Abstract
Epidemiological studies performed over the last decade have demonstrated a positive association between persistent, hepatitis B surface antigen (HBsAg)-positive hepatitis B virus (HBV) infection and B-cell non-Hodgkin lymphoma (NHL), with HBV-infected patients having a 2-3-fold higher risk to develop NHL than non-infected patients. Moreover, there is evidence that also occult HBV infection (HBsAg-negative, HBV DNA-positive) associates with NHL. An association with HBV infection may exist also for other hematological malignancies, but available evidence is much less persuasive than for NHL. In this review article we will discuss available results on the association between HBsAg-positive HBV infection and NHL, as well as the significance of other serological markers of HBV infection in these subjects. We will also discuss the possible etiopathogenic role of HBV, and propose a multifactorial model for lymphomagenesis. Experimental evidence for multifactorial etiopathogenesis has been obtained in recent years for HBV-associated hepatocellular carcinoma (HCC), and we suggest that a similar model may apply to HBV-associated lymphoma as well. Eventually, we will also address some unresolved questions. Two of these are of particular relevance. First, do HBV-positive NHL patients show regression of their hematologic malignancy upon antiviral therapy? A positive answer would represent a direct demonstration of the necessary etiological role of the virus in the development of NHL, as has been shown previously for HCV-associated lymphomas. Second, if HBV plays a necessary role in lymphomagenesis, then expansion of HBV vaccination is expected to reduce the number of incident NHL cases, even though this effect might become evident only after a long time interval. Studies in those countries which have introduced universal HBV vaccination about two decades ago, like Italy, may soon provide results on this important point.
Keywords: Hepatitis B virus, occult infection, anti-HBs antibodies, anti-HBe antibodies, anti-HBc antibodies, non-Hodgkin lymphoma, hematologic malignancies, antiviral therapy, multicausal etiology, vaccination
Hepatitis B virus (HBV)
HBV structure and replication
HBV is a small DNA virus, prototype of the Hepadnaviridae family. HBV is classified into eight genotypes, A to H [1,2]. Each genotype has a distinct geographic distribution. The infectious HBV virion (Dane particle) has a spherical, double-shelled structure 42 nm in diameter, consisting of a lipid envelope containing hepatitis B surface antigen (HBsAg) that surrounds an inner nucleocapsid composed of hepatitis B core antigen (HBcAg) complexed with virally encoded polymerase and the viral DNA genome. The genome of HBV is a partially doublestranded circular DNA. The viral genome encodes four overlapping open reading frames (ORFs: S, C, P, and X). The S ORF encodes the viral surface envelope protein, HBsAg, and can be structurally and functionally divided into the pre-S1, pre-S2, and S regions. The core or C gene encodes the precore and core regions. Multiple in-frame translation initiation codons are a feature of the S and C genes, which give rise to related but functionally distinct proteins. The C ORF encodes either the viral nucleocapsid HBcAg or hepatitis B e antigen (HBeAg) depending on whether translation is initiated from the core or precore regions, respectively. The polymerase is a large protein (about 800 amino acids) encoded by the P ORF. It encompasses a reverse transcriptase domain, which catalyzes genome synthesis, and a ribonuclease H domain, which degrades pregenomic RNA and facilitates replication. The HBV X ORF encodes a 16.5-kd protein which is necessary for productive HBV infection in vivo and may contribute to the oncogenic potential of HBV. HBV replicates through a RNA intermediate and can integrate into the host genome. During replication, HBV undergoes transformation into covalently closed circular DNA (cccDNA), which serves as template for transcription of various viral RNAs and is a stable component of the replication cycle. For this reason, detection of cccDNA intermediates is important because it identifies HBV replication in infected cells.
Lymphotropism of HBV
HBV is by definition a hepatotropic virus because it replicates within hepatocytes. In the present setting and in view of a possible etiologic role of HBV in lymphoma development, it is important to know whether HBV can infect and replicate also in hematopoietic and lymphoid cells. Indeed, there is a large number of experimental findings in favor of this possibility. Thus, extrahepatic sites of HBV are known to exist [3], and HBV nucleic acids have been demonstrated in lymph nodes, spleen, gonads, thyroid gland, kidneys, pancreas and adrenal glands from patients with acute HBV infection [4]. As regards lymphocytes and related cell types, HBV binds to peripheral blood mononuclear cells (PBMC) [5], and infects hematopoietic cells and their progenitors [6,7]. In fact, HBV DNA has been found in all major PBMC subpopulations in acute and chronic HBV infections [8,9], and in patients with a diverse spectrum of serological profiles related to HBV infection [10-13]. HBV DNA replicative intermediates, HBV mRNAs, and expression of HBV-specific proteins - HBsAg and HBcAg - have been detected in PBMCs [14-17]. HBV infection of PBMCs has been demonstrated even in the absence of concomitant liver infection [18], and PBMCs have been shown to harbor HBV DNA for prolonged periods [19]. These observations have suggested that the lymphoid system is an important reservoir of HBV [20]. Eventually, HBV DNA integration in PBMCs has also been demonstrated [8,21,22].
Occult HBV infection
As will be discussed in more detail in the following sections, occult HBV infection plays a still ill-defined, but possibly significant role in lymphomagenesis. Occult HBV infection occurs in subjects who are HBsAg-negative, but positive for HBV DNA. Most of these subjects are positive for anti-HBc (so-called “anti-HBc alone”), but some have been found to be positive for anti-HBc and/or anti-HBs, or even seronegative for all HBV markers [23,24]. Some of these patients have underlying liver disease, suggestive of ongoing hepatocellular injury from persistent HBV infection. In fact, existing evidence supports the notion that occult HBV infection indicates low-level viral replication, which may be responsible for viral transmission. Thus, it has been demonstrated that HBV DNA can persist in the serum and PBMCs of patients for up to 70 months after complete clinical, biochemical, and serological recovery from acute viral hepatitis [25]. Moreover, HBV DNA may persist in the liver even after disappearance of HBV DNA from serum, or in the presence of low levels of circulating HBV DNA [26-28]. Thus, Marusawa et al [29] reported that 13 of 14 healthy donors, serologically negative for HBsAg but positive for anti-HBc, had detectable HBV DNA in their liver specimens. The HBV genomes from these subjects included the replication intermediate of HBV. Livers from these donors, in particular from anti-HBc-positive donors, can lead to the development of hepatitis B in liver transplant recipients [27,30,31]. Overall, complete HBV eradication is now considered to be a rare event, if it occurs at all; a replication-competent HBV DNA probably persists in the liver or lymphocytes or both for many years or even life-long.
