Abstract
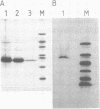
O6-alkylguanine-DNA-alkyltransferase (ATase) activity was increased in rat liver from 80 to 320 fmoles/mg total protein 48 h after administration of 2-acetylaminofluorene at 60 mg/kg body weight. This tissue was used as a source of ATase which was purified by ammonium sulphate precipitation and DNA-cellulose, molecular exclusion and ion exchange chromatography (IEC). IEC purified material showed a major 24 kDa band after polyacrylamide gel electrophoresis (PAGE) with silver staining. Fluorography of purified ATase following incubation with [3H]-methylated substrate DNA and PAGE showed a single band at 24 kDa suggesting that, as with bacterial ATases, the protein itself accepts the alkyl group from O6-alkylguanine in substrate DNA during the repair reaction. Further purification of the protein using reverse phase HPLC resulted in a single peak representing approximately 125,000 fold purification. This was subjected to amino-terminal sequencing and it was found that the protein was blocked at the amino-terminal end: it was cleaved using trypsin or cyanogen bromide and the amino acid sequence of several reverse phase HPLC purified fragments was determined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Margison G. P., Saffhill R. Evidence for the excision repair of O6-n-butyldeoxyguanosine in human cells. Carcinogenesis. 1986 Dec;7(12):1987–1990. doi: 10.1093/carcin/7.12.1987. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent T. P., Dolan M. E., Fraenkel-Conrat H., Hall J., Karran P., Laval L., Margison G. P., Montesano R., Pegg A. E., Potter P. M. Repair of O-alkylpyrimidines in mammalian cells: a present consensus. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1759–1762. doi: 10.1073/pnas.85.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. P., O'Connor P. J., Margison G. P. Effect of acute doses of 2-acetylaminofluorene on the capacity of rat liver to repair methylated purines in DNA in vivo and in vitro. Cancer Res. 1982 Oct;42(10):4203–4209. [PubMed] [Google Scholar]
- Day R. S., 3rd, Babich M. A., Yarosh D. B., Scudiero D. A. The role of O6-methylguanine in human cell killing, sister chromatid exchange induction and mutagenesis: a review. J Cell Sci Suppl. 1987;6:333–353. doi: 10.1242/jcs.1984.supplement_6.22. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. E., Pegg A. E. Extent of formation of O4-methylthymidine in calf thymus DNA methylated by N-methyl-N-nitrosourea and lack of repair of this product by rat liver O6-alkylguanine-DNA-alkyltransferase. Carcinogenesis. 1985 Nov;6(11):1611–1614. doi: 10.1093/carcin/6.11.1611. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Hora J. F., Eastman A., Bresnick E. O6-methylguanine methyltransferase in rat liver. Biochemistry. 1983 Aug 2;22(16):3759–3763. doi: 10.1021/bi00285a007. [DOI] [PubMed] [Google Scholar]
- Lemotte P. K., Walker G. C. Induction and autoregulation of ada, a positively acting element regulating the response of Escherichia coli K-12 to methylating agents. J Bacteriol. 1985 Mar;161(3):888–895. doi: 10.1128/jb.161.3.888-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Butler J., Hoey B. O6-Methylguanine methyltransferase activity is increased in rat tissues by ionising radiation. Carcinogenesis. 1985 Dec;6(12):1699–1702. doi: 10.1093/carcin/6.12.1699. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Brennand J. Cloning of the E. coli O6-methylguanine and methylphosphotriester methyltransferase gene using a functional DNA repair assay. Nucleic Acids Res. 1985 Mar 25;13(6):1939–1952. doi: 10.1093/nar/13.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru G. B., Margison G. P., Chu Y. H., O'Connor P. J. Effects of carcinogens and partial hepatectomy upon the hepatic O6-methylguanine repair system in mice. Carcinogenesis. 1982;3(11):1247–1254. doi: 10.1093/carcin/3.11.1247. [DOI] [PubMed] [Google Scholar]
- Morohoshi F., Hayashi K., Munakata N. Bacillus subtilis gene coding for constitutive O6-methylguanine-DNA alkyltransferase. Nucleic Acids Res. 1989 Aug 25;17(16):6531–6543. doi: 10.1093/nar/17.16.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morten J. E., Margison G. P. Increased O6-alkylguanine alkyltransferase activity in Chinese hamster V79 cells following selection with chloroethylating agents. Carcinogenesis. 1988 Jan;9(1):45–49. doi: 10.1093/carcin/9.1.45. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Kondo H., Kawabata S., Iwanaga S., Sekiguchi M. Purification and structure of the intact Ada regulatory protein of Escherichia coli K12, O6-methylguanine-DNA methyltransferase. J Biol Chem. 1985 Jun 25;260(12):7281–7288. [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E., Perry W., Bennett R. A. Effect of partial hepatectomy on removal of O6-methylguanine from alkylated DNA by rat liver extracts. Biochem J. 1981 Jul 1;197(1):195–201. doi: 10.1042/bj1970195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Roberfroid M., von Bahr C., Foote R. S., Mitra S., Bresil H., Likhachev A., Montesano R. Removal of O6-methylguanine from DNA by human liver fractions. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5162–5165. doi: 10.1073/pnas.79.17.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Scicchitano D., Dolan M. E. Comparison of the rates of repair of O6-alkylguanines in DNA by rat liver and bacterial O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1984 Sep;44(9):3806–3811. [PubMed] [Google Scholar]
- Potter P. M., Kleibl K., Cawkwell L., Margison G. P. Expression of the ogt gene in wild-type and ada mutants of E. coli. Nucleic Acids Res. 1989 Oct 25;17(20):8047–8060. doi: 10.1093/nar/17.20.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter P. M., Wilkinson M. C., Fitton J., Carr F. J., Brennand J., Cooper D. P., Margison G. P. Characterisation and nucleotide sequence of ogt, the O6-alkylguanine-DNA-alkyltransferase gene of E. coli. Nucleic Acids Res. 1987 Nov 25;15(22):9177–9193. doi: 10.1093/nar/15.22.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Sedgwick B. Molecular cloning of a gene which regulates the adaptive response to alkylating agents in Escherichia coli. Mol Gen Genet. 1983;191(3):466–472. doi: 10.1007/BF00425764. [DOI] [PubMed] [Google Scholar]