Abstract
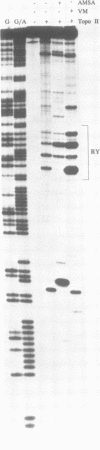
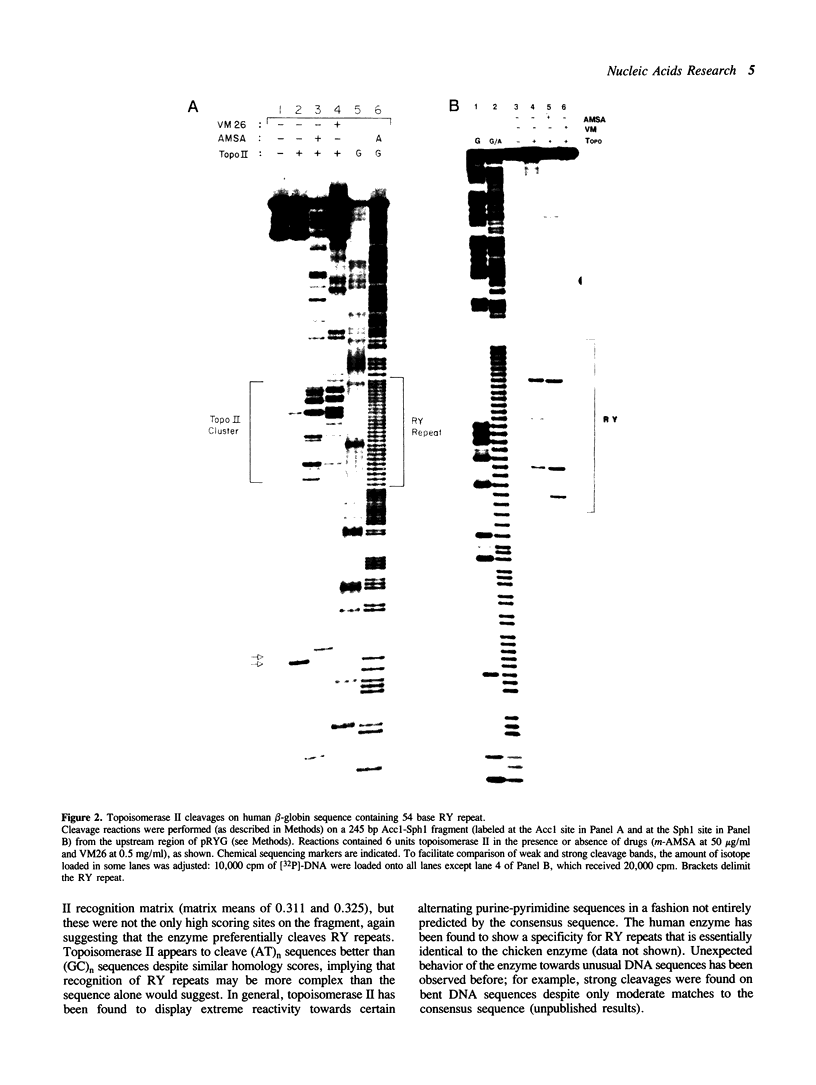
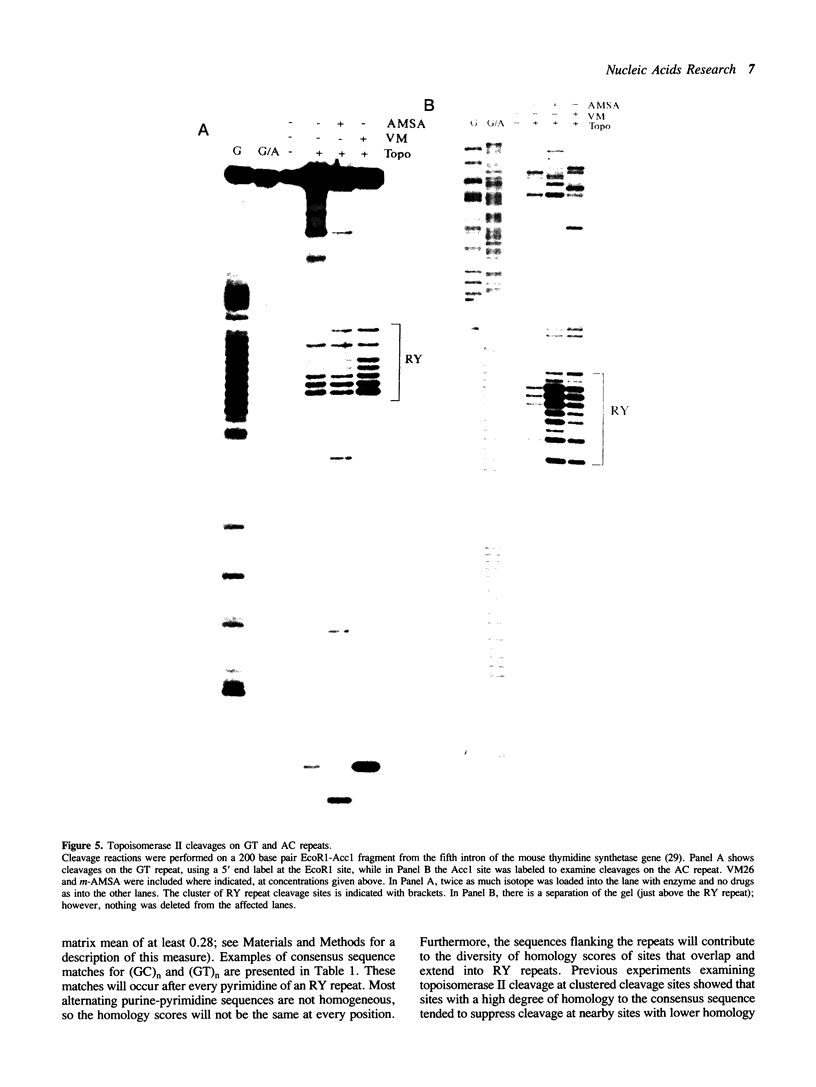
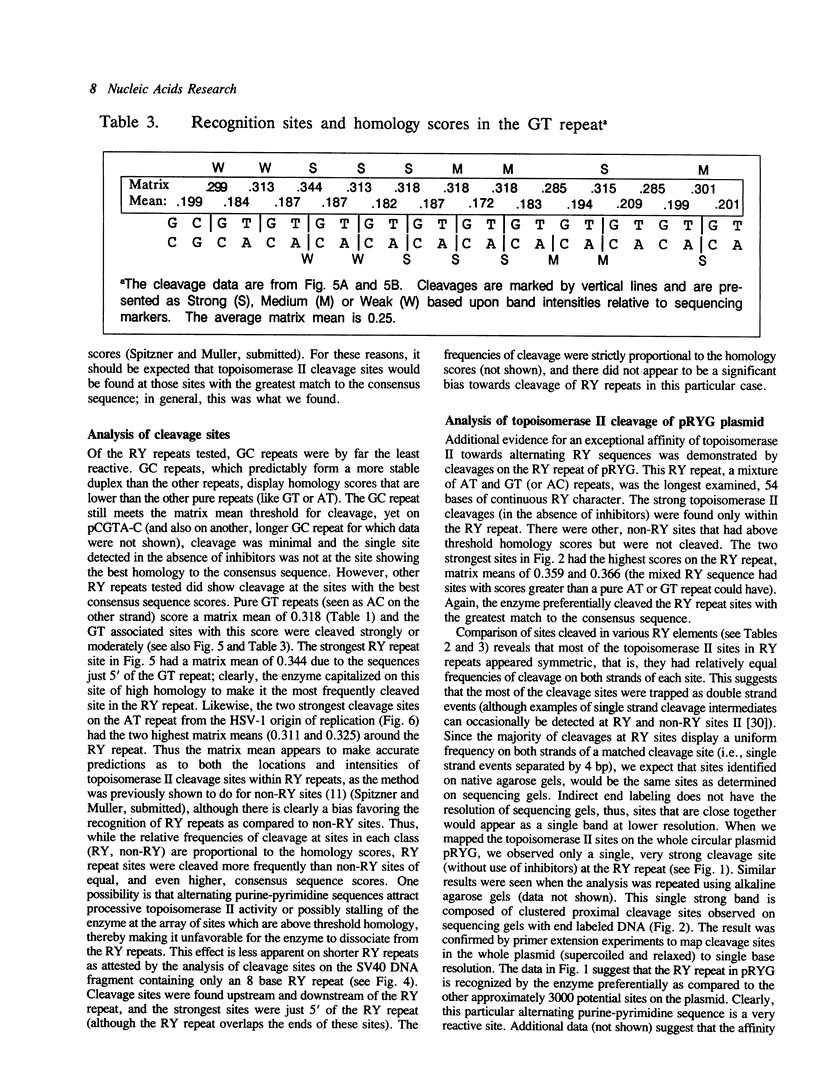
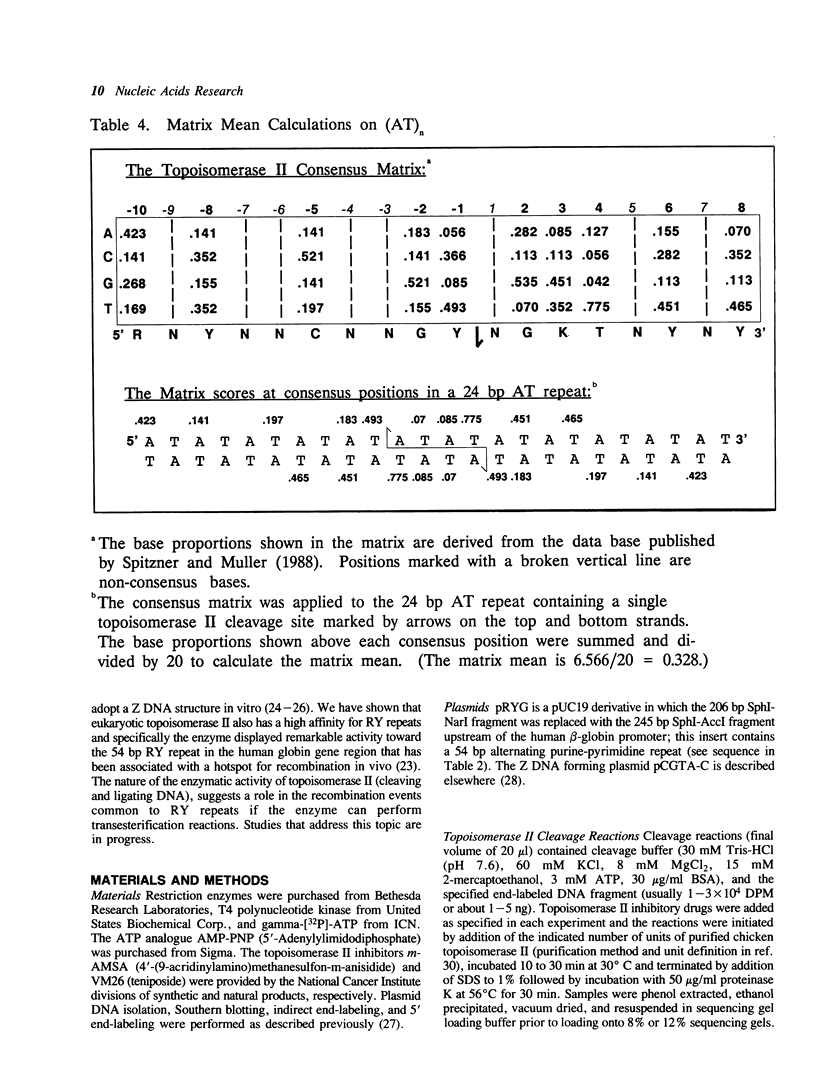
Alternating purine-pyrimidine sequences (RY repeats) demonstrate considerable homology to the consensus sequence for vertebrate topoisomerase II (Spitzner and Muller (1988) Nucleic Acids Res. 16: 1533-1556). This is shown below and positions that can match are underscored. RYRYRYRYRYRYRYRYRY = alternating purine-pyrimidine 18 bp RNYNNCNNGYNGKTNYNY = topoisomerase II consensus sequence (R is purine, Y is pyrimidine, K is G or T.) Topoisomerase II cleavage reactions were performed (in the absence of inhibitors) on a plasmid containing a 54 base RY repeat and the single strong cleavage site mapped to the RY repeat. Analysis of this DNA on sequencing gels showed that the enzyme cleaved a number of sites, all within the 54 base pair RY repeat. Topoisomerase II also made clustered cleavages within other RY repeats that were examined. Quantitative analysis of homology to the consensus sequence, as measured by the match of a site to a matrix of base proportions from the consensus data base (the matrix mean), showed that both the locations and the frequencies of cleavage sites within RY repeats were proportional to homology scores. However, topoisomerase II cleaved RY repeats preferentially in comparison to non-RY sites with similar homology scores. The activity of the enzyme at RY repeats appears to be proportional to the length of the repeat; additionally, GT, AC and AT repeats were better substrates for cleavage than GC repeats.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Rich A. Isolation of Z-DNA binding proteins from SV40 minichromosomes: evidence for binding to the viral control region. Cell. 1985 Jun;41(2):365–374. doi: 10.1016/s0092-8674(85)80009-x. [DOI] [PubMed] [Google Scholar]
