutamine, 25 mM HEPES, 1 mM sodium pyruvate, 5 × 105 M 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin. In separate experiments, purified T cells were labeled with CFSE (Molecular Probes, Eugene, OR) and stimulated with anti-CD3. After stimulation for 3- 5 days, T-cell proliferation was measured with CSFE dilution as described in our previous work [20,21].
T-cell signaling
Immunoprecipitation and Western blotting were performed as previously described [22,23]. Briefly, primed T cells were stimulated with or without anti-CD3ε mAb for different time periods at 37°C on a Thermomixer. Cells were washed in cold PBS containing 0.4mM Na3VO4 and 0.4mM EDTA and lysed on ice for 30 min. Cell lysates were obtained after removal of cell debris by centrifugation at 13,500 rpm for 10 min at 4°C. Proteins in cell lysate were separated in 10% SDS-PAGE gels, transferred to polyvinylidene difluoride membranes, and immunoblotted with mAb specific for phosphotyrosine (Upstate Biotech, Lake Placid, NY), total PLCγ, p-PLCγ (Cell Signaling, Beverly, MA), total ERK, JNK, p-ERK or p-JNK (Santa Cruz, CA). Signal detection was performed by chemiluminescence (Roche Diagnostics, Indianapolis, IN)
BMT
In myeloablative model, B6 mice were exposed to 1200 cGy (split doses) and BALB/c mice to 800 cGy of TBI. T cell depleted-bone marrow (TCD-BM) cells alone or in combination with purified T cells from indicated donors were injected via the tail vein into recipients within 24 hrs after irradiation. Recipient mice were monitored every other day for clinical signs of GVHD, such as ruffled fur, hunched back, lethargy, diarrhea, and mortality. Animals judged to be moribund were sacrificed and counted as GVHD lethality as described in our previous work [21,24].
Statistical analysis
The log-rank test was used to detect statistical differences in recipient survival in GVHD experiments. Student's t test was used to compare percentages or numbers of donor T cells.
Results
T cells deficient for Bim have reduced ability to induce GVHD
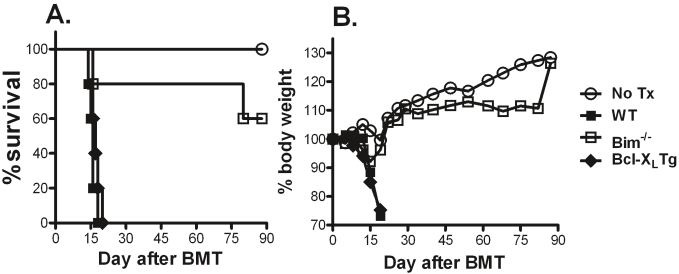
We originally hypothesized that Bim-/- T cells would induce more severe and sustained GVHD considering Bim as a predominant pro-apoptotic molecule. Initial experiments were conducted to compare the ability of Bim+/+ and Bim-/- CD4+ T cells to induce GVHD in non-myeloablative transplantation model: B6 → B6.bm12. Under this condition, donor T cells damage the recipient hemopoietic system, resulting in marrow failure. CD4+ T cells from Bcl-XL Tg mice were also tested as additional controls, because Bcl- XL promotes T-cell survival [16,24]. While Bcl-XL Tg T cells were as capable as WT T cells to induce GVHD, most recipients of Bim-/- T cells survived long-term with little or no weight loss, indicating that their ability to induce GVHD was impaired (Figure 1A and 1B).
Figure 1.
Role of Bcl-XL or Bim in the development of GVHD. B6 bm12 mice were sublethally irradiated (600 cGy) and transferred with 1 × 106 CD4+ T cells from WT, Bcl-XL Tg or Bim-/- B6 donors. Five recipients were used in each group, and the data show recipient survival (A) and weight loss (B) over time.
