Abstract
Depleting oil reserves and growing environmental concerns have necessitated the development of sustainable processes to fuels and chemicals. Here we have developed a general metabolic platform in E. coli to biosynthesize carboxylic acids. By engineering selectivity of 2-ketoacid decarboxylases and screening for promiscuous aldehyde dehydrogenases, synthetic pathways were constructed to produce both C5 and C6 acids. In particular, the production of isovaleric acid reached 32 g/L (0.22 g/g glucose yield), which is 58% of the theoretical yield. Furthermore, we have developed solid base catalysts to efficiently ketonize the bio-derived carboxylic acids such as isovaleric acid and isocaproic acid into high volume industrial ketones: methyl isobutyl ketone (MIBK, yield 84%), diisobutyl ketone (DIBK, yield 66%) and methyl isoamyl ketone (MIAK, yield 81%). This hybrid “Bio-Catalytic conversion” approach provides a general strategy to manufacture aliphatic ketones, and represents an alternate route to expanding the repertoire of renewable chemicals.
Though there are successes in producing fuels1,2,3 and chemicals4,5,6,7,8 from carbohydrates, most industrial chemicals are still petro-based. This can be explained by limitations inherent in current approaches. Catalytic conversion of carbohydrates leads to only a handful of molecules derived from hydroxymethylfurfural9,10. On the other hand, while biosynthesis is highly selective and versatile in generating an amazing array of metabolites11, most petrochemicals cannot be directly produced by microbial fermentation. To expand the scope of cost-effective production of renewable chemicals, we propose a “Bio-Catalytic conversion” approach that involves engineered biosynthesis of novel metabolites and their catalytic conversion to useful chemicals with minimal chemical reaction steps. Here we choose aliphatic ketones to demonstrate the advantage of this strategy.
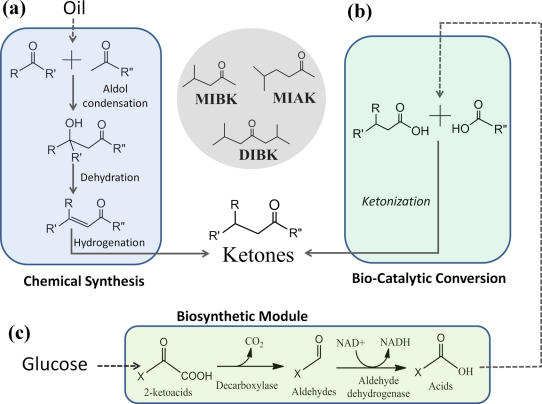
Oxygenated solvents such as aliphatic ketones constitute the largest segment of the solvent market. For example, diisobutyl ketone (DIBK), methyl isoamyl ketone (MIAK) and their alcohol products carbinols have a market volume of around 80 million pounds per year. With an annual production of ∼1 billion pounds, methyl isobutyl ketone (MIBK) is among the top ten most widely used organic solvents in industry12. At present, the manufacturing processes of ketones are relatively complicated. As illustrated in Fig. 1a, the petroleum-derived raw material acetone is chemically processed to MIBK via three reaction steps: (i) aldol condensation to diacetone alcohol, (ii) dehydration to mesityl oxide and (iii) selective hydrogenation of the unsaturated ketone mesityl oxide. Similarly, following the three-step process, the carbonyl compound isobutanal or MIBK reacts with acetone to yield MIAK or DIBK. Although multifunctional catalysts have been developed to enable these reaction series in one pot, the yield to MIBK or DIBK is only around 30% or 10%13. In addition, the operation requires high pressure (10∼100 atm), and the supply of raw materials is dependent on the availability of petroleum feedstock.
Figure 1. Reaction pathways to Ketones.
(a) Current manufacturing starts from petroleum-derived carbonyl compounds and involves three reaction steps (aldol condensation, dehydration, and hydrogenation). The process is unsustainable and the overall yield is low. (b) One of the simplest routes to ketones is ketonization reaction, in which two carboxylic acids cross-condense. (c) A general metabolic platform is developed in E. coli for the biosynthesis of carboxylic acids from glucose. 2-ketoacid decarboxylases catalyze the decarboxylation of 2-ketoacids into aldehydes, which are oxidized to carboxylic acids aided by aldehyde dehydrogenases. The combination of (b) and (c) is the “Bio-catalytic conversion” approach. The process is renewable and cost-effective. Its application is demonstrated here by synthesizing three high-volume industrial ketones MIBK, MIAK and DIBK. R, R’, and R” represent alkyl groups (MIBK, R = R’ = R” = Me).