- Berrios M., Osheroff N., Fisher P. A. In situ localization of DNA topoisomerase II, a major polypeptide component of the Drosophila nuclear matrix fraction. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4142–4146. doi: 10.1073/pnas.82.12.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., Costa de Oliveira R., Laval J. The Escherichia coli O6-methylguanine-DNA methyltransferase does not repair promutagenic O6-methylguanine residues when present in Z-DNA. J Biol Chem. 1985 Jul 25;260(15):8711–8715. [PubMed] [Google Scholar]
- Deng T. L., Li Y., Johnson L. F. Thymidylate synthase gene expression is stimulated by some (but not all) introns. Nucleic Acids Res. 1989 Jan 25;17(2):645–658. doi: 10.1093/nar/17.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laroche T., Falquet J., Boy de la Tour E., Laemmli U. K. Metaphase chromosome structure. Involvement of topoisomerase II. J Mol Biol. 1986 Apr 20;188(4):613–629. doi: 10.1016/s0022-2836(86)80010-9. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Transition of a cloned d(AT)n-d(AT)n tract to a cruciform in vivo. Nucleic Acids Res. 1985 Jun 25;13(12):4343–4363. doi: 10.1093/nar/13.12.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck M. M., Hittelman W. N., Earnshaw W. C. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1086–1090. doi: 10.1073/pnas.85.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Hsieh W. T., Wells R. D. Influence of negative supercoiling and of the proximity of left-handed Z-DNA on the Escherichia coli lactose repressor-operator interaction. J Biol Chem. 1987 Oct 25;262(30):14576–14582. [PubMed] [Google Scholar]
