Abstract
Background
Many new antitumor drugs have been approved in recent years. Their side-effect profiles are distinct from those of older drugs, and their adverse effects are sometimes highly specific, particularly with respect to the skin.
Methods
This article is based on articles retrieved by a selective search in Medline and the database of the American Society of Clinical Oncology (ASCO), as well as on the authors’ personal experience.
Results
Cutaneous adverse effects are among the more common adverse effects of new antitumor drugs: they occur in up to 34% of patients receiving multikinase inhibitors, up to 90% of those receiving selective tyrosine kinase inhibitors (such as EGFR or mutant BRAF inhibitors), and up to 68% of those receiving immunotherapeutic agents (such as CTLA4 inhibitors). These adverse effects can be correlated with therapeutic benefit, but they can also be treatment-limiting because of their severity or visibility.
Conclusion
The recognition and proper management of cutaneous adverse effects is an important part of treatment with new antitumor drugs.
Increased understanding of the pathogenesis of malignant tumors has paved the way for the development of new drugs for medical tumor therapy. In addition to cytotoxic drugs, drugs with specific molecular targets (so-called “targeted therapies”) and new immunological therapeutic approaches are being implemented. Since an increasing number of patients with different types of tumors are being treated with these drugs, doctors from various disciplines are now faced with dealing with the associated adverse events.
The new mechanisms of action of these drugs can lead to clinically unusual and novel adverse events that are associated with the specific targeted structure or mechanism, representing a major therapeutic challenge. In addition to other organs, such adverse events also occur in the skin. Cutaneous adverse events are in fact often in the forefront, for example those that occur with epidermal growth factor receptor (EGFR) inhibitors and mutated BRAF gene inhibitors. These events can lead to changes in dose or treatment modality modification due to their severity, painfulness, and/or psychological discomfort. At the same time, the incidence of cutaneous adverse events can be associated with positive treatment response, as observed for EGFR inhibitors. Optimizing management of these cutaneous adverse events is therefore crucial for the implementation and success of tumor drug therapy for many patients.
This article summarizes current knowledge regarding the presentation and management of cutaneous adverse events of medical tumor therapy. It is based on the evaluation of a selective analysis of published articles from the Medline database, publications from the American Society of Clinical Oncology (ASCO), and the authors’ experience. The data relating to the frequency of cutaneous adverse events, in particular, was based on the current Summary of Product Characteristics and controlled studies. However, since few randomized controlled studies of prophylaxis and treatment of cutaneous adverse events are available, recommendations with a weaker evidence base (such as case reports and expert recommendations) have to be used.
EGFR Inhibitors
EGFR is expressed in many types of solid tumors. Its activation promotes cell proliferation, cell mobility, angiogenesis, and metastasis, but inhibits apoptosis (1).
Tumor therapy uses monoclonal antibodies directed against the extracellular EGFR domains (e.g., cetuximab and panitumumab) or low-molecular-weight, orally administered inhibitors of the intracellular EGFR tyrosine kinase (e.g., erlotinib, gefitinib, and lapatinib), either for monotherapy or in combination with chemoradiotherapy (2).
Unlike conventional chemotherapy, which interferes with RNA and DNA synthesis, EGFR inhibitors have a favorable side effect profile with low hematotoxicity. Since EGFR is also expressed in normal skin and hair follicles, three clinically relevant reaction patterns of skin toxicity are observed following EGFR inhibition, all of which are drug class effects (Figure 1) (3). Frequency, type, and severity of the cutaneous adverse events of EGFR inhibitors vary, depending not only on the therapy duration and the kind of EGFR inhibitor administered, but also on patient-related factors, such as smoker status, immune status, and pharmocogenetic factors like the K-ras mutations that have not yet been clearly defined (4).
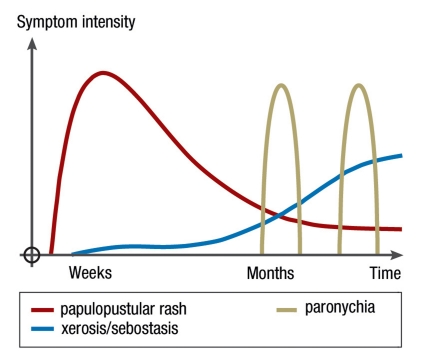
Figure 1.