Association of HBV infection with B-cell non-Hodgkin lymphoma (NHL)
Association of persistent HBsAg-positive HBV infection with NHL
The association of HBV infection with NHL (which, unless otherwise stated, will always refer to B-cell NHL in this review), as determined by persistent, serological HBsAg positivity, has been studied much less intensively than HCV infection. The association of HCV infection with NHL has now been investigated and confirmed in a large number of case-control and cohort studies [e.g. 32, 33]. This is somehow surprising because the first report suggesting an association between HBV infection and chronic lymphoproliferative disorders dates much earlier than the first reports on the association between HCV infection and NHL. Thus, Heimann et al [34] studied 23 patients with chronic lymphoproliferative disorders among whom a high incidence of cirrhoses had been observed. Eleven cases were considered immunocytomas, and six of them showed cirrhosis of the liver. Three of the 6 patients suffering from both cirrhosis and immunocytoma were HBsAg-positive. The authors concluded from these observations that the association between HBV infection and cirrhosis on the one hand and chronic lymphoproliferative disorders on the other may not be purely coincidental. A subsequent piece of evidence in favor of this possibility was brought only 17 years later [35], but still at the same time when the first reports on the association between HCV infection and NHL were published [36,37]. Interestingly, Galun et al [35] performed their work starting from the explicit assumption that the lymphotropism of HBV might lead to neoplastic transformation. These authors retrospectively identified 22 patients who were HBsAg-positive and had extrahepatic malignancies. The patients had 25 tumors, of which 22 were bone marrow-derived.
After these initial studies, still another decade had to elapse before the first case-control study on the association between HBV infection and NHL was published [38]. In this study, 222 patients with newly diagnosed NHL were recruited, as well as 883 patients with non-hematological malignancies or non-malignant conditions. HBsAg was positive in 28 of 222 patients (12.6%) with NHL compared to 53 of 883 (6%) in controls. Adjusted odds ratio (OR) for the association between HBV infection and NHL was 3.30 [95% confidence interval (CI): 1.69-6.45)].
In the following, a large number of retrospective or prospective, case-control or cohort studies [39-51], as well as one meta-analysis [52] have been published. The large majority of these studies confirmed a positive association between HBV infection and NHL. To the best of our knowledge, only two articles did not. In the first, Anderson et al [53] selected from the U.S. Surveillance, Epidemiology, and End Results- Medicare database 61,464 cases with hematopoietic malignancies and 122,531 population-based, matched controls. HBV was not related to any hematopoietic malignancy. The prevalence of HBV infection in the control population, however, was very low (0.2%). As has been observed for HCV [33], at these extremely low levels of HBV prevalence in the general population, a positive association with NHL becomes detectable only when very large cohorts are investigated. The second article [54] reported a case-control study in eight European countries, that included 739 incident cases of NHL, 238 of multiple myeloma (MM), 46 of Hodgkin's lymphoma (HL), and 2,028 matched controls. Nonsignificant associations were found with HBsAg-positivity for NHL, MM, and HL. A significant association, however, was observed between HBsAg-positivity and the combination of NHL, MM, and HL (OR, 2.21; CI, 1.12-4.33). Also this study included subjects from countries with very low prevalence of HBV infection. It is likely that combination of the three lymphoid malignancies allowed to achieve a threshold number of cases that yielded an OR with CIs that did no longer overlap null values.
Some authors found in their studies that HBV infection was associated with a significantly earlier disease (NHL) onset. Thus, Kim et al [38] found that the HBsAg-positive rate was consistently higher for NHL patients in every age group, but the risk of NHL was most evident in the younger HBsAg-positive groups. Wang et al [45] found a median age of 44.5 years for HBsAg-positive NHL patients versus 54 years for HBsAg-negative patients. On the other hand, Lim et al [43] observed a higher prevalence of HBV infection in all age groups.
The impact of HBV infection on the clinical course and prognosis of NHL has been investigated by Wang et al [55]. In their retrospective study, diffuse large B-cell lymphoma (DLBCL) patients were divided into two groups, HBsAg-positive and -negative. The HBsAg-positive patients were further divided in two subgroups based on their hepatic function during chemotherapy. Compared with the HBsAg-negative group, the HBsAg-positive DLBCL group displayed a younger median onset age, and more frequent hepatic dysfunction before and during chemotherapy. However, in both groups the median overall survival (OS) duration and response rates were similar. Lim et al [43] found that the characteristics of HBV-infected patients with lymphoma were similar to those who were HBV-uninfected, including complete remission rate and OS.
While the overall association between HBV infection and NHL seems to be firmly established, results are much less conclusive as regards NHL subtypes. Thus, most studies did not find an association between HBV infection and T-cell NHL, a much rarer entity than NHL [38,45,46,49], but some exceptions have been reported [50,51]. Marcucci et al [42] found a similar prevalence of HBsAg positivity in patients that differed in the clinical course (indolent or aggressive) of NHL. For different histological subtypes of NHL, Engels et al [49] found that HBsAg positivity was associated with increased risk of DLBCL, and other or unknown subtypes, but not with follicular lymphoma. Wang et al [45] and Kang et al [50] did not find a significant difference in HBsAg positivity among different histological subtypes. The scarcity of the data did not allow Nath et al [52] to perform a meta-analysis on histological NHL subtypes.