To address challenges facing the ketone industry, we have developed a new process. Retrosynthetic analysis suggests that the single-step ketonization reaction is one of the simplest routes to ketones (Fig. 1b). In principle, ketonization can utilize a broad range of carboxylic acid feedstocks as long as relevant catalysts are developed to enable the condensation reaction. Moreover, as one of the oldest reactions known in organic chemistry14, ketonization is low-cost, high-yield and environmentally friendly. The current manufacturing processes do not employ ketonization because carboxylic acids are prepared by oxo reaction of olefins, which leads to a higher overall cost. However, if carboxylic acids could be obtained in a sustainable and cost-effective fashion, then the ketonization process regains advantages. Herein, we develop a general metabolic platform in E. coli to biosynthesize carboxylic acids from renewable carbon sources such as glucose (Fig. 1c). The designed synthetic pathway contains a decarboxylase to convert 2-ketoacids (precursors of amino acids) into aldehydes, and an aldehyde dehydrogenase to oxidize aldehydes into carboxylic acids. In this work, the general utility of the hybrid “Bio-Catalytic conversion” approach is demonstrated by the synthesis of MIBK, MIAK and DIBK.
Results
Construct a synthetic metabolic platform for the production of carboxylic acids
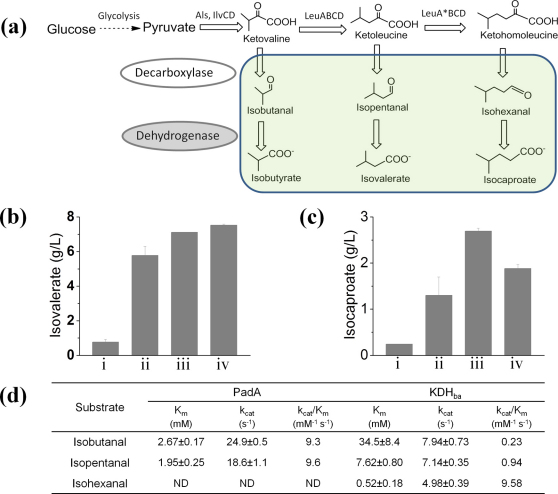
Based on Fig. 1b, isovaleric acid and acetic acid condense to form MIBK; isocaproic acid and acetic acid condense to form MIAK; and self-condensation of isovaleric acid generates DIBK. There exist engineered bacteria to produce acetic acid5. However, although isovaleric acid is a minor metabolite in lactobacillus and yeast (<3 mg/L)15,16, thus far no microorganism has been discovered to accumulate a significant quantity of C5 acid isovalerate or C6 acid isocaproate. To biosynthesize these C5 and C6 acids, we have engineered a synthetic metabolic network in three steps, by (1) amplifying the amino acid biosynthetic pathway to increase the pool of 2-ketoacids, (2) screening for 2-ketoacid decarboxylases that convert ketoacids to aldehydes, and (3) screening for aldehyde dehydrogenases that oxidize aldehydes into carboxylic acids (Fig. 2a).
Figure 2. Engineer E. coli for the biosynthesis of isovalerate and isocaproate.
(a) Construct a synthetic metabolic platform (1) by driving the carbon flux towards 2-ketoacids such as ketoleucine and ketohomoleucine, (2) screening for 2-ketoacid decarboxylases to decarboxylate ketoacids into aldehydes, and (3) screening for aldehyde dehydrogenases to oxidize aldehydes into carboxylic acids. (b) Production level of isovalerate in shake flask by different dehydrogenases. (c) Production level of isocaproate in shake flask by different dehydrogenases. (d) Kinetic parameters of the most productive aldehyde dehydrogenases. i, AldB; ii, AldH; iii, KDHba; iv, PadA.
The first step relies on genetic manipulation of the branched-chain amino acid metabolic pathway. In this pathway, the condensation of two pyruvate molecules leads to ketovaline after a series of biochemical reactions catalyzed by acetolactate synthase (AlsS), ketol-acid reductoisomerase (IlvC) and dihydroxy-acid dehydratase (IlvD). Ketovaline is then elongated to ketoleucine enabled by 2-isopropylmalate synthase (LeuA), isopropylmalate isomerase complex (LeuCD) and isopropylmalate dehydrogenase (LeuB). A LeuA mutant (LeuA*, G462D/S139G/H97L) was reported to catalyze the further elongation of ketoleucine to ketohomoleucine17. Thus we constructed two synthetic operons (Supplementary Fig. S1) that function upstream to drive the carbon flux towards ketoacid synthesis. The first operon for ketoleucine synthesis is composed of 6 genes on a medium-copy plasmid in the transcriptional order leuA-leuB-leuC-leuD-ilvD-alsS. The second operon for ketohomoleucine synthesis is leuA*-leuB-leuC-leuD-ilvD-alsS.
