Abstract
Hematopoietic stem cells (HSCs) that give rise to all blood cell types are important vehicles for cell-based and gene therapies. After isolation from the bone marrow, HSCs are often cultured in laboratory settings for purposes of ex vivo expansion, gene transduction, and bone marrow transplantation for the treatment of various disorders of the blood and immune systems. Here we demonstrate that during in vitro culturing outside of hypoxic bone marrow niches, HSCs may genetically alter even after short durations of time. Lineage- Scal-1+ c-Kit+ (LSK) cells that are enriched with HSCs revealed significant levels of genomic instability following culture, as evidenced by the emergence of aneuploid cells. To further determine the effects of in vitro culturing conditions, whole bone marrow cells were cultured in a hypoxic environment of 3% oxygen, mimicking conditions within the body's bone marrow, following which, cells proved to undergo less genetic alterations. Proper dosages of the antioxidant N-Acetyl-Cysteine (NAC) similarly decreased occurrences of chromosomal change. Furthermore, analysis of aged hematopoietic cells revealed enhanced in vitro normoxic culture-induced chromosomal instability compared to that of young hematopoietic cells due to noted increased oxidative stress in aged cells. These results reveal that in vitro cell culturing does indeed cause genomic instability in hematopoietic cells. Reduced oxygen to physiological levels and additions of antioxidants can be employed as possible strategies to lower oxidative stress and decrease chances of chromosomal transformation. Because hematopoietic cells are commonly processed in laboratory settings before transplantation for patient treatment, our findings also raise a concern on the therapeutic use of cultured hematopoietic cells.
Keywords: Hematopoietic cells, stem cells, oxidative stress, chromosomal instability
Introduction
In vitro maintenance and expansion of human cells, including hematopoietic stem cells (HSCs), embryonic stem cells (ESCs), and bone-marrow-derived mesenchymal stem cells (MSCs), provide an invaluable system for functional analyses and therapeutic applications. However, while past evidence has indicated that ex vivo expansion of ESCs and MSCs can potentially cause genomic instability and malignant transformation [1-4], little to no examination of HSCs has been performed. Given that HSC transplantation is the most often used procedure for patients with diseases of the blood, bone marrow, or certain cancers, it is crucial to understand whether in vitro maintenance of HSCs can also lead to genetic alterations.
The underlying mechanisms by which genomic instability arises in cultured cells have not been fully addressed in previous studies. A better understanding of these mechanisms will promote the development of strategies to prevent the occurrence of genomic abnormalities, and thus, minimize the risk of malignant transformation of cultured stem cells. It has been shown that high oxygen concentrations increase reactive oxygen species (ROS) levels and oxidative stress, which in turn leads to an increased incidence of genomic abnormalities in cultured cardiac stem cells and ESCs [5], whereas karyotypic abnormalities can be suppressed by culture in physiological oxygen or by addition of an optimal concentration of antioxidants [5]. This raises one possibility that optimization of culture conditions by controlling the oxygen levels may reduce the incidence of genomic instability in cultured stem cells.
Materials and methods
Cell sorting
Bone marrow cells were freshly harvested from femurs and tibias following standard procedures. For cell sorting, bone marrow cells were stained with FITC-labeled antibodies for lineage markers including Mac-1, Gr-1, Ter119, CD4, CD8a, CD3, and B220 (BD Biosciences), and c- Kit-APC and Sca-1-PE antibodies. LSK (Lin- Sca- 1+c-Kit+) and LK (Lin- Sca-1-c-Kit+) populations were sorted using BD FACSAria. Sorted cells were cultured in IMDM medium containing Tpo (20 ng/ml), FIt3 ligand (50 ng/ml), SCF (50 ng/ ml), IL-3 (20 ng/ml), IL-6 (20 ng/ml), and 10% fetal bovine serum (FBS) for the indicated periods of time.