Association of other markers of HBV infection with NHL
It is somehow surprising to note that most articles published on the association between HBV infection and NHL rest on the serological detection of HBsAg. This is unfortunate because the complete profiling of antigen and antibody markers of HBV infection is very informative to discriminate between different forms of hepatitis, and it is reasonable to assume that such profiling would be informative also in the present setting. Nevertheless, the few results that have been reported in the literature are interesting and will be summarized in detail in the following sections.
Association of anti-HBs antibodies with NHL
Marcucci et al [42] found in an Italian, hospital-based case-control study, a significantly lower number of anti-HBs-positive, anti-HBc-negative patients among NHL cases than controls. Also Wang et al [45] found significantly lower rates of anti-HBs-positive, anti-HBc-negative patients among cases than controls. Since information on the vaccination status of the patients had not been collected in either study, it could not be excluded that this result was due to a higher prevalence of vaccinated individuals among controls. An alternative explanation, however, is that an anti-HBs response, in the absence of anti-HBc (“anti-HBs alone”), might have been especially effective in the control of HBV replication in lymphoid cells, thereby preventing any contribution of HBV to neoplastic transformation. In fact, an anti-HBs-positive, anti-HBc-negative status in non-vaccinated individuals suggests successful clearance of HBV infection and establishment of effective anti-HBV immunity [45]. In contrast to these results, Kim et al [38] found no significant differences in the anti- HBs-positive rates for the three groups examined in their study (NHL patients, patients with non-hematological malignancies, and subjects with non-malignant conditions). This important point needs clarification because the confirmation of an “anti-HBs alone” response being effective in protecting from HBV-induced lymphomagenesis would represent a further stimulus in accelerating and promoting anti-HBV vaccination, in particular in those areas where HBV infection is endemic.
Association of anti-HBc antibodies with NHL
Anti-HBc is another serological marker of HBV infection that has been investigated in very few of these studies. Marcucci et al [42] detected a significantly higher number of anti-HBc-positive, anti-HBs-negative patients, among NHL patients than controls (adjusted OR, 2.05; CI, 1.24- 3.37). When HBsAg-positive patients were excluded from both populations (so-called “anti- HBc alone” patients), CIs overlapped null value, but were close to achieve statistical significance (adjusted OR, 1.51; CI 0.86-2.67). The serological pattern of anti-HBc alone comprises the large majority of patients with occult HBV infection (HBsAg-negative, HBV DNA positive) [24]. For this reason, these results were the first, indirect evidence that occult HBV infection might be associated with NHL. As will be seen in a subsequent section, this was directly confirmed in later studies. The result of Marcucci et al [42] was later confirmed by Wang et al [45], who found that the prevalence of anti-HBc-positivity, either alone or in combination with anti-HBs-negativity was significantly higher among NHL patients than controls.
Association of HBeAg and anti-HBe antibodies with NHL
Wang et al [45] tested NHL patients and controls also for HBeAg and anti-HBe, two serological markers that have not been reported in any other article. Both of these markers reflect ongoing HBV replication: high-level HBV replication and infectivity in case of HBeAg-positivity; low-level HBV replication and infectivity for anti-HBe-positivity [2]. Wang et al [45] found a higher prevalence of these two markers in cases compared to controls. As we will see in the following, these observations are of significant interest for the pathogenesis of HBV-associated NHL.
Association of occult HBV infection with NHL
The possible association of occult HBV infection with NHL is of particular interest. In fact, as we have seen in a previous section, most studies suggest a positive association between HBsAg-positivity and NHL with an OR in the range of 2- 3. This value, however, may be an underestimate if also occult, HBsAg-negative HBV infection contributes to the association. In fact, although dedicated, population-based studies are still missing, it has been estimated that the occult HBV carrier state is present in a significant fraction of the population in Asian countries [56,57]. Only few studies have investigated the impact of occult HBV infection on the association with NHL. In the first, Chen et al [46] found that among HBsAg-negative subjects, those with NHL had a higher prevalence of occult HBV infection (6%) than those with solid tumors and healthy volunteers (0% and 0.9%, respectively). Rossi et al [58] investigated HBsAg-negative, treatment-naïve and immunocompetent patients with chronic lymphocytic leukemia (CLL), now considered to be a systemic form of NHL. These patients were compared with age- and sex-matched controls for the presence of ≥ 2 HBV DNA sequences (defining occult HBV infection) in PBMCs. Occult HBV infection was observed in 10% of cases and 3% of controls (OR, 3.6; CI 1.37-9.79). Occult infection was not associated with differences on 5-year survival and biological predictors, but patients with CLL and occult infection had significantly lower peripheral lymphocyte count. After 8 years, one occult HBV-positive patient with CLL converted into positive HBsAg serology and developed active hepatitis. Unfortunately, this article did not report parallel results in serum; a comparison between cellular and serological HBV DNA would have been very informative.
We have mentioned in one of the previous sections that most subjects with occult HBV infection fall within the “anti-HBc only”-positive group. Fukushima et al [59] found that 2 out of 48 (4.1%) “anti-HBc only” -positive subjects (representing 35% of lymphoma patients studied) who had intensive chemotherapy, including steroids and rituximab, underwent HBV reactivation. These results are consistent with previous ones obtained by Hui et al [57], who reported that 8 of 244 (3.3%) occult HBV carriers among malignant lymphoma patients developed HBV reactivation. These results show that a sizeable fraction of “anti-HBc only” lymphoma patients, presumably carriers of occult HBV infection, can undergo HBV reactivation. It is unclear, however, if “anti-HBc only” -patients that do not undergo HBV reactivation harbor the virus in one or more cell types, and if yes, whether the virus or genetic components of it are present in a form that may contribute to lymphomagenesis.