Cell division and expansion of Bim-/- T cells is compromised in response to alloantigens in vivo
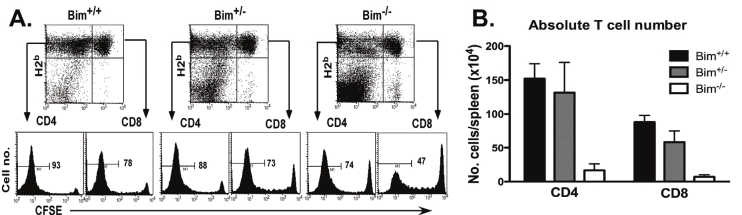
To investigate the mechanism by which Bim-/- T cells cause significantly less GVHD than WT T cells, we compared T-cell division and expansion of Bim+/+, Bim+/- and Bim-/- T cells in allogeneic BALB/c recipients. Four days after adoptive transfer, the percentages of Bim+/+ and Bim-/- CD4+ T cells that had divided (CSFElow) were 94 ± 5 and 74 ± 5, respectively (p < 0.05); and the percentages of Bim+/+ and Bim-/- CD8+ T cells that had divided (CSFElow) were 77 ± 8 and 46 ± 5, respectively (p < 0.01) (Figure 2A). By counting the absolute number of T cells in recipient spleens, we found that the numbers of CD4+ or CD8+ Bim-/- T cells were significantly lower than those of Bim+/+ T cells (p < 0.01) (Figure 2B), indicating that Bim increases T-cell expansion in vivo.
Figure 2.
Bim-/- T cells have impaired expansion in response to alloantigen in vivo. Purified T cells from Bim+/+, Bim+/- or Bim-/- B6 mice were labeled with CFSE and then injected into lethally irradiated BALB/c mice at 2 × 106 cells/ mouse. T-cell division and expansion in recipient spleen was determined 4 days after cell transfer. (A) Expression of H2Kb (y-axis) and CD8 (x-axis) is shown in recipient spleen cells (top panel); CFSE profiles are shown on gated donor CD4 (H2Kb+CD8-) or CD8 (H2Kb+CD8+) T cells (bottom panel). (B) Absolute numbers of donor CD4 or CD8 T cells (average ± 1 SD, n = 3 - 4 recipients in each group) are shown in recipient spleen. The data represent 1 of 4 replicate experiments.
Bim plays a distinct role in homeostasis-driven or alloantigen-induced T cell response
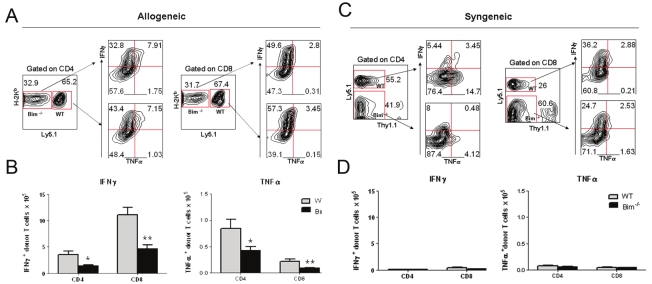
To distinguish the effects of Bim on homeostatic proliferation from those of alloantigen-driven proliferation, we designed a competitive repopulation experiment. We purified T cells from normal B6 mice expressing the Ly5.1 congenic marker and from B6 Bim-/- mice, mixed them at a 1:1 ratio and transferred the mixture into either allogeneic BALB/c or syngenic B6 mice expressing the Thy1.1 congenic marker. Four days after cell transfer, the presence of WT and Bim-/- T cells was measured in recipient spleens (Figure 3A and 3C). In syngeneic B6 recipients, there were 31.22 ± 6.25 % WT cells (Thy1.1- Ly5.1+) and 49.58 ± 4.99 % Bim-/- cells (Thy1.1- Ly5.1-) (p = 0.03). In allogeneic BALB/c recipients, there were 63.6 ± 1.18 % WT cells (H2b+Ly5.1-) and 30.9 ± 5.15 % Bim-/- cells (H2b+Ly5.1-) (p = 0.0009). These data indicated that Bim promotes alloantigen-driven T cell proliferation, and not homeostatic proliferation.
Figure 3.
Bim affects cytokine secretion differentially through homeostasis-driven or alloantigen-induced proliferation. Lethally irradiated Thy1.1 (Ly5.1-) (syngeneic) or BALB/c (allogeneic) recipients were infused with B6 purified WT (Ly5.1+) or Bim-/- (Ly5.1-) T cells. Four days after cell transfer, recipient splenocytes were analyzed for expression of surface markers and intracellular cytokines that were measured in splenocytes from allogeneic (A) or syngenic (C) recipients. The absolute number of IFN-γ and TNF-α producing cells were shown in the spleen of allogeneic (B) or syngenic (D) recipients. Results shown are representative of three replicate experiments with 3 mice per group.