Identify promiscuous aldehyde dehydrogenases to oxidize isopentanal and isohexanal
In the Ehrlich pathway, 2-keto acids are normally processed to aldehydes by 2-ketoacid decarboxylases, and then to alcohols by alcohol dehydrogenases16. To shift the products from alcohols to acids, we need to construct nonnatural metabolic pathways to oxidize rather than reduce aldehydes. While the natural Ehrlich pathway enzyme 2-ketoacid decarboxylase (KIVD) from Lactococcus lactis18 has been shown to convert a wide variety of 2-ketoacids into aldehydes, suitable aldehyde dehydrogenases for oxidation of isopentanal and isohexanal are not previously known. As suggested by our previous work on producing isobutyrate19, we chose the following aldehyde dehydrogenases as candidate enzymes: E. coli acetaldehyde dehydrogenase AldB; E. coli 3-hydroxypropionaldehyde dehydrogenase AldH; E. coli phenylacetaldehyde dehydrogenase PadA; and Burkholderia ambifaria α-ketoglutaric semialdehyde dehydrogenase KDHba. These enzymes were individually cloned after KIVD to build an expression cassette on a high copy plasmid (Supplementary Fig. S1).
The complete biosynthetic pathways were then introduced into an E. coli strain AKO1 (BW25113, ΔyqhD)19. Shake flask fermentation was performed at 30°C for 48 hours in M9 minimal medium containing 40 g/L glucose. The choice of aldehyde dehydrogenases affected the fermentation outcome (Fig. 2b & 2c, i-iv). AldB could only produce either 0.8 g/L isovalerate or 0.24 g/L isocaproate, which suggests that its activity towards both isopentanal and isohexanal is low. In comparison, AldH, KDHba and PadA significantly increased the production level of C5 target to 5.8 g/L, 7.1 g/L and 7.5 g/L for the isovalerate pathway. For the isocaproate pathway, AldH, KDHba and PadA could produce 1.3 g/L, 2.7 g/L, and 1.9 g/L C6 target. It is interesting to discover that these enzymes are promiscuous enough to catalyze the oxidation of isopentanal or isohexanal, although these enzymes’ natural functions do not include the biosynthesis of isovalerate or isocaproate.
Both PadA and KDHba were purified and assayed in vitro to characterize their enzymatic activities (Fig. 2d). The Michaelis–Menten constant (Km) and the catalytic rate constant (kcat) of PadA for isopentanal were determined to be 1.95 mM and 18.6 s−1. Therefore, PadA possesses strong catalytic activity towards the noncognate substrate isopentanal. PadA also has a similarly high activity towards the smaller substrate isobutanal (Fig. 2d). Unlike PadA, KDHba, has a decreasing Michaelis–Menten constant (Km) as the size of the substrates increases: towards isobutanal, Km is 34.5 mM; and the number is 0.52 mM for the bulkier compound isohexanal. On the other hand, the catalytic rate constant (kcat) is similar for all three aldehydes. Therefore, the specificity constant kcat/Km of KDHba towards isohexanal is 10-fold higher and 42-fold higher than those towards isopentanal and isobutanal, which could explain why KDHba was the best enzyme discovered for the synthesis of isocaproate instead of isobutyrate and isovalerate (supplementary Table S3).
Engineer selectivity of 2-ketoacid decarboxylases to increase production
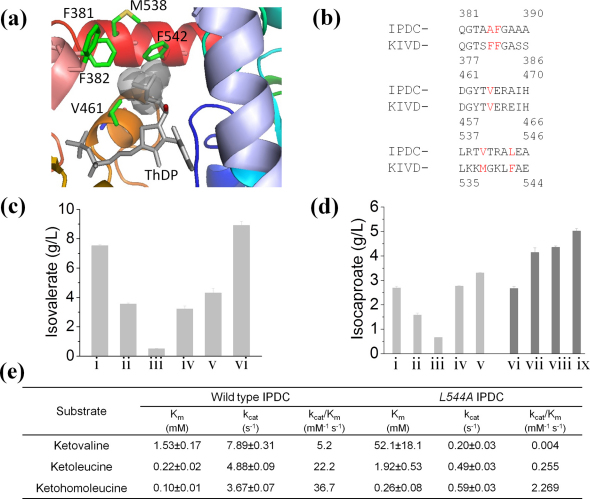
While the discovery of PadA and KDHba has conferred optimal activity in the oxidation step, the promiscuous nature of KIVD decarboxylase does not allow good selectivity in the decarboxylation step. Intermediate byproducts such as isobutyrate were present in the fermentation broth (supplementary Table S3). To increase the production of isovalerate or isocaproate, we examined the effect of engineering KIVD selectivity. According to the crystal structure (PDB: 2VBG), amino acid residues Phe381, Phe382, Val461, Met538 and Phe542, in combination with the cofactor thiamine diphosphate (ThDP), delineate the active site of KIVD (Fig. 3a)20. Mutation V461A was reported to decrease the activity of KIVD towards smaller substrates17. To further increase the selectivity towards bigger substrate, another four mutations F381L, F382L, M538A or F542L on top of V461A were performed.