Intensity and time-course of the most common cutaneous adverse events during EGFR inhibition
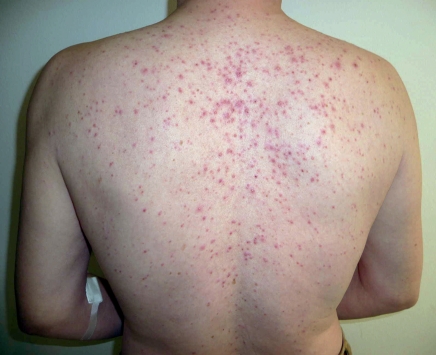
The earliest and most common cutaneous adverse events are papulopustular, follicular exanthems, often referred to as skin rashes or as „acneiform“ that, in contrast to acne, does not present with comedones (blackheads). This immunologically mediated and often stigmatizing and painful rash usually occurs initially on the face, chest, and upper back (Figure 2), but can also occur anywhere on the entirety of the skin and the hair regions of the head. The eruption slowly resides after several weeks, so that usually only moderate erythema and follicular papules remain even after long-term EGFR inhibitor therapy in the absence of dermatological therapy. The severity levels have been classified by the US National Cancer Institute (NCI) in a catalog of common toxicity criteria (CTC) (Table 1), and the progress of the rash can be evaluated using a precise dermatological severity index score (5). Incidence and severity of papulopustular rashes are associated with a better prognosis and are therefore considered to be predictive indicators for the response of a tumor to EGFR inhibitor (4). After discontinuation of EGFR inhibitor, papulopustular lesions usually heal completely. After the onset of massive inflammation, isolated cases of scarring or perifollicular xanthoma have been described (6).
Figure 2.
Papulopustular rash during treatment with the EGFR-inhibitor cetuximab
Table 1. Classification of severity of cutaneous adverse events (as defined by the National Cancer Institute Common Toxicity Criteria, version 4.03).
| Papulopustular (acneiform) rash | Maculo-papular rash | Hand-foot syndrome | |
| Grade 1 | <10% body-surface area, with or without symptoms of pruritus or tenderness | <10% body-surface area, with or without symptoms (e.g. pruritus, tightness, or burning) | Minimal skin changes (e.g., erythema, edema, or hyperkeratosis) without pain |
| Grade 2 | 10–30% body-surface area, with or without symptoms of pruritus or tenderness; with psychosocial impact; limiting instrumental activities of daily living | 10–30% body-surface area with or without symptoms (e.g. pruritus, tightness, or burning), limiting instrumental activities of daily living | Skin changes (e.g., peeling, blisters, bleeding, edema, or hyperkeratosis) with pain; limiting practical activities |
| Grade 3 | >30% body-surface area, with or without symptoms of pruritus or tenderness; limiting self-care activities of daily living; associated with local superinfection with oral antibiotics indicated | >30% body-surface area with or without associated symptoms; limiting self-care activities of daily living | Severe skin changes (e.g., peeling, blisters, bleeding, edema, or hyperkeratosis) with pain; limiting self-care activities |
| Grade 4 | Covering any percent of the body-surface area; with or without symptoms of pruritus or tenderness; associated with extensive superinfection with IV antibiotics indicated; life-threatening consequences | ||
| Grade 5 | Death |
The second group of clinically significant adverse events appear insidiously and gain clinical relevance first after 1 to 2 months of therapy for many patients; these include:
sebostasis
epidermal atrophy
xerosis cutis
itchy, dry eczema
vulnerability of the skin to fissures, especially on the fingers, toes, and heels (figure 1).
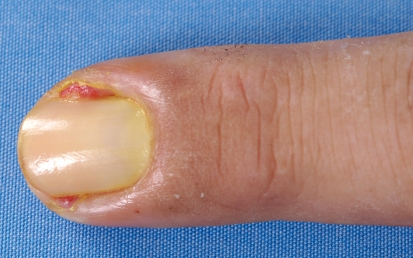
The third major group consists mainly of painful periungual inflammation (paronychia) that usually arises from the nail wall and is sometimes associated with abundant formation of granulation tissue (Figure 3). This affects only about 10% to 30% of patients, suggesting the presence of an infectious cofactor. In almost all smears, gram-positive or gram-negative bacteria, and sometimes also Candida albicans, are detectable (7).
Figure 3.