- Jaworski A., Hsieh W. T., Blaho J. A., Larson J. E., Wells R. D. Left-handed DNA in vivo. Science. 1987 Nov 6;238(4828):773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Klysik J., Singleton C. K., Zarling D. A., Jovin T. M., Hanau L. H., Erlanger B. F., Wells R. D. Intervening sequences in human fetal globin genes adopt left-handed Z helices. J Biol Chem. 1984 Jun 10;259(11):7268–7274. [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Wells R. D. Left-handed DNA. Cloning, characterization, and instability of inserts containing different lengths of (dC-dG) in Escherichia coli. J Biol Chem. 1982 Sep 10;257(17):10152–10158. [PubMed] [Google Scholar]
- Lafer E. M., Sousa R., Rosen B., Hsu A., Rich A. Isolation and characterization of Z-DNA binding proteins from wheat germ. Biochemistry. 1985 Sep 10;24(19):5070–5076. doi: 10.1021/bi00340a017. [DOI] [PubMed] [Google Scholar]
- Lagravère C., Malfoy B., Leng M., Laval J. Ring-opened alkylated guanine is not repaired in Z-DNA. 1984 Aug 30-Sep 5Nature. 310(5980):798–800. doi: 10.1038/310798a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M. J., Blaho J. A., Kilpatrick M. W., Wells R. D. Consecutive A X T pairs can adopt a left-handed DNA structure. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5884–5888. doi: 10.1073/pnas.83.16.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Mehta V. B. DNase I hypersensitivity is independent of endogenous topoisomerase II activity during chicken erythrocyte differentiation. Mol Cell Biol. 1988 Sep;8(9):3661–3669. doi: 10.1128/mcb.8.9.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T. Quantitation of eukaryotic topoisomerase I reactivity with DNA. Preferential cleavage of supercoiled DNA. Biochim Biophys Acta. 1985 Mar 20;824(3):263–267. doi: 10.1016/0167-4781(85)90057-0. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Spitzner J. R., DiDonato J. A., Mehta V. B., Tsutsui K., Tsutsui K. Single-strand DNA cleavages by eukaryotic topoisomerase II. Biochemistry. 1988 Nov 1;27(22):8369–8379. doi: 10.1021/bi00422a012. [DOI] [PubMed] [Google Scholar]
- Murphy K. E., Stringer J. R. RecA independent recombination of poly[d(GT)-d(CA)] in pBR322. Nucleic Acids Res. 1986 Sep 25;14(18):7325–7340. doi: 10.1093/nar/14.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Kohn K. Topological complexes between DNA and topoisomerase II and effects of polyamines. Biochemistry. 1989 Feb 7;28(3):995–1002. doi: 10.1021/bi00429a012. [DOI] [PubMed] [Google Scholar]
- Sander M., Hsieh T., Udvardy A., Schedl P. Sequence dependence of Drosophila topoisomerase II in plasmid relaxation and DNA binding. J Mol Biol. 1987 Mar 20;194(2):219–229. doi: 10.1016/0022-2836(87)90370-6. [DOI] [PubMed] [Google Scholar]
- Semenza G. L., Malladi P., Surrey S., Delgrosso K., Poncz M., Schwartz E. Detection of a novel DNA polymorphism in the beta-globin gene cluster. J Biol Chem. 1984 May 25;259(10):6045–6048. [PubMed] [Google Scholar]
- Sinden R. R., Kochel T. J. Reduced 4,5',8-trimethylpsoralen cross-linking of left-handed Z-DNA stabilized by DNA supercoiling. Biochemistry. 1987 Mar 10;26(5):1343–1350. doi: 10.1021/bi00379a021. [DOI] [PubMed] [Google Scholar]
- Spitzner J. R., Muller M. T. A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids Res. 1988 Jun 24;16(12):5533–5556. doi: 10.1093/nar/16.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Tanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986 May;5(5):1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]