Figure 3. Engineer decarboxylation selectivity to increase production.
(a) Crystal structure of KIVD active site. (b) Sequence alignment of KIVD and Salmonella typhimurium IPDC. (c) Production level of isovalerate by different decarboxylases. (d) Production level of isocaproate by different decarboxylases. (e) Kinetic parameters of the most productive aldehyde dehydrogenases. i, WT KIVD. ii, F381L/V461AKIVD. iii, F382L/V461AKIVD. iv, M538A/V461AKIVD. v, F542L/V461AKIVD. vi, WT IPDC. vii, V465A IPDC. viii, V540A IPDC. ix, L544A IPDC.
For the isovalerate pathway, these KIVD mutants reduced the isobutyrate concentration from 0.7 g/L to a range of 0.1 to 0.2 g/L during fermentation (Supplementary Table S2). However, there was no improvement in the production of isovalerate (Fig. 3c, i–iv). Without success in engineering KIVD, we then cloned another enzyme, indolepyruvate decarboxylase (IPDC) from Salmonella typhimurium, whose activity was not previously investigated. The combination of IPDC and PadA produced 8.90 g/L isovalerate (Fig. 3c, vi) from 40 g/L glucose, which represents a yield of 0.22 g/ g glucose that is 58% of the theoretical maximum.
For the isocaproate pathway, the fermentation data indicated that M538A/V461A and F542L/V461A KIVD mutants enhanced isocaproate production (Fig. 3d, i–v). Compared to wild type KIVD, isocaproate production by M538A/V461A mutant did not increase significantly; while production by F542L/V461A mutant increased by 23% to 3.30 g/L. Nevertheless, the yield of isocaproate was only 25.6% of the theoretical maximum. Given the unsatisfactory performance of engineered KIVD mutants, we investigated the effect of engineering IPDC. Based on the protein sequence alignment between IPDC and KIVD, Ala385, Phe386, Val465, Val540 and Leu544 are the corresponding active-site residues of IPDC (Fig. 3b). Since V461A, M538A and F542L mutations in KIVD were helpful in increasing isocaproate production, accordingly, single-site mutations V465A, V540A and L544A were performed on IPDC. Fermentation results demonstrated that V465A, V540A and L544A IPDC mutants produced 4.14, 4.35 and 5.02 g/L isocaproate respectively (Fig. 3d, vii–ix). Thus isocaproate produced by L544A IPDC was 52% higher than that produced by F542L/V461A KIVD, 88% higher than that produced by wild type IPDC. These results suggest that F542 of KIVD or L544 of IPDC plays a critical role in determining the substrate selectivity towards ketohomoleucine.
Enzymatic assays (Fig. 3e) indicate that, compared to KIVD21, for the smaller substrate ketovaline, IPDC has a significantly lower kcat (7.89 s−1 versus 48 s−1) and a comparable Km (1.53 mM versus 2.8 mM); for ketoleucine, IPDC still has a lower kcat (4.88 s−1 versus 49 s−1), but a much lower Km (0.22 mM versus 3.7 mM). Thus, the specificity constant kcat/Km of IPDC towards ketoleucine is 4-fold higher than that towards ketovaline, which could explain the better performance of IPDC over KIVD with respect to isovalerate production. However, for wild type IPDC, the selectivity constant (kcat/Km) towards ketoleucine and ketohomoleucine are 22.2 and 36.7 mM−1 s−1 (Fig. 3e) respectively, indicating that wild type IPDC has similar activity for both C6 and C7 ketoacids. In comparison, the L544A mutation dramatically increases the selectivity of IPDC towards the C7 ketoacid ketohomoleucine, which is 10 times higher than that towards ketoleucine, and 567 times higher than that towards ketovaline. The consequence is L544A mutant produced the largest amount of isocaproate while the production of isobutyrate and isovalerate (derived from ketovaline and ketoleucine) were reduced (supplementary Table S3).