To explore the mechanisms involved with the distinct effects of Bim on T-cell allogenic and homeostatic proliferation, we measured intracellular cytokines of WT and Bim-/- CD4 T cells after transfer to recipient mice. In allogeneic recipients, CD4 and CD8 Bim-/- T cells produced less IFNγ than their WT counterparts (Figure 3A). Furthermore, there were significantly lower numbers of IFNγ+ and TNFα+ Bim-/- than WT T cells (Figure 3B). After transfer into syngenic recipients, WT or Bim-/- donor T cells produced a comparably low amount of IFNγ and TNFα on both CD4+ and CD8+ T cells (Figure 3D), and the percentage of IFNγ+ and TNFα+ T cells detected by intra-cytoplasmic staining were comparable (Figure 3C). These data indicate that Bim preferentially promoted alloantigen-driven proliferation.
Bim-/- T cells had reduced ability to induce GVHD but partially retained the graft-versus-leukemia (GVL) effect
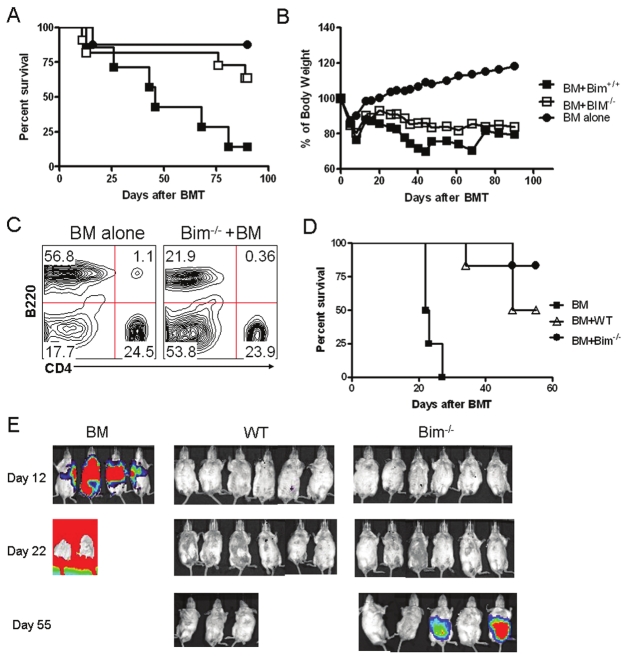
In the clinical hematopoietic cell transplantation (HCT) setting, GVHD typically refers to the epithelial damage induced by donor T cells in major and/or minor histocompatibility complex-mismatched recipients that are lethally irradiated and reconstituted with hemopoietic cells plus peripheral T cells from the donor. Thus, the role of Bim was evaluated in a myeloablated BMT model, where GVHD lethality is induced through epithelial damage. BALB/c mice were lethally irradiated and infused with BM plus CD4+ and CD8+ T cells from either WT or Bim-/- B6 mice. While 90% BALB/c recipients of WT T cells died from GVHD, only 27% recipients of Bim-/- T cells died (Figure 4A and 4B, p < 0.01). The recipients of BM plus Bim-/- T cells had similar T-cell reconstitution but compromised B-cell reconstitution as compared to those of BM alone (Figure 4C). These results indicated that Bim-/- T cells are severely impaired in their ability to induce acute GVHD.
Figure 4.
Bim deficiency does not induce GVHD but promotes GVT. Lethally irradiated BALB/c recipients were transplanted with C57BL/6 TCD-BM alone or plus 1 × 106 total T cells from WT or Bim-/- mice. Recipient survival (A) and weight change (B) are shown with 12 mice from WT or Bim-/- group and 8 mice from TCD-BM alone group. (C) Expression of B220 and CD4 on donor T and B cells was shown in the spleen of recipients of BM alone and BM plus Bim-/- T cells after completion of the experiment on day 100. Lethally irradiated BALB/c mice received TCD-BM cells alone or plus 1 × 106 naïve T cells from WT or Bim-/- donors. Recipients were given 2 × 103 A20 tumor cells with luciferase transgene at the same time of transplantation. Recipient survival (D) and tumor growth (E) were monitored with in vivo BLI.