Paronychia with granulation tissue during treatment with the EGFR-inhibitor cetuximab
The prophylaxis and treatment of adverse events should be adjusted based on the experiential knowledge of the individual situation (2– 4). On initiation of an EGFR inhibitor therapy, patients should begin an emollient-based therapy with a topical preparation containing urea and should avoid activities that are mechanically damaging. The rationale for the treatment of a papulopustular rash is based on its similarity to the acne-related skin diseases, especially papulopustular acne and rosacea. Mild cases of papulopustular rashes can be treated with metronidazole or topical erythromycin containing preparations. For moderate cases, a combination of nadifloxacin cream and a topically applied glucocorticosteroid, such as prednicarbate cream, or an oral antibiotic therapy (with tetracycline, minocyline, or doxycycline), have proven effective. For the majority of patients, the papulopustular rash can be satisfactorily treated this way, resolving in either complete healing or regression to a grade 1 rash (Table 1) (4). Currently, prophylactic long-term oral antibiotic medication is not generally recommended (2– 4). Patients with severe or unusual manifestations, or skin reactions that do not respond to standard treatment, should be examined by an experienced dermatologist (4). Fissures on the finger tips or toes can be treated with tetracycline-containing ointment or hydrocolloid dressing or, where appropriate, closed with a cyanoacrylate adhesive.
Paronychia treatment is usually antiseptic, anti-inflammatory, and, depending on the findings, antibiotic or antifungal, although surgical procedures are sometimes required (7).
Multikinase, c-Kit, BRAF, and MEK inhibitors
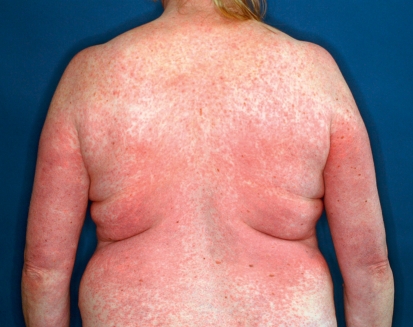
Targeted therapies can cause cutaneous adverse events that are either specifically related to the drug’s mechanism of action or non-specific. Examples of non-specific reactions are maculo-papular rash, itching (often associated with xerosis cutis), and reversible alopecia (Table 2). The trigger factor leading to maculo-papular rashes (Figure 4) can often be identified by the temporal relationship obtained from the medical history and clinical progression. It should be noted that alternative trigger factors, such as infections or other drugs, should also be considered. Test procedures have not yet been established for most drugs. Therapy is analogous to the treatment protocols for other drug-induced rashes and depends on the course and severity of the event (Table 1). Topical and systemic glucocorticosteriods can be used, while keeping in mind that dose reduction or discontinuation of the suspected trigger substance may be necessary.
Table 2. Cutaneous adverse events by targeted therapies (++ very frequent [≥ 10%], + frequent [≥ 1%], +/– occasionally [≥ 0.1%], –seldom/never [<0.1%]).
| Target structure (reference) | Main indications | Substances | Cutaneous adverse events | Frequency |
| EGFR inhibitors (2, 3, 4) | Carcinomas of lung, | Erlotinib, | Papulopustular rash, | ++ |
| pancreas, | gefitinib, | perifollicular xanthoma, | +/– | |
| gastrointestinal tract, | lapatinib, | xerosis cutis/pruritus, | ++ | |
| breast; | cetuximab, | eczema craquele, | + | |
| squamous cell carcinomas | panitumumab | fissures/rhagades, | ++ | |
| of the head and neck | paronychia, | ++ | ||
| hypertrichosis, | + | |||
| hair follicle abnormalities | + | |||
| Multikinase inhibitors (8, 9, 13, 14) | Renal cell carcinoma, | Sorafenib, | Maculo-papular rash, | ++ |
| hepatocellular carcinoma | sunitinib, | hand-foot syndrome, | ++/pazopanib + | |
| pazopanib | hair discoloration, | ++/sorafenib – | ||
| skin discoloration, | ++ (only sunitinib) | |||
| xerosis cutis/pruritus, | + | |||
| facial erythema, | + | |||
| alopecia, | + | |||
| epithelial skin tumors, | + (only sorafenib) | |||
| subungual splinter hemorrhages | + | |||
| BCR/ABL c-kit (15, 17) | Certain leukemia entities, | Imatinib, | Maculo-papular rash, | ++ |
| gastrointestinal stromal tumor | nilotinib, | periorbital edema, | ++ (only imatinib) | |
| xerosis cutis/pruritus, | + | |||
| dasatinib | light sensitivity, | + | ||
| alopecia, | + | |||
| pigmentation disorders, | + | |||
| pustules/folliculitis | +/– | |||
| Mutated BRAF (15, 16) | In clinical trials, | Vemurafenib (PLX4032, RG7204, RO5185426), GSK2118436 | Maculo-papular rash, | ++ |
| with focus on melanoma | light sensitivity, | ++ (only vemurafenib) | ||
| epithelial skin tumors, | ++ | |||
| alopecia, | + | |||
| hand-foot syndrome | + | |||
| MEK (17) | In clinical trials, | Selumetinib (AZD6244) GSK1120212CI-1040 (PD184352) | Papulopustular rash, | ++ |
| with focus on melanoma | xerosis/pruritus, | + | ||
| paronychia, | + | |||
| fissures/rhagade | + |
Source: Summary of Product Characteristics and given references
Figure 4.
Maculo-papular rash during treatment with the multikinase inhibitor sorafenib
For pruritus, possible triggers should be investigated primarily by analyzing the medical history and clinical examinations. Pruritus is often associated with xerosis cutis, which can be treated with a consistent skin moisturizing regimen.
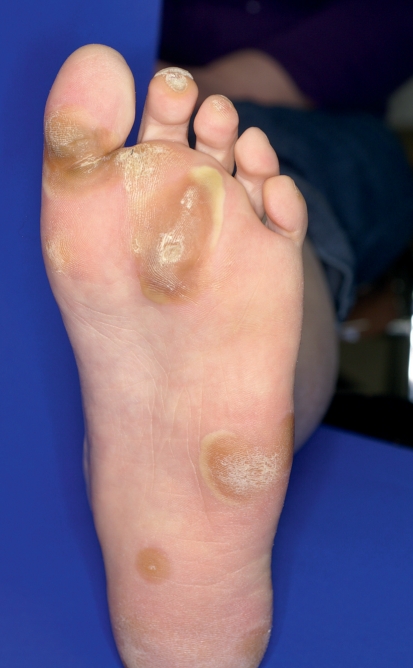
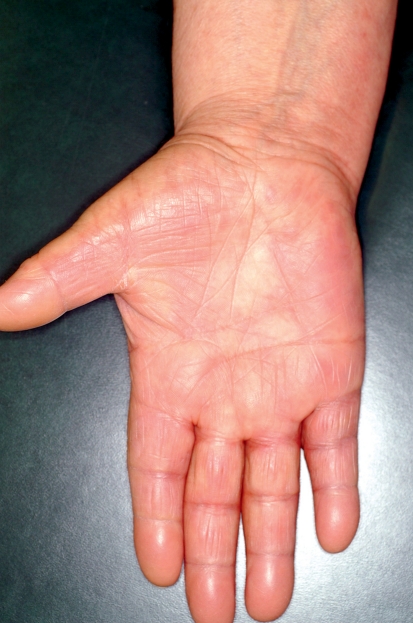
The multikinase inhibitors (MKI) sorafenib and sunitinib often provoke cutaneous side effects that are specifically associated with their mechanisms of action (Table 2). Since these drugs target different kinases, they also have different side effect spectra (8). The most common side effect for both drugs is the hand-foot syndrome (HFS), which differs from the chemotherapy-associated HFS (9). With MKI, a painful, callus-like hyperkeratosis, sometimes with blistering and inflammation in its peripheral region, often appears almost exclusively in the palmoplantar areas, especially those under mechanical stress (Figure 5). This is associated with the inhibition of vascular endothelial growth factor (VEGF) receptors. In contrast, cytostatic-associated HFS that begins within a few days after starting cytostatic therapy displays sensory symptoms (dysesthesia) on the palms and soles, with sharply demarcated erythema sometimes showing edema (Table 1; Figure 6). In grade 3 severity, further symptoms ranging from blistering and desquamation of the stratum corneum, to the development of large-area erosion and ulceration, can also be observed (Table 1) (9, 10). Additional areas that may be affected include the dorsal parts of the hands and feet, intertriginous areas, and skin in contact with tight clothes, suggesting that a possible triggering mechanism is the excretion in sweat of cytostatic or toxic metabolics (9, 10).
Figure 5.
Hand-foot syndrome during treatment with the multikinase inhibitor sorafenib
Currently, there are few recommendations from controlled studies on the management of MKI- or cytostatic-associated HFS. The guiding principles for management are based on detailed explanations about the disease and its preventive measures, such as:
treatment of previously-existing skin conditions (e.g., eczema or fungal infections)
consistent moisurizing skin care
avoidance of mechanical stress
regular removal of sweat with warm water.
Two studies using docetaxel therapy have shown that cooling of the hands and feet, perhaps through reactive vasoconstriction, significantly reduced the frequency and severity of HFS (9). Recent prospective studies have found that treating externally with urea- and lactic acid–containing preparations, or systemically administering vitamin B6, were not beneficial for the prophylaxis of capecitabine-associated HFS (11, 12). Topical glucocorticosteroid-containing preparations are recommended to treat inflammatory lesions on the hands and feet.
In severe cases of HFS, it is recommended that the dose is reduced and the associated therapy is 1 severity, it is usually possible to attempt renewed treatment (9).
Epithelial skin tumors have been described in association with sorafenib treatment; these fall within the clinical and histological spectrum of actinic keratosis, keratoacanthoma, atypical keratoacanthoma, cutaneous squamous cell carcinoma, and, in rare cases, basal cell carcinoma (13– 15). In some retrospective case series, the incidence has been estimated at 6% to 7%, but this remains to be verified by prospective studies (13). These epithelial skin tumors have a very good prognosis. Metastasis or local recurrence after surgical removal has not been described, and spontaneous regression is possible, especially after discontinuation of sorafenib therapy (13). The incidence of epithelial skin tumors is likely due to the effect of sorafenib on the tyrosine kinase BRAF, since the MKI sunitinib, which does not target BRAF, is not associated with the development of skin tumors. In contrast, studies in which the highly selective inhibitors of the mutated BRAF, vemurafenib and GSK2118436, were used in malignant melanoma therapies revealed that up to 30% of the treated patients developed epithelial skin tumors (Table 2) (15, 16). The skin tumors resulting from sorafenib and selective BRAF inhibitor treatment are clinically, histologically, and prognostically comparable. In light of this, patients treated with sorafenib or a BRAF inhibitor should undergo regular dermatological examinations, and newly developed skin changes should be removed at an early stage and histopathologically examined.
Sunitinib and other blockers of the tyrosine kinase c-Kit often lead to a hypopigmentation of the hair or skin (Table 2) (17), likely due to the effects of the receptor on the melanocytes of the hair follicles. The intense yellow discoloration of the skin with sunitinib is due to a metabolite of sunitinib that has a yellow color (8). No treatment is possible for this.
MEK inhibitors inhibit the same signaling pathways as do the EGFR inhibitors and can therefore cause similar cutaneous adverse events (Table 2) (18).
Immunotherapy with CTLA-4 blockade
Immunostimulatory cytokines, such as interferon alpha and interleukin-2, have been approved for years as a drug therapy for different tumor diseases (especially for renal cell carcinoma, malignant melanomas, and certain lymphomas and leukemias). They have a well known cutaneous side-effect profile, which includes:
drug-induced maculo-papular rash
xerosis cutis and pruritus
alopecia
triggering inflammatory skin diseases (such as psoriasis or other autoimmune dermatoses)
necrosis at the injection site (19).
Currently, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blockade plays an important role. Two anti-CTLA-4 antibodies, ipilimumab and tremelimumab, are in clinical trials for different tumor types in advanced stages, including malignant melanoma, lung carcinoma, prostate cancer, and renal cell carcinoma. Ipilimumab is at a more developed stage than tremelimumab and was approved in March 2011 in the USA, and in July 2011 in Europe, for the treatment of metastatic melanoma.
CTLA-4 is a surface protein that is expressed by activated T lymphocytes; binding of the antigen-presenting cells by the costimulatory molecules B7-1 and B7-2 leads to the down-regulation of the T cells. Under physiological conditions, this mechanism is important for preventing an excessive T-cell–mediated immune response. In tumor therapy, an enhanced T-cell–mediated immune response is desirable for tumor therapy, provided that it is directed against the structures of the tumor cells. Thus, the two antibodies mentioned above were developed for CTLA-4 blockade in order to enhance the T-cell–mediated anti-tumor immune response. Both substances have a similar spectrum of side effects. Intervening with the regulatory mechanisms of the T-cell–mediated immune response often leads to excessive immune reactions with respect to autoimmune-related infections, in particular enterocolitis (with the clinical symptom of diarrhea), hepatitis (which is usually first recognized by increased levels of liver enzymes), and hypophysitis (with the clinical symptom of headache) (20– 22). These side effects are often severe and may necessitate therapy interruption or discontinuation. Comprehensive algorithms have been developed to manage these side effects (23).
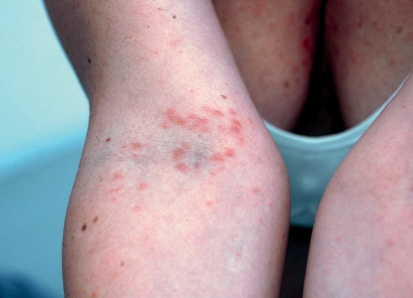
Adverse effects on the skin usually occur in the form of a rash that usually has a macular, maculo-papular, or urticarial morphology (Figure 7) and is often accompanied by severe itching (20, 24). For ipilimumab, these skin lesions have been observed in up to 68% of all treated patients; however, fewer than 5% of these patients develop a severity of grade 3 (Table 1) (22, 23). The rash usually occurs 3 to 4 weeks after administration of ipilimumab but can also occur either very early in therapy or after therapy has ended, and it gets worse after each ipilimumab dose (23). It usually is self-limiting within the first 2 to 3 months of treatment, is well tolerated using topical treatment with glucocorticosteroids and antipruritic compounds (such as ThesitTM), and seldom leads to therapy interruption or discontinuation (23). The prophylactic application of topical steroidal cream is therefore not recommended.
Figure 7.
Papular urticarial rash during treatment with the anti-CTLA-4-antibody ipilimumab
Conclusions
New medical tumor therapies are frequently associated with cutaneous adverse events. Early intervention is critical in treating them. However, unified therapy standards and guidelines based on studies are generally lacking. With early diagnosis and appropriate treatment based on available evidence, cutaneous adverse effects should not limit the tumor therapy for most patients. Successful management requires not only the efforts of the primary attending physician but also an intense interdisciplinary collaboration involving dermatologists, who are experts on the classification and treatment of cutaneous adverse events.
Figure 6.
Hand-foot syndrome (palmar-plantar erythrodysesthesia) during treatment with pegylated liposome-encapsulated doxorubicin
Key Messages.
Cutaneous adverse events (AE) in anti-tumor drug therapy are common (up to 34% for multikinase inhibitors, 90% for EGFR inhibitors, and 68% for anti-CTLA-4 antibodies).
Cutaneous AE in anti-tumor drug therapy may correlate to tumor response.
Cutaneous AE in anti-tumor drug therapy may be clinically unusual and severe.
Cutaneous AE in anti-tumor drug therapy require special knowledge in diagnosis and management.
Cutaneous AE in anti-tumor drug therapy generally are not therapy-limiting if properly managed.
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD.
Footnotes
Conflict of interest statement
Prof. Gutzmer received lecture honoraria, consulting fees, meeting and continuing education participation fees, travel and accommodation reimbursement, and grant support from Roche Pharma, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Merck Serono, MSD, Hermal Almirall-Hermal, Celgene, Lilly, Amgen, and EISAI.
Prof. Wollenberg received lecture honoraria, consultant fees, and grant support from Amgen, Merck Serono, and Roche Pharma.
Prof. Ugurel received lecture honoraria, meeting and continuing education participation fees, travel and accommodation reimbursement, and grant support from Amgen, Bristol-Myers Squibb, Novartis, Merck Serono, and Roche.
Prof. Homey received consultant fees, lecture honoraria, and grant support from Merck Serono and Roche Pharma.
Prof. Ganser received lecture honoraria and consultant fees from Galen, Genzyme, Amgen, and Novartis.
Prof. Kapp received consultant fees, meeting and continuing education participation fees, travel and accommodation reimbursement, and grant support from Bassilea, Alk Abello, GlaxoSmithKline, Novartis, Celgene, Lilly, Roche Pharma, EISAI, Jansen, and Genentech.
References
- 1.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 2.Potthoff K, Hofheinz R, Hassel JC, et al. Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann Oncol. 2011;22:524–535. doi: 10.1093/annonc/mdq387. [DOI] [PubMed] [Google Scholar]
- 3.Wollenberg A, Kroth J, Hauschild A, Dirschka T. Hautreaktionen unter EGFR-Inhibitoren - Klinik und Management. Dtsch Med Wochenschr. 2010;135:149–154. doi: 10.1055/s-0029-1244831. [DOI] [PubMed] [Google Scholar]
- 4.Gutzmer R, Becker JC, Enk A, et al. Management kutaner Nebenwirkungen von FGFR-Inhibitoren: Empfehlungen eines deutschen Expertengremiums für den primär behandelnden Arzt. J Dtsch Dermatol Ges. 2011;9:195–203. [Google Scholar]
- 5.Wollenberg A, Moosmann N, Klein E, Katzer K. A tool for scoring of acneiform skin eruptions induced by EGF receptor inhibition. Exp Dermatol. 2008;17:790–792. doi: 10.1111/j.1600-0625.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- 6.Eames T, Kroth J, Flaig MJ, Ruzicka T, Wollenberg A. Perifollicular xanthomas associated with epidermal growth factor receptor inhibitor therapy. Acta Derm Venereol. 2010;90:202–203. doi: 10.2340/00015555-0792. [DOI] [PubMed] [Google Scholar]
- 7.Eames T, Grabein B, Kroth J, Wollenberg A. Microbiological analysis of epidermal growth factor receptor inhibitor therapy-associated paronychia. J Eur Acad Dermatol Venereol. 2010;24:958–960. doi: 10.1111/j.1468-3083.2009.03516.x. [DOI] [PubMed] [Google Scholar]
- 8.Wollenberg A, Staehler M, Eames T. Kutane Nebenwirkungen der Multikinaseinhibitoren Sorafenib und Sunitinib. Hautarzt. 2010;61:662–667. doi: 10.1007/s00105-010-1942-7. [DOI] [PubMed] [Google Scholar]
- 9.Degen A, Alter M, Schenck F, et al. Das Hand-Fuß-Syndrom als Nebenwirkung der medikamentösen Tumortherapie - Klassifikation und Management. J Dtsch Dermatol Ges. 2010;8:652–661. [Google Scholar]
- 10.Farr KP, Safwat A. Palmar-plantar erythrodysesthesia associated with chemotherapy and its treatment. Case Rep Oncol. 2011;4:229–235. doi: 10.1159/000327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang YK, Lee SS, Yoon DH, et al. Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: results of a randomized, double-blind, placebo-controlled study. J Clin Oncol. 2010;28:3824–3829. doi: 10.1200/JCO.2010.29.1807. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Qin R, Menon SP, et al. Placebo-controlled trial to determine the effectiveness of a urea/lactic acid-based topical keratolytic agent for prevention of capecitabine-induced hand-foot syndrome: North Central Cancer Treatment Group Study N05C5. J Clin Oncol. 2010;28:5182–5187. doi: 10.1200/JCO.2010.31.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 14.Degen A, Satzger I, Voelker B, et al. Does basal cell carcinoma belong to the spectrum of sorafenib-induced epithelial skin cancers? Dermatology. 2010;221:193–196. doi: 10.1159/000317081. [DOI] [PubMed] [Google Scholar]
- 15.Robert C, Arnault JP, Mateus C. RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol. 2011;23:177–182. doi: 10.1097/CCO.0b013e3283436e8c. [DOI] [PubMed] [Google Scholar]
- 16.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahler KC, Hauschild A. Hautveränderungen durch „targeted therapies“ bei onkologischen Patienten: Kutane Nebenwirkungen zielgerichteter Therapie bei onkologischen Patienten. Hautarzt. 2009;60:433–440. doi: 10.1007/s00105-009-1754-9. [DOI] [PubMed] [Google Scholar]
- 18.Schad K, Baumann CK, Zipser MC, et al. Mitogen-activated protein/extracellular signal-regulated kinase kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clin Cancer Res. 2010;16:1058–1064. doi: 10.1158/1078-0432.CCR-09-1766. [DOI] [PubMed] [Google Scholar]
- 19.Trefzer U, Hofmann M, Sterry W. Kutane Nebenwirkungen klinisch relevanter Zytokintherapien. Dtsch Med Wochenschr. 2003;128:1782–1787. doi: 10.1055/s-2003-41708. [DOI] [PubMed] [Google Scholar]
- 20.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahler KC, Hauschild A. Behandlung und Nebenwirkungsmanagement des metastasierten Melanoms mit CTLA-4-Antikörpern. J Dtsch Dermatol Ges. 2011;9:277–286. doi: 10.1111/j.1610-0387.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 24.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166–172. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]