Bioreactor fermentation and purification
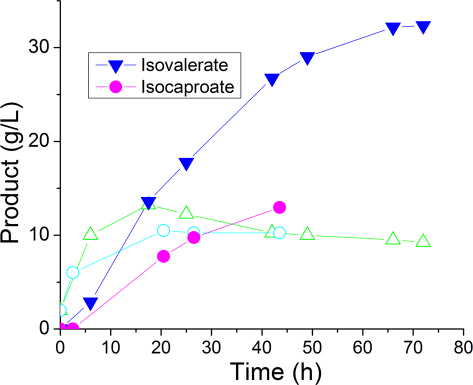
To explore the possibility of scale-up production, we translated the fermentation process from shake flasks to a 1-liter bioreactor (Fig. 4). The cul-ture produced 32.3 g/L isovalerate in 66 hours. The longer chain carboxylic acid isocaproate had a production level of 13 g/L after 43 hours. The difference in production titer might be due to the higher toxicity associated with longer carbon chain22. Nevertheless, it is encouraging to find out that the concentration of protonated isovalerate and isocaproate in the fermentation broth is already greater than their solubility limit (25 g/L and 11 g/L respectively), which should greatly facilitate the purification process. Thus we not only have developed novel metabolic pathways to biosynthesize C5 and C6 acids, but also have pushed their production levels to be among the highest reported for new biobased products1,3,23,24,25,26,27. Since isovalerate is protonated under acidic conditions and becomes hydrophobic, we used this feature to develop a simple purification method. The pH of the fermentation broth was adjusted to 3.0, and then solvents were applied to extract isovaleric acid out of the aqueous phase. Among the solvents tested, hexane and oleyl alcohol appear to be suitable for the extraction purpose: isovaleric acid has a high distribution coefficient Kd in both solvents; the solvents are water insoluble (minimal loss during extraction); the boiling points of both solvents are very different from that of isovaleric acid (facile separation through distillation). While the purification procedure was optimized for isovalerate production here, this strategy can be applied to manufacturing isocaproate and other aliphatic carboxylic acids as well.
Figure 4. Production of isovalerate and isocaproate in a 1-L bioreactor.
Time courses of the fermentation profile. Solid symbol denotes the concentration of carboxylic acids. Open symbol denotes the corresponding dry biomass weight. Triangle, isovalerate; Circle, isocaproate.
Catalytic conversion to ketones
With success in engineered biosynthesis, we began to explore catalysts for the ketonization of isovaleric acid (IVA) or isocaproic acid (ICA) to produce MIBK, MIAK and DIBK. While these particular reactions were not investigated previously, some studies have shown that ceria-based systems are active on chemically similar substrates such as acetic acid and propionic acid28,29. Accordingly, CeO2 could be an effective catalyst for the ketonization of IVA and ICA. We prepared catalysts with various weight ratios of CeO2 supported on different carriers (Al2O3, TiO2 and ZrO2).
Among the three catalyst systems, the TiO2-supported CeO2 catalysts exhibited the highest efficiency for the production of MIBK and MIAK (Table 1, entries 1–3 and 18–20). Temperature was observed to have an effect on the reaction, with the highest yield of MIBK and MIAK occurring at 693 K (Table 1, entries 3–6 and 20–24). When the temperature was below 613 K, the conversion was not complete. However, when temperature was above 693 K, the yields of MIBK, DIBK and MIAK all decreased, possibly due to an increase in cracking reactions at high temperatures. Not surprisingly, the MIBK yield was proportional to the CeO2 content (Table 1, entries 3, 7 and 8). The molar ratio of substrates was also an important factor for the ketonization reactions to form asymmetric ketones30. Results showed that when the ratio of acetic acid to IVA was increased from 1∶1 to 4∶1, the yield of asymmetric product MIBK increased substantially from 41% to 84%; whereas the yield of symmetric product DIBK decreased from 20% to 11% (Table 1, entries 3 and 9–11). Similarly, the highest yield (81%) of MIAK was achieved by increasing the ratio of acetic acid/ICA to 4∶1 (Table 1, entry 28). Notably, acetic acid was completely converted in all cases, and acetone is a valuable byproduct whose price is twice that of acetic acid, so an excess of acetic acid is more economical for the manufacturing process.
Table 1. Ketonization of isovaleric acid or isocaproic acid for MIBK, DIBK and MIAK production.