Association of HBV infection with hematological malignancies other than NHL
Data on the association of HBV infection with hematological malignancies other than NHL are very scarce. In a case-control study, Kang et al [50] found HBV infection to be associated also with HL and acute myeloid leukemia. In the large cohort study published by Engels et al [49], HBsAg-positivity was not significantly increased for HL, MM, or various leukemias. Lim et al [43] did not observe a higher prevalence of HBV infection in patients with HL compared to the general population. Takai et al [41] did not find a higher prevalence of HBV infection in patients with acute leukemia than in the general Japanese population (1.2%). Overall, evidence in favor of an association between HBV infection and hematological malignancies other than NHL is not conclusive, similar to what has been observed for HCV infection [60]. Given the relative rarity of these disorders it is questionable whether future studies can be powered enough to allow drawing definitive conclusions.
Possible etiopathogenic role of HBV in lymphomagenesis
As we have already discussed in a previous publication [61], a positive association of HBV and NHL in treatment-naïve patients strongly suggests, although does not conclusively demonstrate, a causal relationship between HBV infection and NHL development. This causal relationship may be the result of different, HBV-driven events (Figure 1).
Figure 1.
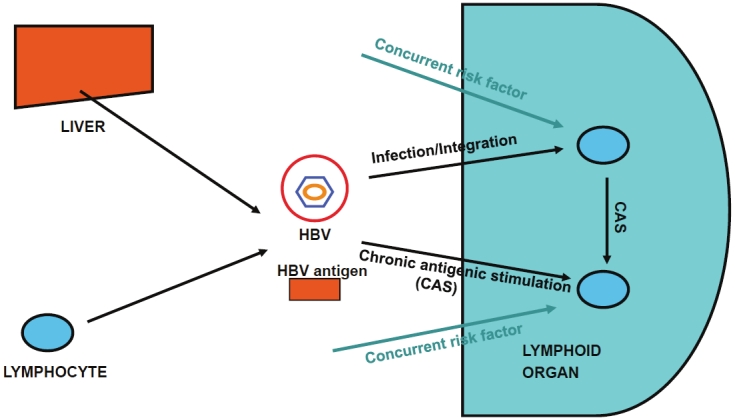
A simplified view of the etiopathogenic role of HBV in lymphomagenesis. HBV particles or HBV antigens can be synthesized and assembled by hepatocytes and, probably, also by lymphocytes. HBV particles can then infect other lymphocytes homing in lymphoid organs. Infection can give rise to other infectious particles or integrate in the host genome, leading to overexpression of cellular oncogenes or downregulation of the expression of tumor suppressor genes. Moreover, HBV antigens can also induce chronic antigenic stimulation (CAS). Infection, integration, and CAS are proposed to be causally linked to lymphomagenesis in concurrence to other as yet undefined risk factors (other lymphotropic viruses, other chronic antigenic stimuli?).
The first of these events is neoplastic development as a direct result of infection. As we have discussed in a previous section, there is ample evidence that HBV can infect and, probably, replicate within lymphocytes. While infection may represent a causal event for neoplastic transformation, this is not necessarily the consequence of viral replication. In fact, HBV can integrate in the host genome, and this could lead to overexpression of cellular oncogenes or downregulation of the expression of tumor suppressor genes [62,63].
The second mechanism is chronic antigenic stimulation. This is a mechanism similar to that most commonly evoked to explain HCV-driven lymphomagenesis [61]. This mechanism does not necessarily foresee infection of lymphocytes. In fact, infection of hepatocytes, with production of infective virus and viral antigens, would be sufficient to support chronic, antigen-driven stimulation and proliferation of B-lymphocytes. This may predispose to genetic aberrations like translocation or overexpression of proto-oncogenes, or induction of double-strand DNA breaks, events which would then autonomously induce neoplastic transformation and proliferation. This consideration raises another question: does HBV contribute only to the initial event that leads to neoplastic transformation or is it required also to support continuous neoplastic proliferation? In case of HCV it has been shown that a significant fraction of anti- HCV-positive lymphoma patients respond to antiviral therapy [64], a result that speaks clearly in favor of ongoing viral replication as a necessary event to support neoplastic proliferation. It is surprising to note that similar therapeutic trials have not yet been reported for HBV-associated NHL. Evidence suggesting that ongoing viral replication may be required to support neoplastic proliferation comes from the study of Wang et al [45] that has already been discussed under section “Association of anti-HBc antibodies with NHL”. These authors found a higher prevalence of HBeAg and anti-HBe in NHL cases than controls, which are considered markers of ongoing, high-level and low-level viral replication, respectively. Even more direct evidence in support of this possibility comes from an interesting report of Zhang et al [65]. These authors reviewed 203 consecutive patients who underwent liver transplantation for benign liver disease. The patients comprised 144 patients with hepatitis B and 59 patients without hepatitis B. After liver transplantation, 36 of the 144 patients with hepatitis B experienced HBV reactivation, while the remaining 108 patients did not. Importantly, four patients (11.1%) with HBV reactivation developed posttransplant lymphoproliferative disease, compared to only one patient (0.9%) without HBV reactivation (p = 0.007). Moreover, HBV DNA was detected in lymphoma cells in all four patients who experienced HBV reactivation.
A difficult issue to address when discussing the etiological role of HBV in lymphomagenesis, is the contribution of occult HBV infection. Population studies on the prevalence of occult HBV infection are still lacking and, in any case, even when these studies will become available they will likely inform only about the serological prevalence of HBV DNA, thus neglecting other sanctuaries that could harbor HBV DNA even in the absence of serologically detectable HBV DNA, like PBMCs or hepatocytes. Nevertheless, it has been estimated that a significant fraction of the population in Asian countries [56,57] may be carrier of occult HBV infection. It appears obvious that if also occult HBV infection is associated with increased risk of NHL, as suggested in some studies [46], then a higher number of incident NHL cases than heretofore estimated, could be attributable to HBV infection.
While it appears possible that HBV infection, whether overt or occult, could be responsible for a higher number of NHL cases than previously thought, it appears obvious that the relative risk to develop a NHL is relatively modest for a carrier of HBsAg-positive HBV infection, about 2-3 times more than for non-infected subjects. In order to explain this relatively modest risk, we have proposed a multifactorial model for lymphomagenesis, in which HBV or HCV infection plays a necessary, but not sufficient etiological role [61]. It is interesting to note that several studies that have been performed in hepatocellular carcinoma (HCC)-affected HBV or HCV carriers are in agreement with this model. In one of these studies, Chen et al [66] found a more than 100-fold increased risk to develop HCC for HBV or HCV carriers with both obesity and diabetes, indicating synergistic effects of metabolic factors and hepatitis. Synergistic interactions were found also between heavy alcohol consumption and chronic HBV or HCV infection [67]. While it appears unlikely that hepatic risk factors like alcohol consumption, obesity and diabetes, may synergize with HBV also in the development of NHL, it appears reasonable to assume that other risk factors may concur with HBV in the development of NHL. Such lymphomagenic risk factors might be other lymphotropic viruses or chronic antigenic stimuli like those underlying autoimmune diseases.
Conclusions – What next?
When we go over to critically consider what we have learnt so far on the association between HBV infection and NHL, we are left with one certainty and with many questions.
The certainty concerns the existence of a positive association between HBV infection and NHL. This association covers both overt, HBsAg-positive, as well as occult, HBsAg-negative infection, although the impact of the latter is very difficult to quantify.
When considering the unanswered questions, there is a long list that would deserve clarification. First, we have no conclusive data on the association of HBV infection with specific subtypes of NHL. Second, we have no conclusive data on the association between HBV infection and hematologic malignancies other than NHL. It appears questionable, however, whether studies can be set up that are powered enough to answer these questions. Third, it would be of interest to quantify the impact of occult infection on the association of HBV with NHL. This is also a challenging undertaking because of the difficulty to detect all patients with occult infection, given that a fraction of them can harbor the virus in sites that escape serological HBV DNA detection, the most commonly used method to detect occult HBV infection.
In addition, however, there are two questions of great relevance from a prophylactic and therapeutic point of view. The first is whether HBV infection, and active viral replication, play a necessary etiological role in the development of NHL and, possibly, other hematologic malignancies. The observation [45] of a higher prevalence of HBeAg or anti-HBe among NHL patients than controls suggests that active viral replication is indeed associated with NHL. The most immediate way to answer this question, however, would be to investigate whether HBV-positive NHL patients undergo clinical responses (partial or complete) upon treatment with antiviral therapy. This approach has allowed to assign an undisputable etiological role to HCV infection in lymphomagenesis [68,69]. For this reason, it is most surprising to note that similar studies have not yet been published on HBV-positive NHL patients. Studies of this kind appear very urgent, even if reporting negative results.
Another important question is to know whether anti-HBV vaccination protects from HBV-associated NHL and, possibly, other hematological malignancies. The observation that the serological “anti-HBs alone” profile is negatively associated with NHL development [42,45] suggests that this might be indeed the case, even if these studies could not formally exclude the possibility of a biased distribution of vaccinated individuals among cases and controls. It has been pointed out that HBV vaccination, even if effective, would have a limited effect on NHL incidence because of the small magnitude of the association [49]. To this regard, there are two points to consider, however. First, the uncertain impact of occult HBV infection, which could increase the magnitude of the association. Second, as has been shown for HCV [70], HBV might sustain lymphomagenesis and induce gene rearrangements which then could sustain neoplastic proliferation independently of persistent HBV infection. For this reason, a fraction of HBV-negative cases might have become negative after an initial phase of positivity. It is obvious that it would be very difficult to quantify these cases, but vaccination would allow to answer also this question. Expansion of HBV vaccination, however, is expected to reduce the number of incident NHL cases after a considerable number of years [39], due to presumably long incubation times. Dedicated studies, particularly in those countries which have mandated HBV vaccination since a sufficient number of years (e.g. for 20 years in Italy) may, in some years, provide results that give an answer to this important point.
Acknowledgments
This study was supported in part by the “Fondazione Banca Nazionale delle Comunicazioni (BNC)” (Rome, Italy). The researchers participating to the study are independent from the supporter of the study.
References
- 1.Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317:489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ. Hepatitis B: The virus and disease. Hepatology. 2009;49:S13–S21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciesek S, Helfritz FA, Lehmann U, Becker T, Strassburg CP, Neipp M, Ciner A, Fytili P, Tillmann HL, Manns MP, Wedemeyer H. Persistence of occult hepatitis B after removal of the hepatitis B virus-infected liver. J Infect Dis. 2008;197:355–360. doi: 10.1086/525286. [DOI] [PubMed] [Google Scholar]
- 4.Yoffe B, Burns DK, Bhatt HS, Combes B. Extrahepatic hepatitis B virus DNA sequences in patients with acute hepatitis B infection. Hepatology. 1990;12:187–192. doi: 10.1002/hep.1840120202. [DOI] [PubMed] [Google Scholar]
- 5.Neurath AR, Strick N, Sproul P, Ralph HE, Valinsky J. Detection of receptors for hepatitis B virus on cells of extrahepatic origin. Virology. 1990;176:448–457. doi: 10.1016/0042-6822(90)90014-i. [DOI] [PubMed] [Google Scholar]
- 6.Romet-Lemonne JL, McLane MF, Elfassi E, Haseltine WA, Azocar J, Essex M. Hepatitis B virus infection in cultured human lymphoblastoid cells. Science. 1983;221:667–669. doi: 10.1126/science.6867736. [DOI] [PubMed] [Google Scholar]
- 7.Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest. 1986;78:411–417. doi: 10.1172/JCI112591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontisso P, Poon MC, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in mononuclear blood cells. Br Med J. 1984;288:1563–1566. doi: 10.1136/bmj.288.6430.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouffard P, Lamelin JP, Zoulim F, Lepot D, Trepo C. Phytohemagglutinin and concanavalin A activate hepatitis B virus in peripheral blood mononuclear cells of patients with chronic hepatitis B virus infection. J Med Virol. 1992;37:255–262. doi: 10.1002/jmv.1890370404. [DOI] [PubMed] [Google Scholar]
- 10.Calmus Y, Marcellin P, Beaurain G, Chatenoud L, Brechot C. Distribution of hepatitis B virus DNA sequences in different peripheral blood mononuclear cell subsets in HBs antigen-positive and negative patients. Eur J Clin Invest. 1994;24:548–552. doi: 10.1111/j.1365-2362.1994.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 11.Malave LC, Gorrino MT, Campelo C, Lardelli P, Cisterna R. Detection of hepatitis B virus DNA and determination of surface antigen expression in peripheral blood mononuclear cells from patients with AIDS. Eur J Clin Microbiol Infect Dis. 1994;13:267–271. doi: 10.1007/BF01974550. [DOI] [PubMed] [Google Scholar]
- 12.Oesterreicher C, Hammer J, Koch U, Pfeffel F, Sunder-Plassmann G, Petermann D, Müller C. HBV and HCV genome in peripheral blood mononuclear cells in patients undergoing chronic hemodialysis. Kidney Int. 1995;48:1967–1971. doi: 10.1038/ki.1995.498. [DOI] [PubMed] [Google Scholar]
- 13.Cabrerizo M, Bartolomé J, Caramelo C, Barril G, Carreño V. Molecular analysis of hepatitis B virus DNA in serum and peripheral blood mononuclear cells from hepatitis B surface antigen-negative cases. Hepatology. 2000;32:116–123. doi: 10.1053/jhep.2000.8541. [DOI] [PubMed] [Google Scholar]
- 14.Yoffe B, Noonan CA, Melnick JL, Hollinger FB. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986;153:471–477. doi: 10.1093/infdis/153.3.471. [DOI] [PubMed] [Google Scholar]
- 15.Baginski I, Chemin I, Bouffard P, Hantz O, Trepo C. Detection of polyadenylated RNA in hepatitis B virus-infected peripheral blood mononuclear cells by polymerase chain reaction. J Infect Dis. 1991;163:996–1000. doi: 10.1093/infdis/163.5.996. [DOI] [PubMed] [Google Scholar]
- 16.Roisman FR, Castello A, Fainboim H, Morelli A, Fainboim L. Hepatitis B virus antigens in peripheral blood mononuclear cells during the course of viral infection. Clin Immunol Immunopathol. 1994;70:99–103. doi: 10.1006/clin.1994.1016. [DOI] [PubMed] [Google Scholar]
- 17.Stoll-Becker S, Repp R, Glebe D, Schaefer S, Kreuder J, Kann M, Lampert F, Gerlich WH. Transcription of hepatitis B virus in peripheral blood mononuclear cells from persistently infected patients. J Virol. 1997;71:5399–5407. doi: 10.1128/jvi.71.7.5399-5407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feray C, Zignego AL, Samuel D, Bismuth A, Reynes M, Tiollais P, Bismuth H, Brechot C. Persistent hepatitis B virus infection of mononuclear blood cells without concomitant liver infection. The liver transplantation model. Transplantation. 1990;49:1155–1158. doi: 10.1097/00007890-199006000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Mason A, Yoffe B, Noonan C, Mearns M, Campbell C, Kelley A, Perrillo RP. Hepatitis B virus DNA in peripheral-blood mononuclear cells in chronic hepatitis B after HBsAg clearance. Hepatology. 1992;16:36–41. doi: 10.1002/hep.1840160108. [DOI] [PubMed] [Google Scholar]
- 20.Pontisso P, Vidalino L, Quarta S, Gatta A. Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev. 2008;8:13–17. doi: 10.1016/j.autrev.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Murakami Y, Minami M, Daimon Y, Okanoue T. Hepatitis B virus DNA in liver, serum, and peripheral blood mononuclear cells after the clearance of serum hepatitis B virus surface antigen. J Med Virol. 2004;72:203–214. doi: 10.1002/jmv.10547. [DOI] [PubMed] [Google Scholar]
- 22.Umeda M, Marusawa H, Seno H, Katsurada A, Nabeshima M, Egawa H, Uemoto S, Inomata Y, Tanaka K, Chiba T. Hepatitis B virus infection in lymphatic tissues in inactive hepatitis B carriers. J Hepatol. 2005;42:806–812. doi: 10.1016/j.jhep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Liang TJ, Blum HE, Wands JR. Characterization and biological properties of a hepatitis B isolated from a patient without HBV serologic markers. Hepatology. 1990;12:204–212. doi: 10.1002/hep.1840120205. [DOI] [PubMed] [Google Scholar]
- 24.Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 25.Michalak TI, Pasquinelli C, Guilhot S, Chisari FV. Hepatitis B virus persistnce after recovery from acute viral hepatitis. J Clin Invest. 1994;93:230–239. doi: 10.1172/JCI116950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loriot MA, Marcellin P, Walker F, Boyer N, Degott C, Randrianatoavina I, Erlinger , Benhamou JP. Persistence of hepatitis B virus DNA in serum and liver from patients with chronic hepatitis B after loss of HBsAg. J Hepatol. 1997;27:251–258. doi: 10.1016/s0168-8278(97)80168-7. [DOI] [PubMed] [Google Scholar]