Karyotypic analysis
To prepare metaphase spreads, fresh or cultured LSK cells, LK cells, or whole bone marrow cells were treated with colcemid (0.05 μg/ml) at 37°C for 2 hours. Cells were harvested, suspended in pre-warmed 75 mM KCl hypotonic solution, and incubated at 37°C for 10 min. The cells were then fixed in Carnoy’s solution (75% methanol and 25% acetic acid) at room temperature for 15 min, washed twice with fixative, and dropped onto pre-chilled microscope slides. The slides were dried and stained in DAPI (4’,6- diamidino-2-phenylindole) (1 μg/ml) for 10 min. Chromosomes in each metaphase cell (non-megakaryocyte) were enumerated under a fluorescence microscope using an 100x oil objective.
ROS measurement
To measure cellular ROS levels, bone marrow cells freshly harvested were loaded with 2’-7’- dichlorofluorescein diacetate (DCF-DA) (5 μM) at 37°C for 15 min. ROS (H2O2) levels were then quantified by measuring DCF-DA fluorescence intensity using flow cytometry.
Results
To determine the impact of in vitro culture on hematopoietic stem cells (HSCs), we isolated LSK cells (Lin- Sca-1+c-Kit+, a population enriched with HSCs) from mouse bone marrow samples and cultured them under normoxic conditions (20% O2) for two and six days. The chromosome numbers were determined by traditional karyotypic analysis. Assessment of LSK cells after the two day culture noted nearly 50% aneuploid cells (cells with more or less than 40 chromosomes seen in normal cells) (Figure 1A and 1B). Following six days of culture, even greater levels of abnormalities were observed (Figure 1B). To further confirm these results, sorted LK cells (Lin- c-Kit+ cells composed primarily of myeloid progenitors) and whole bone marrow cells were also cultured for various periods of time, and similar results were obtained (Figure 1C and 1D). These observations suggest that in vitro culture of hematopoietic cells under normoxic conditions causes significant chromosomal instability.
Figure 1.
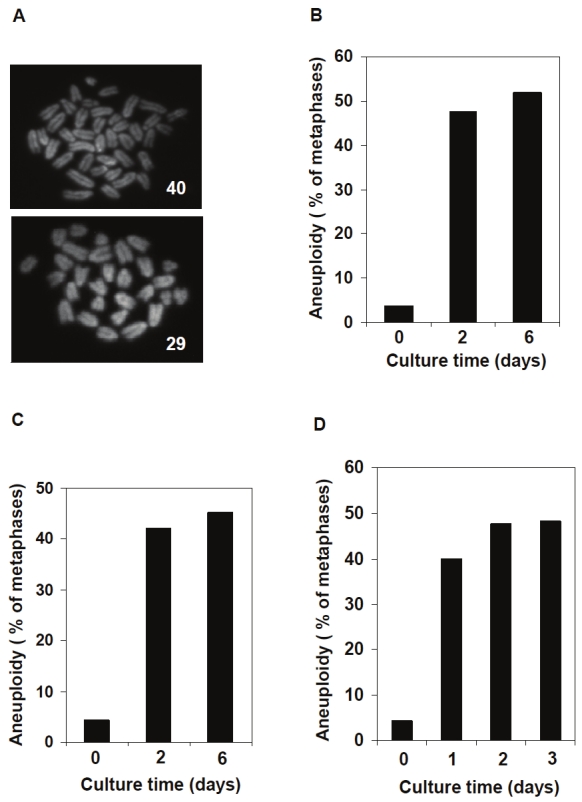
Aneuploidy in hematopoietic cells cultured under normoxic conditions. LSK cells that are enriched with HSCs and LK cells (myeloid progenitors) were sorted from freshly harvested bone marrow cells. A. Representative metaphase spreads with normal (top) and abnormal (bottom) chromosome numbers prepared from cultured LSK cells. B. Sorted LSK cells were cultured under normoxic conditions for two and six days. Karyotypic analyses were performed as described in Materials and methods. Fresh whole bone marrow cells were included as the Day 0 control due to the scarcity of mitotic cells in fresh LSK cells. C. Sorted LK cells were cultured under normoxic conditions for two and six days. Karyotypic analyses were performed as described above. D. Whole bone marrow cells were cultured under normoxic conditions for one, two, and three days. Karyotypic analyses for non-megakaryocytes were performed. For all the experiments shown, at least 100 metaphase spreads were counted for each group.
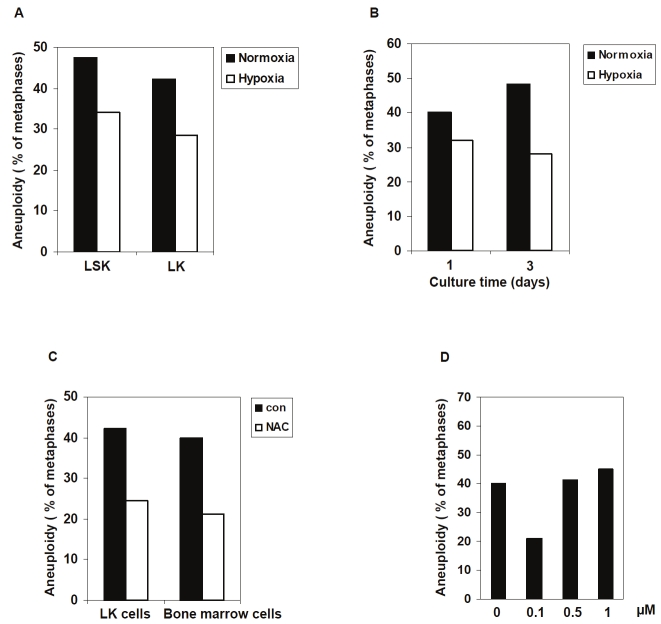
Once cells are isolated from the body and cultured in an environment with a higher oxygen concentration (20%), they are exposed to greater levels of oxidative stress and resultantly generate more ROS. Recent evidence has revealed that HSCs in the bone marrow are located in a hypoxic environment known as the stem cell niche [6-9]. The niche protects stem cells from oxidative stress and the accumulation of ROS and other free radicals, which are known to cause DNA damage and induce genomic instability [10-11]. As a result, only the physiological levels of intracellular ROS can maintain genomic stability in stem cells [5,12]. It has also been shown that low oxygen levels can enhance the survival and self-renewal of HSCs during in vitro culture [13-14]. Thus, these studies now raise the possibility that chromosomal instability of in vitro cultured LSK cells might be caused by the dramatically different oxygen environment compared to that of the stem cell niche. To test this hypothesis, we cultured LSK cells under hypoxic (3% oxygen) and normoxic (20% oxygen) conditions, and karyotypic analyses were performed after 2 days. The percentage of aneuploid LSK cells was decreased when cells were cultured in the lower oxygen concentration (Figure 2A), indicating that the physiological oxygen level is required for maintaining the chromosomal integrity of hematopoietic cells. The chromosome numbers of LK cells and bone marrow cells were also compared under states of hypoxia and normoxia. Similarly, the frequency of chromosomal instability was reduced after hypoxic culture (Figure 2A and 2B), indicating that karyotypic abnormalities in cultured hematopoietic cells are closely related to oxidative stress.
Figure 2.
Aneuploidy in cultured hematopoietic cells is associated with oxidative stress. LSK cells that are enriched with HSCs and LK cells (myeloid progenitors) were sorted from freshly harvested bone marrow cells. A. LSK cells and LK cells sorted were cultured under normoxic (20% oxygen) or hypoxic (3% oxygen) conditions for two days. Karyotypic analyses were performed as described in Materials and methods. B. Whole bone marrow cells were cultured under normoxic or hypoxic conditions for one and three days. Karyotypic analyses for non-megakaryocytes were performed as described above. C. LK cells sorted or whole bone marrow cells were cultured in the presence of the antioxidant N-Acetyl-Cysteine (NAC) (0.1 μM) for two days. Karyotypic analyses were performed as described above. D. LK cells sorted were cultured in the presence of the antioxidant N-Acetyl-Cysteine (NAC) at the indicated concentrations for two days. Karyotypic analyses were performed as described above. For all the experiments, at least 100 metaphase spreads were counted for each group of each cell type.
To further confirm these results, hematopoietic cells cultured under normoxic conditions were treated with antioxidant N-acetyl-L-cysteine (NAC) and their numbers of chromosomes were also determined. Consistent with the results obtained under hypoxic conditions, the percentage of aneuploid cells was dramatically lower compared to untreated control cells (Figure 2C). However, the rescue effect was only found at a low dosage (0.1 μM) but not at higher dosages (0.5 and 1 μM) (Figure 2D). This may be explained by the recent finding that optimal genomic stability of hematopoietic cells is maintained only in a narrow range of ROS levels. Higher dosages of antioxidants suppress ROS to subphysiological levels which thus may not be sufficient to activate the DNA repair pathway to maintain genomic stability [5].
Oxidative stress-induced DNA damage and genomic instability increase with age and are considered as major causal factors in cancer and other age-related diseases [15-17]. Consistent with this thought, bone marrow cells from 2-year -old mice showed more aneuploidy than those of 2-month-old mice immediately after isolation from the body (Figure 3A). Interestingly, the difference between young and old mice was even more apparent after in vitro culture for one day under normoxic conditions, indicating that cells from old mice are more prone to oxidative stress-induced chromosomal instability. Given that ROS are a major source of oxidative stress, the levels of ROS in bone marrow cells from young and old mice were determined by 2’, 7’- dichlorfluorescein-diacetate (DCF-DA) staining and flow cytometric analysis. After one day culture, the ROS levels increased in cells from both age groups of mice (Figure 3B). However, the cells derived from old mice accumulated more ROS, thereby inducing the higher frequency of aneuploidy previously noted (Figure 3B). To further confirm that the chromosomal instability was caused by oxidative stress, bone marrow cells from young and old mice were treated with antioxidant NAC, and the numbers of chromosomes were again counted after karyotypic analyses. Addition of NAC decreased the occurrences of aneuploidy in cells from both young and aged mice, further suggesting that chromosomal instability in cultured hematopoietic cells is related to oxidative stress (Figure 3C).
Figure 3.
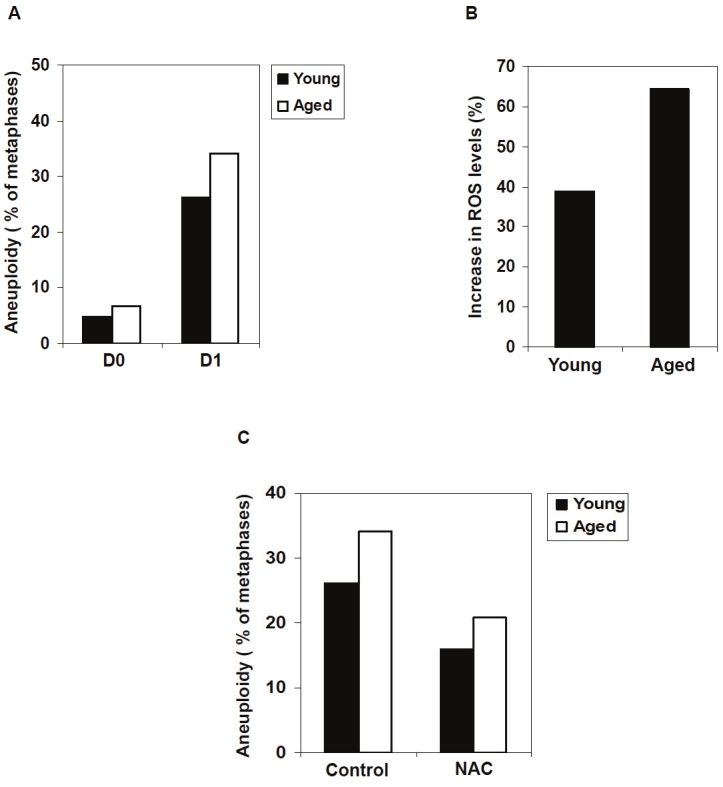
The cells derived from aged mice are more prone to oxidative stress-induced chromosomal instability. A. Bone marrow cells were freshly harvested from 2-month-old or 24-month-old mice. Bone marrow cells were cultured under normoxic conditions for one day. Karyotypic analyses for non-megakaryocytes in fresh (D0) or cultured (D1) cells were performed as described in Materials and methods. At least 100 metaphase spreads were counted for each group. B. Bone marrow cells were freshly harvested from 2- month-old or 24-month-old mice. The cells were cultured under normoxic conditions for one day. ROS levels in the fresh and cultured cells were measured as described in Materials and methods. The relative increase in ROS levels following the culture for each group of the cells was determined. C. Bone marrow cells freshly harvested from 2-month-old or 24-month-old mice were cultured in the presence of NAC (0.1 μM) under normoxic conditions for one day. Karyotypic analyses for non-megakaryocytes were performed. At least 100 metaphase spreads were counted for each group.
Discussion
In this report, we present evidence that in vitro culture causes significant chromosomal instability in hematopoietic cells and that this effect appears to be attributable to oxidative stress imposed on the cells. This finding has important clinical implications. For clinical applications of stem cells, an adequate number of cells are necessary so an extensive expansion ex vivo is required. Previous studies have indicated that ex vivo expansion of ESCs and MSCs can potentially cause genomic instability [1-4], while little is known about the HSCs. We have now shown that in vitro expansion of HSCs also generated chromosomal instability. These data suggest that regular monitoring of these cells will be critical for future therapeutic purposes. Our finding is especially important in protecting the chromosomal regularity of HSCs from older patients because of their increased susceptibility to oxidative stress-induced genomic instability. Previous studies demonstrated that ROSlow HSCs retained long term self-renewal ability through a serial transplantation assay, whereas this capacity decreased in ROShigh HSCs [18]. Our finding that the cells from old mice accumulated more ROS and showed higher chromosomal instability than those from young mice (Figure 3B) indicates that these cells are also prone to genomic alterations beside loss of the self-renewal ability. It has been previously shown that low oxygen levels can enhance the survival and self-renewal of HSCs in in vitro culture [13-14]. We now provide evidence that reducing oxygen concentrations to physiological levels or adding proper dosages of antioxidants decreases in vitro culture induced aneuploidy, providing potential strategies to limit genomic alterations when expanding hematopoietic cells in vitro. Further determination of the mechanisms by which chromosomal instability is induced under oxidative stress will notably improve the clinical application of hematopoietic stem cells.
Acknowledgments
This work was supported by The National Institutes of Health grants HL068212 and DK092722 (to C.K.Q.).
References
- 1.Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 2.Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, Heath PR, Holden H, Andrews PW. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 3.Foudah D, Redaelli S, Donzelli E, Bentivegna A, Miloso M, Dalpra L, Tredici G. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome Res. 2009;17:1025–1039. doi: 10.1007/s10577-009-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, Huso DL, Semenza GL. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849–10854. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 5.Li TS, Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 8.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 10.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 13.Eliasson P, Rehn M, Hammar P, Larsson P, Sirenko O, Flippin LA, Cammenga J, Jonsson JI. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Exp Hematol. 2010;38:301–310. doi: 10.1016/j.exphem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 16.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 18.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]