| Entry | Catalyst | Feed Ratioa | Temperature (K) | Conversion (%)b | Yieldb,c |
|---|---|---|---|---|---|
| MIBK production | |||||
| 1 | 18%CeO2/Al2O3 | 3.4∶1 | 693 | 98 | 65(8, 42) |
| 2 | 18%CeO2/ZrO2 | 3.4∶1 | 693 | 98 | 57(15, 47) |
| 3 | 18%CeO2/TiO2 | 3.4∶1 | 693 | 98 | 74(15, 55) |
| 4 | 18%CeO2/TiO2 | 3.4∶1 | 613 | 92 | 35(2, 30) |
| 5 | 18%CeO2/TiO2 | 3.4∶1 | 653 | 98 | 65(4, 49) |
| 6 | 18%CeO2/TiO2 | 3.4∶1 | 733 | 98 | 70(8, 42) |
| 7 | 10%CeO2/TiO2 | 3.4∶1 | 693 | 98 | 68(13, 46) |
| 8 | 25%CeO2/TiO2 | 3.4∶1 | 693 | 99 | 77(14, 44) |
| 9 | 18%CeO2/TiO2 | 1∶1 | 693 | 86 | 41(20, 34) |
| 10 | 18%CeO2/TiO2 | 2∶1 | 693 | 94 | 59(19, 50) |
| 11 | 18%CeO2/TiO2 | 4∶1 | 693 | 99 | 84(11, 67) |
| DIBK production | |||||
| 12 | 18%CeO2/TiO2 | - | 693 | 64 | 34 |
| 13 | 18%CeO2/Al2O3 | - | 693 | 74 | 14 |
| 14 | 18%CeO2/ZrO2 | - | 693 | 91 | 50 |
| 15 | 18%CeO2/ZrO2 | - | 733 | 98 | 66 |
| 16 | 10%CeO2/ZrO2 | - | 733 | 98 | 62 |
| 17 | 25%CeO2/ZrO2 | - | 733 | 98 | 60 |
| MIAK production | |||||
| 18 | 18%CeO2/Al2O3 | 3.8∶1 | 693 | 96 | 62(9, 43) |
| 19 | 18%CeO2/ZrO2 | 3.8∶1 | 693 | 98 | 71(12, 48) |
| 20 | 18%CeO2/TiO2 | 3.8∶1 | 693 | 98 | 80(11, 57) |
| 21 | 18%CeO2/TiO2 | 3.8∶1 | 633 | 88 | 37(4, 36) |
| 22 | 18%CeO2/TiO2 | 3.8∶1 | 663 | 97 | 71(6, 40) |
| 23 | 18%CeO2/TiO2 | 3.8∶1 | 723 | 98 | 68(9, 46) |
| 24 | 18%CeO2/TiO2 | 3.8∶1 | 753 | 99 | 58(8, 45) |
| 25 | 10%CeO2/TiO2 | 3.8∶1 | 693 | 96 | 60(9, 49) |
| 26 | 25%CeO2/TiO2 | 3.8∶1 | 693 | 99 | 82(12, 58) |
| 27 | 18%CeO2/TiO2 | 2∶1 | 693 | 90 | 40(17, 43) |
| 28 | 18%CeO2/TiO2 | 4∶1 | 693 | 99 | 81(9, 62) |
aMolar ratio of acetic acid to isovaleric acid or isocaproic acid.
bDetermined by GC, based on isovaleric acid or isocaproic acid.
cThe data in the brackets are yield of DIBK, acetone, respectively. DIBK or acetone is the byproduct from the self-coupling of isovaleric acid or acetic acid.
We also investigated the self-condensation of IVA to form DIBK. Unlike MIBK production, highest IVA conversion and DIBK yield were obtained with the ZrO2-supported CeO2 catalyst (Table 1, entries 12–14). The IVA conversion was not complete at 693 K. This suggests that increased chain length lowers reaction activity, which was reported before on a similar type of reaction31. Another reason for the lower yield comparing with MIBK production is that the gasification of pure IVA is more difficult than the mixture of IVA and acetic acid, which will result in the insufficient contact with the catalyst bed and the generation of coke. We then raised the reaction temperature to 733 K, and achieved almost complete conversion of IVA and an improved DIBK yield of 66% (Table 1, entry 15). ZrO2-supported catalysts with different CeO2 loadings were also examined. It was found that the 18% CeO2/ZrO2 catalyst performed best, and a decrease in yield was observed with 25% CeO2 loading (Table 1, entries 15, 16 and 17). This is possibly a consequence of increased side reactions such as aldol condensation with high CeO2 loadings.
Discussion
In this work, we have designed a synthetic metabolic platform to synthesize iso-branched C4–C6 carboxylic acids. The biosynthetic module employs two biochemical steps, decarboxylation and oxidation, to channel 2-ketoacids from amino acid pathways into carboxylic acids. The generated metabolites C5 and C6 acids serve as new types of platform chemicals. They could be chemically reduced by H2 to alcohols to be used as advanced fuels17, or esterified to be green solvents as alternatives of ethyl lactate32. Here, as a general strategy to simplify the manufacturing of aliphatic ketones33, we have developed CeO2 catalysts to condense the biosynthesized carboxylic acids into ketones upon single-step decarboxylative coupling.