To assess whether the adoptive transfer of Bim-/- T cells could mediate graft-versus-tumor (GVT) activity, we challenged the allogeneic BMT recipients with A20-Luc mouse lymphoma cells on day 0 after transplantation and measured the development of tumor by in vivo bioluminescence imaging (BLI). Notably, we found GVT activity in recipients of Bim-/- T cells, which resulted in a significant delay in tumor growth compared with the bone marrow-only group and a subsequent significant survival benefit (Figure 4D, 4E; P < 0.001). All mice that received WT T cells rejected the tumor at the expense of severe GVHD, whereas fewer animals in Bim-/- T cell group suffered from GVHD (Figure 4E; P < 0.05). The GVL activity in recipients of Bim-/- T cells was associated with secretion of Th1-type cytokines (including IFN-γ and TNF-α) by Bim-/- T cells, compared with bone marrow-derived donor T cells. Increased concentrations of IFN-γ, which has been shown to be a critical cytokine for GVT effects after allogeneic HCT, were also detected in Bim-/- T cells (data not shown).
Bim-/- T cells have a selective defect in CD69 expression and PLCγ phosphorylation
Because Bim-/- T cells were impaired in alloantigen- driven expansion, we hypothesized that Bim may be required for optimal TCR-signaling. Splenocytes from WT and Bim-/- mice were labeled with CFSE and stimulated with anti-CD3 in vitro. Bim-/- T cells divided slower than WT T cells (Figure 5A). Subsequently, we measured CD69 expression, an early T-cell activation marker. Twenty hours after anti-CD3 stimulation, 60% WT T cells expressed CD69 whereas only 30% Bim-/- T cells expressed CD69 (Figure 5B, left histogram). As a positive control, PMA plus ionomycin induced the same levels of CD69 expression on WT as well as Bim-/- T cells (Figure 5B, right histogram). These data demonstrated that Bim-/- T cells were defective in TCRsignaling.
Figure 5.
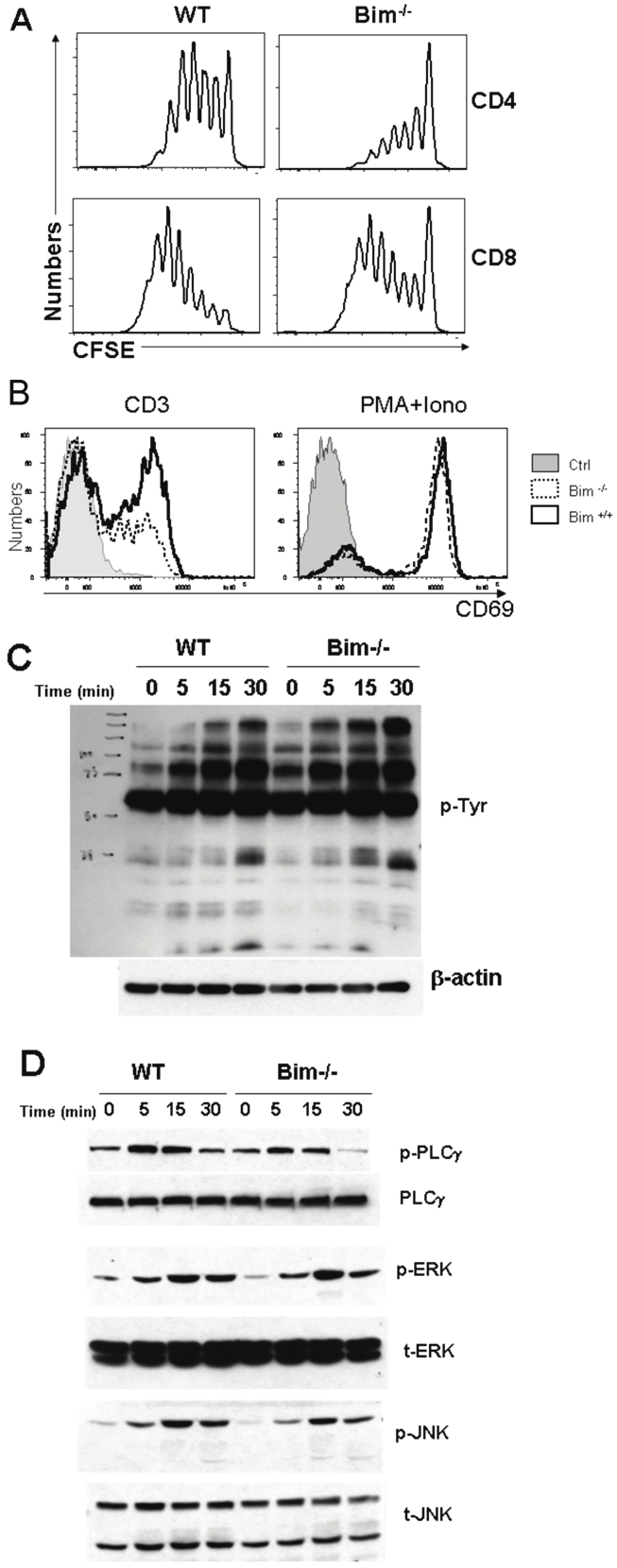
Bim deficiency down-regulates TCR-induced CD69 expression. (A) T cell proliferation after anti-CD3 stimulation. Splenocytes from WT or Bim-/- B6 mice were labeled with CFSE and stimulated with anti-CD3 Ab for 3 days. CFSE dilution was measured by FACS analysis on CD4+ (top panel) and CD8+ cells (bottom panel). (B) CD69 expression. T cells from WT or Bim-/- B6 mice were cultured with medium alone (Ctrl), or stimulated by anti-CD3 mAb (left) or PMA + ionomycin (right) for 20 hrs. Cells were harvested and stained for CD69 expression, and the data represent 1 of 3 replicate experiments. (C) Total protein tyrosine phosphorylation was measured on the T cells from WT and Bim-/- mice stimulated with or without anti-CD3 mAb at 10μg/ml for 5, 15 and 30 min. (D) Tyrosine phosphorylation of PLCγ, ERK and JNK were analyzed by western-blot. Representative data from more than 3 similar experiments are shown.
To further explore whether TCR-signaling was affected by Bim, we measured overall tyrosine phosphorylation upon TCR-engagement and found that WT vs. Bim-/- T cells had similar levels of tyrosine phosphorylation after anti-CD3 stimulation (Figure 5C, p-PLCγ). Phosphorylation of ERK and JNK was not different between these two types of T cells, indicating that the MAPK signaling pathways are normal in Bim-/- T cells. In contrast, PLCγ phosphorylation was markedly reduced at 5 or 10 minutes and not sustained in Bim-/- T cells at 30 minutes after TCR engagement (Figure 5D). Because recruitment and activation of PLCγ1 is a key step in the T-cell activation process triggered by the TCR, defect in activation of PLCγ1 may account for the compromised ability of Bim-/- T cells to respond to alloantigen and induce GVHD.
Discussion
The current study provides evidence that Bim is required for optimal alloantigen-induced T-cell activation in vivo. Using murine models of allogeneic BMT, we observed that Bim-/- T cells are severely impaired in the induction of GVHD (Figure 1 and 4). Ludwinski et al. recently found that Bim-/- mice are resistant to autoimmune encephalomyelitis and diabetes, and concluded that Bim is paradoxically required for the activation of autoreactive T cells [25]. Our study showed that Bim is required for the activation and expansion of alloreactive T cells. Our data demonstrate that Bim plays a critical role in T-cell activation and expansion.
We were interested in understanding how Bim affects T-cell signaling that is initiated on the cell surface and required for the T-cell response. Bim-/- T cells divided slower than Bim+/+ T cells in vivo (Figure 2). Bim-/- B cells also have reduced division and entry into the cell cycle upon stimulation with anti-IgM or LPS [26]. Delayed B-cell cycle entry in Bim-deficiency was shown to be associated with increased expression and delayed degradation of the Cip/Kip subfamily CdK inhibitor, p27kip1 [26]. It is likely that Bim also plays a role in TCR-mediated degradation of p27kip1 in T cells, because Bim-/- T cells also express elevated levels of p27kip1 before and after anti-CD3 stimulation [25]. Ludwinski et al recently reported that Bim-/- T cells have a selective defect in the Ca2+/NFAT signaling pathway [25]. We extended their study and found that Bim-/- T cells are impaired in the upregulation of CD69 expression and phosphorylation of PLCγ upon TCR engagement (Figure 5B). PLCγ activation leads to Ca2+-influx, which in turn facilitates CD69 up-regulation [27]. Both studies agree that Bim has no effect on MAPK signaling pathway, including ERK and JNK (Figure 5D). In addition, reduced CD69 expression and PLCγ activation could be the reason for decreased Bim-/- T cells proliferation in response to TCR engagement (Figure 5D).
Strong evidence indicates that multiple apoptosis pathways are operative in transplantation settings. While massive apoptosis of alloreactive T cells occurs during the development of GVHD [28], neither the loss of Fas nor the gain of Bcl-XL impairs T cell death and thus accelerates the disease [29-31]. Likewise, Bim-deficiency or Bcl-2 transgene does not affect T-cell mediated rejection of allogeneic islet graft, nor does it interfere with tolerance induction by costimulatory blockade [32]. In agreement with these published findings, our data show that Bim-deficiency did not significantly promote survival of alloreactive T cells in vivo, instead it reduced T-cell expansion by limiting cell division. Since the liver is a major organ for the apoptosis of activated T cells, we observed 2-3 fold fewer Bim-/- than WT T cells in the liver (Figure 3B). We also found that WT and Bim-/- T cells underwent a similar rate of apoptosis in allogeneic recipients in vivo (data not shown). Thus, the reduction of dividing Bim-/- T cells was neither due to accelerated apoptosis nor trafficking to the liver. Consistently, Bim and Puma have overlapping functions with Bim in vivo [33], and Fas, Bak or Bax also compensates the loss of Bim for downsizing T-cell responses in vivo as well [34-37].
In contrast to allogeneic recipient, Bim-deficiency enhanced T-cell expansion in syngenic recipients (Figure 3), consistent with the notion that Bim is required for hematopoietic homeostasis [38,39]. Furthermore, we found that Bim-/- T cells started to accumulate in allogeneic recipient over time, and in fact the number of Bim-/- and WT T cells were comparable after 2 weeks post BMT (data not shown). We interpreted that, in the absence of Bim, the strong homeostatic proliferation of non-alloreactive T cells would eventually offset the weak alloantigen-driven proliferation of alloreactive T cells. However, GVHD development was still diminished because those non-alloreactive T cells were not pathogenic to the host. Moreover, we also found that Bim-/- T cells not only rescues hosts from GVHD but also preserve the GVT activity. We proved this finding by using the BLI to serially monitor the clearance of allogeneic tumors in GVHD model system.
The current study demonstrated that T cells deficient for Bim are impaired in the induction of GVHD, primarily because Bim-deficiency limits division and expansion of alloreactive T cells in vivo. Together with the recent observation by others that deletion of Bim in hematopoietic cells renders mice resistant to autoimmune diseases [25], our studies uncover a novel aspect of Bim function in T-cell activation and provide an important implication for our understanding of the mechanisms of T-cell activation and tolerance under allogeneic transplantation.
Acknowledgment
We thank Drs. P Marrack for providing Bim-/- mice and CB Thompson for Bcl-XL Tg mice. We are grateful for the technical assistance provided by Flow Cytometry and Mouse Core Facility at the Moffitt Cancer Center. This work was supported in part by National Institutes of Heath Grants CA118116 and CA143812 to X-ZY, and CA076292 and AI082498 to CA.
Authorship and conflict of interest statements
YY participated in experimental design, performed research, collected, analyzed and interpreted data, performed statistical analysis, and drafted and revised the manuscript; JY, CI and KK performed research, collected and analyzed data, and edited the manuscript; CA participated in experimental design, interpreted data, and revised the manuscript; X-ZY designed research, analyzed and interpreted data, performed statistical analysis, and revised the manuscript. The authors have no conflicting financial interests.