- 27.Uemoto S, Sugiyama K, Marusawa H, Inomata Y, Asonuma K, Egawa H, Kiuchi T, Miyake Y, Tanaka K, Chiba T. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living related liver transplants. Transplantation. 1998;66:494–499. doi: 10.1097/00007890-199802270-00007. [DOI] [PubMed] [Google Scholar]
- 28.Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, Omura M, Hikiji K, Kato M. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology. 2003;37:1172–1179. doi: 10.1053/jhep.2003.50171. [DOI] [PubMed] [Google Scholar]
- 29.Marusawa H, Uemoto S, Hijikata M, Ueda Y, Tanaka K, Shimotohno K, Chiba T. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31:488–495. doi: 10.1002/hep.510310232. [DOI] [PubMed] [Google Scholar]
- 30.Lowell JA, Howard TK, White HM, Shenoy S, Huettner PC, Brennan DC, Peters MG. Serological evidence of past hepatitis B infection in liver donor and hepatitis B infection in liver allograft. Lancet. 1995;345:1084–1085. doi: 10.1016/s0140-6736(95)90819-6. [DOI] [PubMed] [Google Scholar]
- 31.Dickson RC, Everhart JE, Lake JR, Wei Y, Seaberg EC, Wiesner RH, Zetterman RK, Pruett TL, Ishitani MB, Hoofnagle JH. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. Gastroenterology. 1997;113:1668–1674. doi: 10.1053/gast.1997.v113.pm9352871. [DOI] [PubMed] [Google Scholar]
- 32.Mele A, Pulsoni A, Bianco E, Musto P, Szklo A, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Liso V, Andrizzi C, Pusterla S, Core F, Maresca M, Rapicetta M, Marcucci F, Franceschi S. Hepatitis C virus and B-cell non- Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996–999. doi: 10.1182/blood-2002-10-3230. [DOI] [PubMed] [Google Scholar]
- 33.Duberg AS, Nordström M, Törner A, Reichard O, Strauss R, Janzon R, Bäck E, Ekdahl K. Non- Hodgkin's lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology. 2005;41:652–659. doi: 10.1002/hep.20608. [DOI] [PubMed] [Google Scholar]
- 34.Heimann R, Ray MB, Desmet VJ. HBsAg, chronic lymphoproliferative disorders, and cirrhosis of liver. J Clin Path. 1977;30:817–821. doi: 10.1136/jcp.30.9.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galun E, Ilan Y, Livni N, Ketzinel M, Nahor O, Pizov G, Nagler A, Eid A, Rivkind A, Laster M, Ron N, Blum HE, Shouval D. Hepatitis B virus infection associated with hematopoietic tumors. Am J Pathol. 1994;145:1001–1007. [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longombardo G, Lombardini F, Greco F, Capochiani E, Mazzonio A, Mazzaro C, Pasero G. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol. 1994;88:392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 37.Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, Sulfaro S, Franzin F, Tulissi P, Moretti M, Santini GF. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84:3047–3053. [PubMed] [Google Scholar]
- 38.Kim JH, Bang YJ, Park BJ, Yoo T, Kim CW, Kim TY, Heo DS, Lee HS, Kim NK. Hepatitis B virus infection and B-cell non-Hodgkin's lymphoma in a hepatitis B endemic area: A case-control study. Jpn J Cancer Res. 2002;93:471–477. doi: 10.1111/j.1349-7006.2002.tb01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crook PD, Jones ME, Hall AJ. Mortality of hepatitis B surface antigen-positive blood donors in England and Wales. Int J Epidemiol. 2003;32:118–124. doi: 10.1093/ije/dyg039. [DOI] [PubMed] [Google Scholar]
- 40.Iwata H, Matsuo K, Takeuchi K, Kishi Y, Murashige N, Kami M. High incidences of malignant lymphoma in patients infected with hepatitis B or hepatitis C virus. Haematologica. 2004;89:368–370. [PubMed] [Google Scholar]
- 41.Takai S, Tsurumi H, Ando K, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K, Takahashi T, Oyama M, Onishi H, Tomita E, Takami T, Imawari M, Moriwaki H. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158–165. doi: 10.1111/j.1600-0609.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 42.Marcucci F, Mele A, Spada E, Candido A, Bianco E, Pulsoni A, Chionne P, Madonna E, Cotichini R, Barbui A, De Renzo A, Dore F, Iannitto E, Liso V, Martino B, Montanaro M, Pagano L, Musto P, Rapicetta M. High prevalnce of hepatitis B virus infection in B cell non-Hodgkin lymphoma. Haematologica. 2006;91:554–557. [PubMed] [Google Scholar]
- 43.Lim ST, Fei G, Quek R, Lim LC, Lee LH, Ya SP, Loong S, Tao M. The relationship of hepatitis B virus infection and non-Hodgkin's lymphoma and its impact on clinical characteristics and prognosis. Eur J Haematol. 2007;79:132–137. doi: 10.1111/j.1600-0609.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 44.Ulcickas Yood M, Quesenberry Jr CP, Guo D, Caldwell C, Wells K, Shan J, Sanders L, Skovron ML, Iloeje U, Manos MM. Incidence of non- Hodgkin's lymphoma among individuals with chronic hepatitis B virus infection. Hepatology. 2007;46:107–112. doi: 10.1002/hep.21642. [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Xu Rh, Han B, Shi YX, Luo HY, Jiang WQ, Lin TY, Huang HQ, Xia ZJ, Guan ZZ. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109:1360–1364. doi: 10.1002/cncr.22549. [DOI] [PubMed] [Google Scholar]
- 46.Chen MH, Hsiao LT, Chiou TJ, Liu JH, Gau JP, Teng HW, Wang WS, Chao TC, Yen CC, Chen PM. High prevalence of occult hepatitis B virus infection in patients with B-cell non-Hodgkin's lymphoma. Ann Hematol. 2008;87:475–480. doi: 10.1007/s00277-008-0469-9. [DOI] [PubMed] [Google Scholar]
- 47.Park SC, Jeong SH, Kim J, Han CJ, Kim YC, Choi KS, Cho JH, Lee M, Jung HH, Ki SS, Chang YH, Lee SS, Park YH, Lee KH. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin's lymphoma in Korea. J Med Virol. 2008;80:960–966. doi: 10.1002/jmv.21168. [DOI] [PubMed] [Google Scholar]
- 48.Becker N, Fortuny J, Alvaro T, Nieters A, Maynadié M, Foretova L, Staines A, Brennan P, Boffetta P, Cocco PL, de Danjose S. Medical history and risk of lymphoma: results of a European case-control study (EPILYMPH) J Cancer Res Clin Oncol. 2009;135:1099–1107. doi: 10.1007/s00432-009-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11:827–834. doi: 10.1016/S1470-2045(10)70167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang J, Cho JH, Suh CW, Lee DH, Oh HB, Sohn YH, Chi HS, Park CJ, Jang SS, Lee KH, Lee JH, Lee JH, Lee SW, Chung YH, Kim TH, Shin HR, Huh J. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol. 2011;90:159–164. doi: 10.1007/s00277-010-1055-5. [DOI] [PubMed] [Google Scholar]
- 51.Kim YM, Jeong SH, Kim JW, Lee SH, Hwang JH, Park YS, Kim N, Lee JS, Kim HY, Lee DH. Chronic hepatitis B, non-Hodgkin's lymphoma, and effect of prophylactic antiviral therapy. J Clin Virol. 2011;51:237–241. doi: 10.1016/j.jcv.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Nath A, Agarwal R, Malhotra P, Varma S. Prevalence of hepatitis B virus infection in non- Hodgkin's lymphoma. A systematic review and meta-analysis. Intern Med J. 2010;40:633–641. doi: 10.1111/j.1445-5994.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 53.Anderson LA, Pfeiffer R, Warren JL, Landgren O, Gadalla S, Berndt SI, Ricker W, Parsons R, Wheeler W, Engels EA. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17:3069–3075. doi: 10.1158/1055-9965.EPI-08-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franceschi S, Lise M, Trépo C, Berthillon P, Chuang SC, Nieters A, Travis RC, Vermeulen R, Overvad K, Tjønneland A, Olsen A, Bergmann MM, Boeing H, Kaaks R, Becker N, Trichopoulou A, Lagiou P, Bamia C, Palli D, Sieri S, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita B, Peeters PH, Rodríguez L, Barroso LL, Dorronsoro M, Sánchez MJ, Navarro C, Barricarte A, Regnér S, Borgquist S, Melin B, Hallmans G, Khaw KT, Wareham N, Rinaldi S, Hainaut P, Riboli E, Vineis P. Infection with Hepatitis B and C Viruses and Risk of Lymphoid Malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2011;20:208–214. doi: 10.1158/1055-9965.EPI-10-0889. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Xu RH, Luo HY, Zhang DS, Jiang WQ, Huang HQ, Sun XF, Xia ZJ, Guan ZZ. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B-cell lymphoma. BMC Cancer. 2008;8:115. doi: 10.1186/1471-2407-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hui CK, Sun J, Au WY, Lie AK, Yueng YH, Zhang HY, Lee NP, Hou JL, Liang R, Lau GK. Occult hepatitis B virus infection in hematopoietic stem cell donors in a hepatitis B virus endemic area. J Hepatol. 2005;42:813–819. doi: 10.1016/j.jhep.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, Leung N, Luk JM, Lie AK, Kwong YL, Liang R, Lau GK. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Rossi D, Sala L, Minisini R, Fabris C, Falleti E, Cerri M, Burlone ME, Toniutto P, Gaidano G, Pirisi M. Occult hepatitis B virus infection of peripheral blood mononuclear cells among treatment-naïve patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:604–611. doi: 10.1080/10428190902777673. [DOI] [PubMed] [Google Scholar]
- 59.Fukushima N, Mizuta T, Tanaka M, Yokoo M, Ide M, Hisatomi T, Kuwahara N, Tomimasu R, Tsuneyoshi N, Funai N, Sueoka E. Retrospective and prospective studies of hepatitis B virus reactivation in malignant lymphoma with occult HBV carrier. Ann Oncol. 2009;20:2013–2017. doi: 10.1093/annonc/mdp230. [DOI] [PubMed] [Google Scholar]
- 60.Bianco E, Marcucci F, Mele A, Musto P, Cotichini R, Sanpaolo MG, Iannitto E, De Renzo A, Martino B, Specchia G, Montanaro M, Barbui AM, Nieddu R, Pagano L, Rapicetta M, Franceschi S, Mandelli F, Pulsoni A. for the GIMEMA study group on HCV and hematologic diseases. Prevalence of hepatitis C virus infection in lymphoproliferative diseases other than B-cell non-Hodgkin's lymphoma, and in myeloproliferative diseases: an Italian multicenter case-control study. Haematologica. 2004;89:70–76. [PubMed] [Google Scholar]
- 61.Marcucci F, Mele A. Hepatitis viruses and non- Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792–1798. doi: 10.1182/blood-2010-06-275818. [DOI] [PubMed] [Google Scholar]
- 62.Natoli G, Avantaggiati ML, Chirillo P, Puri PL, Ianni A, Balsano C, Levrero M. Ras- and Raf-dependent activation of c-jun transcriptional activity by the hepatitis B virus transactivator pX. Oncogene. 1994;9:2837–2843. [PubMed] [Google Scholar]
- 63.Wang XW, Gibson MK, Vermeulen W, Yeh H, Forrester K, Sturzbecher HW, Hoeijmakers JH, Harris CC. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995;55:6012–6016. [PubMed] [Google Scholar]
- 64.Gisbert JP, García-Buey L, Pajares JM, Moreno- Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653–662. doi: 10.1111/j.1365-2036.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- 65.Zhang A, Zhang M, Shen Y, Wang W, Zheng S. Hepatitis B virus reactivation is a risk factor for development of post-transplant lymphoproliferative disease after liver transplantation. Clin Transplant. 2009;23:756–760. doi: 10.1111/j.1399-0012.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- 66.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 67.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 68.Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub P, Brechot C, Varet B, Troussard X. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;34:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 69.Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, Lazzaro A, Trabacchi E, Anselmi , Arcari AL, Moroni C, Bertè R, Lazzarino M, Cavanna L. Role of antihepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non -Hodgkin's lymphoma: A multicenter Italian experience. J Clin Oncol. 2005;23:468–473. doi: 10.1200/JCO.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Zuckerman E, Zuckerman T, Sahar D, Streichman S, Attias D, Sabo E, Yeshurun D, Rowe JM. bcl-2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infection. Br J Haematol. 2011;112:364–369. doi: 10.1046/j.1365-2141.2001.02573.x. [DOI] [PubMed] [Google Scholar]