This research opens a new path for the production of renewable chemicals. The “Bio-Catalytic conversion” strategy takes advantage of the benefits of both microbial fermentation and catalytic synthesis. The process and raw material requirements are simplified in comparison to the current chemical manufacturing process. While this approach can be easily extended to other aliphatic ketones, we envision that the concept has broader implications. Currently, to produce industrial chemicals such as plastics, fertilizers, and pharmaceuticals, 99% of the feedstock materials come from petroleum or natural gas34. The established chemical synthesis routes are optimized based on the availability and cost of petroleum stock. In the future, we should design more simplified manufacturing routes based on chemical precursors that can be efficiently biosynthesized from renewable carbohydrates.
Methods
Strains and plasmids
The AKO1 strain is BW25113 with ΔyqhD. Construction of plasmids is described in detail in the Supplementary information.
Culture and production conditions
For shake flask experiments, overnight culture was diluted 25 fold into 5 ml fresh medium in a 125-ml conical flask (0.5 g CaCO3 was added to buffer the pH). Production was induced with 0.1 mM IPTG at 30°C. For bioreactor fermentation (Fig. 4a), production started once OD600 reached 8.0 with the addition of 0.2 mM IPTG. The pH was controlled at 7.0 and the temperature was kept at 30°C. Dissolved oxygen (DO) level was maintained at 10% air saturation. Fermentation products were analyzed with an Agilent 1260 HPLC.
Ketonization experiments
Supported CeO2 catalysts were prepared by impregnation supports with an aqueous solution of Ce(NO3)3·6H2O via incipient technique. All the ketonization runs were carried out in a typical fixed bed tubular quartz reactor with the mixture of 1 g catalyst and 2 g quartz granules. Reactants were dosed using a microdosing pump with liquid hourly space velocity(LHSV) = 1 cm3 gcatalyst−1 h−1, and nitrogen was used as carried gas at a flow rate of 20 cm3 min−1. Prior to the reaction the catalyst sample was preheated at the reaction temperature in the N2 flow for 30 min. The effluent product was condensed by an ice trap. The liquid products were analyzed by Shimadzu GC-2014 GC- FID.
Author Contributions
M.X., J.D., Y.F. and K.Z. designed experiments; M.X., J.D., A.W., M.Z., J.Z., S.P., H.L. performed the experiments; M.X., J.D., Y.F. and K.Z. analyzed the data; and M.X., J.D., Y.F. and K.Z. wrote the paper.
Supplementary Material
supplement
Acknowledgments
This work was supported by start-up funds from the University of Minnesota, and the National Basic Research Program of China (2012CB215306).
Footnotes
The University of Minnesota has filed US provisional patent on this technology.
References
- Atsumi S., Hanai T. & Liao J. C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451, 86–89 (2008). [DOI] [PubMed] [Google Scholar]
- Roman-Leshkov Y., Barrett C. J., Liu Z. Y. & Dumesic J. A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 447, 982–985 (2007). [DOI] [PubMed] [Google Scholar]
- Steen E. J. et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463, 559–562 (2010). [DOI] [PubMed] [Google Scholar]
- Holm M. S., Saravanamurugan S. & Taarning E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 328, 602–605 (2010). [DOI] [PubMed] [Google Scholar]
- Causey T. B., Zhou S., Shanmugam K. T. & Ingram L. O. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc. Natl. Acad. Sci. USA 100, 825–832 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha W., Shao Z., Frost J. W. & Zhao H. Rational pathway engineering of type I fatty acid synthase allows the biosynthesis of triacetic acid lactone from D-glucose in vivo. J. Am. Chem. Soc 126, 4534–4535 (2004). [DOI] [PubMed] [Google Scholar]
- Yan Y., Chemler J., Huang L., Martens S. & Koffas M. A. G. Metabolic engineering of anthocyanin biosynthesis in Escherichia coli. Appl. Environ. Microbiol. 71, 3617–3623 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer B. A., Admiraal S. J., Gramajo H., Cane D. E. & Khosla C. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291, 1790–1792 (2001). [DOI] [PubMed] [Google Scholar]
- Zhao H., Holladay J. E., Brown H. & Zhang Z. C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316, 1597 (2007). [DOI] [PubMed] [Google Scholar]
- Huber G. W., Iborra S. & Corma A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2006). [DOI] [PubMed] [Google Scholar]
- Stephanopoulos G., Aristidou A. A., Nielsen J. H. & Nielsen J. Metabolic engineering: principles and methodologies, (Academic Pr, 1998). [Google Scholar]
- Organisation for Economic Cooperation and Development Existing Chemicals Database.