References
- 1.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grayson JM, Weant AE, Holbrook BC, Hildeman D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J Virol. 2006;80:8627–8638. doi: 10.1128/JVI.00855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic Bcl-2 family member Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3- only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 10.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;7:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 12.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic Bcl-2 family member Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell T, Kappler J, Marrack P. Bystander virus infection prolongs activated T cell survival. J Immunol. 1999;162:4527–4535. [PubMed] [Google Scholar]
- 14.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 15.Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 16.Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wagner EF, Cook SJ. Activation of ERK1/2 by ΔRaf-1: ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene. 2003;22:1281–1293. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- 17.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marani M, Hancock D, Lopes R, Tenev T, Downward J, Lemoine NR. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene. 2004;23:2431–2441. doi: 10.1038/sj.onc.1207364. [DOI] [PubMed] [Google Scholar]
- 19.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Liu C, Djeu JY, Zhong B, Peters T, Scharffetter-Kochanek K, Anasetti C, Yu XZ. β2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111:954–962. doi: 10.1182/blood-2007-05-089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenzuela JO, Iclozan C, Hossain MS, Prlic M, Hopewell E, Bronk CC, Wang J, Celis E, Engelman RW, Blazar BR, Bevan MJ, Waller EK, Yu XZ, Beg AA. PKCtheta is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119:3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu XZ, Levin SD, Madrenas J, Anasetti C. Lck is required for activation-induced T cell death after TCR ligation with partial agonists. J Immunol. 2004;172:1437–1443. doi: 10.4049/jimmunol.172.3.1437. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Iclozan C, Yamazaki T, Yang X, Anasetti C, Dong C, Yu XZ. Abundant c-Fas-associated death domain-like interleukin-1- converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iclozan C, Yu Y, Liu C, Liang Y, Yi T, Anasetti C, Yu XZ. (2009) T helper17 cells are sufficient but not necessary to induce acute graft-versus- host disease. Biol Blood Marrow Transplant. 2010;16:170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwinski MW, Sun J, Hilliard B, Gong S, Xue F, Carmody RJ, DeVirgiliis J, Chen YH. Critical roles of Bim in T cell activation and T cell-mediated autoimmune inflammation in mice. J Clin Invest. 2009;119:1706–1713. doi: 10.1172/JCI37619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craxton A, Draves KE, Clark EA. Bim regulates BCR-induced entry of B cells into the cell cycle. Euro J immunol. 2007;37:2715–2722. doi: 10.1002/eji.200737327. [DOI] [PubMed] [Google Scholar]
- 27.Graber M, Bockenstedt LK, Weiss A. Signaling via the inositol phospholipid pathway by T cell antigen receptor is limited by receptor number. J Immunol. 1991;146:2935–2943. [PubMed] [Google Scholar]
- 28.Brochu S, Rioux-Masse B, Roy J, Roy DC, Perreault C. Massive activation-induced cell death of alloreactive T cells with apoptosis of bystander postthymic T cells prevents immune reconstitution in mice with graft-versus-host disease. Blood. 1999;94:390–400. [PubMed] [Google Scholar]
- 29.Li XC, Strom TB, Turka LA, Wells AD. T cell death and transplantation tolerance. Immunity. 2001;14:407–416. doi: 10.1016/s1074-7613(01)00121-2. [DOI] [PubMed] [Google Scholar]
- 30.Drobyski WR, Komorowski R, Logan B, Gendelman M. Role of the passive apoptotic pathway in graft-versus-host disease. J Immunol. 2002;169:1626–1633. doi: 10.4049/jimmunol.169.3.1626. [DOI] [PubMed] [Google Scholar]
- 31.Li XC, Li Y, Dodge I, Wells AD, Zheng XX, Turka LA, Strom TB. Induction of allograft tolerance in the absence of Fas-mediated apoptosis. J Immunol. 1999;163:2500–2507. [PubMed] [Google Scholar]
- 32.Lehnert AM, Murray-Segal L, Cowan PJ, d'Apice AJ, O'Connell PJ. Blockade of the passive cell death pathway does not prevent tolerance induction to islet grafts. Transplantation. 2007;83:653–655. doi: 10.1097/01.tp.0000255592.09784.ba. [DOI] [PubMed] [Google Scholar]
- 33.Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutcheson J, Scatizzi JC, Bickel E, Brown NJ, Bouillet P, Strasser A, Perlman H. Combined loss of proapoptotic genes Bak or Bax with Bim synergizes to cause defects in hematopoiesis and in thymocyte apoptosis. J Exp Med. 2005;201:1949–1960. doi: 10.1084/jem.20041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK rd, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 39.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]