- Chen Y. Z., Hwang C. M. & Liaw C. W. One-step synthesis of methyl isobutyl ketone from acetone with calcined Mg/Al hydrotalcite-supported palladium or nickel catalysts. Appl. Catal. A: Gen. 169, 207–214 (1998). [Google Scholar]
- Friedel C. Ueber sg gemischte Acetone. Justus Liebigs Ann. Chem. 108, 122–125 (1858). [Google Scholar]
- Lambrechts M. G. & Pretorius I. S. Yeast and its importance to wine aroma-a review. S. Afr. J. Enol. Vitic. 21, 97–129 (2000). [Google Scholar]
- Hazelwood L. A., Daran J. M., van Maris A. J. A., Pronk J. T. & Dickinson J. R. The Ehrlich Pathway for Fusel Alcohol Production: a Century of Research on Saccharomyces cerevisiae Metabolism? Appl. Environ. Microbiol. 74, 2259–2266 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Sawaya M. R., Eisenberg D. S. & Liao J. C. Expanding metabolism for biosynthesis of nonnatural alcohols. Proc. Natl. Acad. Sci. USA 105, 20653–20658 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Plaza M., Fernandez de Palencia P., Pelaez C. & Requena T. Biochemical and molecular characterization of alpha-ketoisovalerate decarboxylase, an enzyme involved in the formation of aldehydes from amino acids by Lactococcus lactis. FEMS Microbiol. Lett. 238, 367–374 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang K., Woodruff A. P., Xiong M., Zhou J. & Dhande Y. K. A Synthetic Metabolic Pathway for Production of the Platform Chemical Isobutyric Acid. ChemSusChem 4, 1068–1070 (2011). [DOI] [PubMed] [Google Scholar]
- Berthold C. L. et al. Structure of the branched-chain keto acid decarboxylase (KdcA) from Lactococcus lactis provides insights into the structural basis for the chemoselective and enantioselective carboligation reaction. Acta. Cryst. 63, 1217–1224 (2007). [DOI] [PubMed] [Google Scholar]
- Yep A., Kenyon G. L. & McLeish M. J. Determinants of substrate specificity in KdcA, a thiamin diphosphate-dependent decarboxylase. Bioorg. Chem. 34, 325–336 (2006). [DOI] [PubMed] [Google Scholar]
- Warnecke T. & Gill R. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb. Cell Fact. 4, 25 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H. et al. Metabolic engineering of Escherichia coli for direct production of 1, 4-butanediol. Nat. Chem. Biol. 7, 445–452 (2011). [DOI] [PubMed] [Google Scholar]
- Qian Z. G., Xia X. X. & Lee S. Y. Metabolic engineering of Escherichia coli for the production of cadaverine: a five carbon diamine. Biotechnol Bioeng. 108, 93–103 (2011). [DOI] [PubMed] [Google Scholar]
- Moon T. S., Dueber J. E., Shiue E. & Prather K. L. J. Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metab. Eng. 12, 298–305 (2010). [DOI] [PubMed] [Google Scholar]
- Dellomonaco C., Clomburg J. M., Miller E. N. & Gonzalez R. Engineered reversal of the B-oxidation cycle for the synthesis of fuels and chemicals. Nature 476, 355–359 (2011). [DOI] [PubMed] [Google Scholar]
- McKenna R. & Nielsen D. R. Styrene biosynthesis from glucose by engineered E. coli. Metab. Eng. 13, 544–554 (2011). [DOI] [PubMed] [Google Scholar]
- Renz M. Ketonization of carboxylic acids by decarboxylation: mechanism and scope. Eur. J. Org. Chem. 2005, 979–988 (2005). [Google Scholar]
- Gaertner C. A., Serrano-Ruiz J. C., Braden D. J. & Dumesic J. A. Ketonization Reactions of Carboxylic Acids and Esters over Ceria− Zirconia as Biomass-Upgrading Processes. Ind. Eng. Chem. Res. 49, 6027–6033 (2010). [Google Scholar]
- Hendren T. S. & Dooley K. M. Kinetics of catalyzed acid/acid and acid/aldehyde condensation reactions to non-symmetric ketones. Catal. Today 85, 333–351 (2003). [Google Scholar]
- Nagashima O., Sato S., Takahashi R. & Sodesawa T. Ketonization of carboxylic acids over CeO2-based composite oxides. J. Mol. Catal. A: Chem. 227, 231–239 (2005). [Google Scholar]
- Datta R. & Henry M. Lactic acid: recent advances in products, processes and technologies a review. J. Chem. Technol. Biotechnol. 81, 1119–1129 (2006). [Google Scholar]
- Goh E.-B., Baidoo E. E. K., Keasling J. D. & Beller H. R. Engineering of Bacterial Methyl Ketone Synthesis for Biofuels. Appl. Environ. Microbiol. 78, 70–80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane J. & Robinson S. Survey of Alternative Feedstocks for Commodity Chemical Manufacturing. http://info.ornl.gov/sites/publications/files/Pub8760.pdf (2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplement