Abstract
The microbial composition and in vitro fermentation characteristics of human milk oligosaccharides (HMO), lacto-N-neotetraose (LNnT), a 2:1 mixture of polydextrose (PDX) and galactooligosaccharides (GOS), and short-chain fructooligosaccharides (scFOS) by pooled ascending colonic microbiota from 9- and 17-d-old formula-fed (FF) and sow-reared (SR) piglets were assessed. pH change and gas, SCFA, and lactate production were determined after 0, 2, 4, 8, and 12 h of incubation. In most donor groups, the pH change was greater for scFOS fermentation and lower for PDX/GOS than for other substrates. LNnT fermentation produced larger amounts of gas, total SCFA, acetate, and butyrate than did the other substrates, whereas HMO and scFOS produced higher amounts of propionate and lactate, respectively. In general, pH change, total SCFA, acetate, and propionate production were greater in pooled inoculum from FF and 9-d-old piglets, whereas SR-derived inoculum produced higher amounts of butyrate and lactate after 4 h fermentation. Gut microbiota were assessed by 16S ribosomal RNA V3 gene denaturing gradient gel electrophoresis analysis and real-time qPCR. Microbial structures differed among the 4 groups before fermentation, with higher counts of Bifidobacterium in SR piglets and higher counts of Clostridium cluster IV, XIVa, and Bacteroides vulgatus in FF piglets. Lactobacillus counts were higher in 9-d-old piglets than in 17-d-old piglets, regardless of diet. Bifidobacterium, Bacteroides, and clostridial species increased after 8 and 12 h fermentation on most substrates. In summary, piglet diet and age affect gut microbiota, leading to different fermentation patterns. HMO have potential prebiotic effects due to their effects on SCFA production and microbial modulation.
Introduction
Human milk is the optimal nutrient source for infants and contains a remarkable array of functional components. A unique aspect of human milk compared with that of other species is its high content and complexity of HMO8, which averages 20 g/L in colostrum and 5–10 g/L in mature milk (1, 2). Most HMO are composed of variable combinations of 5 monosaccharides: glucose, galactose, fucose, N-acetylglucosamine, and N-acetylneuraminic acid. More than 200 distinct oligosaccharides have been identified (3). The major components of HMO are 2′ fucosyl-lactose, lacto-N-tetraose, and LNnT (2, 4).
HMO are resistant to host hydrolases, and >90% of HMO are undigested and arrive intact in the large intestine (5). HMO have been shown to modulate proliferation, differentiation, and apoptosis of intestinal cells in vitro (6, 7) and act as decoys to prevent adherence of pathogens to host epithelial cells (8, 9). Another contribution of HMO to intestinal health is in its prebiotic effect on infant gut microbiota. HMO can be metabolized by single gut bacteria, such as Bifidobacterium infantis (10), Bifidobacterium longum subsp., and Bacteroides vulgatus (11), and can stimulate the growth of beneficial bacteria, e.g., bifidobacteria, during in vitro fermentation using fecal microbiota from weaned FF infants (12). Microbial populations change during infancy (13), which also will influence the fermentation characteristics of HMO. Therefore, studying the effects of diet and age on neonatal gut microbiota and fermentation activity, especially of dietary carbohydrates such as HMO, is critical for evaluating the putative beneficial effect of HMO on intestinal health.
The neonatal piglet is an ideal model for human infant nutrition research due to similarities in anatomy, physiology, and gastrointestinal tract metabolism (14, 15). Furthermore, the fermentation profiles of some types of oligosaccharides have been examined in pig models (14, 16). Hence, the purpose of the present study was to use the piglet model to investigate the effects of diet (SR vs. FF) and age (9 and 17 d old) on microbial population and the fermentation characteristics and the prebiotic properties of pooled HMO; LNnT, a predominant HMO; and several commercially used prebiotics, such as PDX, GOS, and scFOS, by pooled ascending colonic microbiota. The changes in pH, gas, and SCFA production were monitored in an in vitro batch culture system. Microbial population changes were analyzed by 16S rRNA gene V3 region DGGE analysis and group-specific qPCR.
Materials and Methods
Substrates and HMO isolation
The substrates used for the in vitro fermentation experiment were as follows: crude HMO isolated from pooled preterm human milk (mean gestational age: 24 ± 2.3 wk) provided by Paula Meier (Rush University, Chicago, IL), LNnT (Abbott Nutrition), a 2:1 combination of PDX (Litesse; Danisco) and GOS (Vivinal GOS; FrieslandCampina Domo) (PDX/GOS), and scFOS (GTC Nutrition). HMO were isolated by using an adaptation of the method described by Gnoth et al. (5). Briefly, pooled preterm human milk (2 L) was defatted by centrifugation (Beckman J-6B centrifuge; Beckman) at 3000 × g at 4°C for 20 min. The aqueous phase was mixed with 2 volumes of 95% ethanol at 4°C overnight and then centrifuged at 15,000 × g at 0°C for 30 min to precipitate crude protein. The supernatant was evaporated to remove ethanol and concentrated by lyophilization. The concentrated supernatant (100 mL) was applied to a column (100 cm × 50 mm i.d.; GE Healthcare) containing medium-grade Sephadex G25 (Sigma) to remove protein and lactose. The oligosaccharide-containing fractions collected from the Sephadex G25 run were applied to a protein A affinity column (Protein A-Sepharose CL4B; Sigma) to remove immunoglobulin. Then the eluent was reapplied on the same Sephadex G25 column twice for further purification. Finally, the crude HMO were run through Detoxin-Gel Endoxin Removing Gel (Thermo Fisher Scientific) to eliminate endotoxin. The composition of the isolated HMO was analyzed by HPLC-chip time-of-flight MS (17).
Donors and sample collection
We used 9- and 17-d-old FF (FF9: n = 4; FF17: n = 4) and SR (SR9: n = 5; SR17: n = 5) piglets as donors in this study. Individual piglets in each group were used to analyze the effects of diet and age on microbial population before fermentation. Pooled ascending colonic contents from each group were used for fermentation study. The mean body weights were 2.79 ± 0.17 kg and 5.27 ± 0.37 kg for the 9- and 17-d-old piglets, respectively. Piglets were raised as follows: late gestation sows were obtained from the Swine Research Center at the University of Illinois, Urbana, and were transported to the Edward R. Madigan Laboratory animal facility and housed in farrowing crates. All piglets were vaginally delivered. SR groups remained with the sow for the duration of the study. FF piglets were colostrum-deprived and housed in metabolism cages individually in environmentally controlled rooms (25°C) with a 12-h light/dark cycle. To provide passive immunity, FF piglets received 5 mL maternal serum per kg BW by oral gavage at birth, and 8, 24, and 36 h postpartum, and were fed daily a nonmedicated sow milk replacer formula (183 g/L) (Advance Liquiwean; Milk Specialties) (Supplemental Table 1). The feeding rate increased as follows: from 250 mL/kg BW on d 1, 270 mL/kg BW on d 2, 300 mL/kg BW on d 3, 320 ml/kg BW on d 4, 340 mL/kg BW on d 5, to 360 mL/kg BW on d 6 and thereafter. Piglets were weighed each morning.
On d 9 and 17, 3 piglets from each donor group were killed at the same time for the in vitro fermentation experiment, and samples were processed within 20 min of the time of death to maintain maximum viability of the anaerobic bacteria. Piglets were sedated with Telazol (tiletamine HCl and zolazepam HCl, 3.5 mg/kg BW; Pfizer Animal Health), and then killed by an intravenous injection of sodium pentobarbital (Fatal Plus, 72 mg/kg BW; Vortech Pharmaceuticals). Fresh ascending colonic contents were collected immediately in plastic bags, which were expressed to remove excess air, and transferred to the laboratory within 5 min for the fermentation experiment. In addition, a small amount (~1 g) of colonic contents of each individual piglet was placed into a 2-mL tube and stored in liquid nitrogen for further microbial composition analysis.
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana (protocol 09185).
In vitro fermentation model
Gas production and pH measurement.
Before the fermentation experiments, 80 mg (DM basis) of each substrate were weighted in triplicate into 16-mL Balch tubes. The composition of the in vitro medium has been previously described (18) with the exclusion of the SCFA mix. An aliquot (7 mL) of medium was aseptically transferred into Balch tubes, capped with butyl rubber stoppers, and sealed with aluminum caps. The tubes were stored at 4°C overnight to hydrate the substrates, and prewarmed in a 37°C water bath for 30 min before inoculation.
Ascending colonic contents from 3 individual piglets from each group were pooled as sources of inocula. A pooled sample better represents microbial diversity across treatments and minimizes the animal variation within groups. In addition, pooling samples can reduce the lab labor, cost, and analysis complexity, which is more realistic in in vitro studies in which multiple treatments or substrates are tested. This has been widely used in both human and animal in vitro research (19–21). Equal amounts (~3.3 g) of contents from each pig were mixed together and diluted 1:10 (wt:vol) in an anaerobic dilution solution (22). The samples were blended in a Waring blender (Waring Products) under a CO2 stream, filtered through 4 layers of sterile cheesecloth, and sealed in 125 mL serum bottles under CO2. Appropriate oligosaccharides and blank tubes were aseptically inoculated with 1 mL diluted contents and incubated at 37°C with periodic mixing for 0, 2, 4, 8, and 12 h. At each time point of incubation, the tubes were removed from the 37°C incubator and processed immediately for pH, gas production, and SCFA production measurements. Gas production was determined by fluid displacement (water with 5% HCl and resazurin) at equal pressure using a manometer (23). Total gas production was calculated as gas production (mL) from substrates minus gas production (mL) from the blank divided by substrate weight expressed on a DM basis. The pH was measured using a standard pH meter (Denver Instrument Co). The pH change was calculated as the pH values of the tubes containing the substrates minus the pH values of the blank tube. A 3-mL subsample was taken from each tube for SCFA analysis. Another 1-mL sample was stored at −80°C for further microbial analysis.
SCFA and lactate analyses.
Sample preparation for SCFA and lactate analyses was performed as previously described (18). The SCFA concentrations were analyzed by a Hewlett-Packard 5890A Series ΙΙ gas chromatograph and a glass column (180 cm × 4 mm i.d.), which was packed with 10% SP-1200/1% H3PO4 on 80/100 mesh Chromosorb WAW (Superlco). Oven temperature, detector temperature, and injector temperature were 125°, 175°, and 180°C, respectively. SCFA production was calculated as SCFA concentrations of substrate-containing tubes minus the SCFA content of blank tubes divided by substrate weight expressed on a DM basis. Lactate concentration was analyzed using a spectrophotometric method adapted from Barker and Summerson (24).
Microbial analysis.
The ascending colonic contents from individual piglets (FF9 and FF17: n = 4/group; SR9 and SR17: n = 5/group) were used to analyze the structural differences in gut microbiota among piglet groups before fermentation using 16S rRNA V3 gene DGGE analysis. The pooled samples from 3 piglets from each group were used to study the changes in pooled microbial populations during fermentation using DGGE and group-specific qPCR.
Bacterial DNA was extracted by using a bead beating method followed by purification with a QIAamp stool mini-kit (Qiagen). One milliliter of frozen pooled sample was thawed on ice and centrifuged at 9000 × g for 5 min. Cell pellets were suspended in lysis buffer (ASL buffer) in a QIAamp kit. Triplicate tubes were pooled and transferred into 2-mL microfuge tubes with glass matrix E (MP Biomedical) and agitated for 30 s in a bead beater at a speed of 6 meters per second (Fastprep; MP Biomedical). For individual piglet samples, 200 mg of frozen sample was weighed into the tube with glass matrix E, and 1 mL ASL buffer added, then shaken in the bead beater as described above. The DNA purification procedures were used according to manufacturer’s instruction. The concentration of extracted DNA was measured by using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies).
DGGE.
The bacterial universal V3 region of the 16S rRNA gene was amplified as previously described (25). To minimize heteroduplex formation during PCR amplification, a reconditioning PCR (i.e., 5 additional PCR cycles using fresh reagent mixture) (26) was performed before DGGE analysis. The PCR products were analyzed by electrophoresis on 1% (wt:vol) agarose gel in 0.2 mol/L ethidium bromide to verify a single product of the expected size was obtained. Nucleic acid concentrations were measured by using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies).
Parallel DGGE was performed with a Dcode System apparatus (Bio-Rad) following the protocol described by the manufacturer. PCR products of 16S rRNA gene V3 regions were electrophoresed in 8% (wt:vol) polyacrylamide gels. The DGGE gel contained a linear 28 to 55% denaturant gradient (100% denaturant corresponds to 7 mol/L urea and 40% deionized formamide). The same amount of DNA (250 ng) from each sample was parallel loaded in each lane of the DGGE gel. Electrophoresis was performed in 1× Tris-acetate-EDTA buffer at a constant voltage of 200 V and a temperature of 60°C for 240 min. The DNA bands were stained by SYBR green I (Sigma) and photographed with a Gel Doc XR+ System (Bio-Rad).
The DGGE bands that increased in intensity during fermentation were excised from original gels and identified by sequencing as previously described (25). The nearest neighbors of the sequences were found by RDPquery (27) by using the RDP II online database. The sequences of excised DGGE bands are available in the GenBank database under accession numbers JN810694–JN810701. The DGGE marker was made by the mixture of randomly selected bands.
Real-time qPCR.
The density changes in specific bacterial groups during fermentation were analyzed by real-time qPCR. Concentrations of total bacteria (28), Lactobacillus spp. (29), Bifidobacterium spp. (30), and Clostridium cluster IV and XIVa (31) were measured at 0-, 8-, and 12-h fermentation time points. One DGGE band (band_5) whose intensity increased during fermentation also was verified quantitatively by qPCR (32). The primer sequences and annealing temperatures are listed in Supplemental Table 2. qPCR was performed on an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems) with the use of a Power SYBR Green PCR Master mix (Applied Biosystems). Triplication was performed for each sample. The data were analyzed by SDS v2.3 software (Applied Biosystems).
Statistical analysis
In vitro fermentation and qPCR data were analyzed by using the SAS Proc Mixed procedure (SAS Institute) with a Tukey adjustment. For qPCR data, the log-transformed bacterial counts (log10 copies/g content) were used for statistical analysis. Treatments included substrate, donor, and time of fermentation. Therefore, substrate, donor, time of fermentation, substrate × donor, substrate × time, donor × time, and substrate × donor × time were used in the statistical model. For in vitro data, all treatment least-squares means were compared with each other. For qPCR data, the bacterial densities at 8 and 12 h were compared only with those at 0 h for each substrate. Model assumptions of normality and homogeneity of variance of data were tested before running Proc Mixed (33). The results did not indicate a violation of the normality and the equal variance of the residuals assumption. Least-squares means were reported along with the pooled SEM for all response criteria. A probability of P ≤ 0.05 was considered as significant.
The DGGE banding patterns were processed by Quantity One software (Bio-Rad). To analyze the structural differences of gut microbiota among the 4 pig groups, PCA of DGGE profiles was applied using Matlab (The Mathworks). PCA is a multivariate projection method that uses an orthogonal transformation to convert a set of possibly correlated variables into a set of uncorrelated variables (called principal components), and visualizes the relations between variables (34). It has been widely used in gut microbial ecology analysis (25, 34).
Results
HMO composition.
The oligosaccharide composition of the isolated HMO was characterized by HPLC-Chip time-of-flight MS analysis (Supplemental Table 3). Eighty-one different types of oligosaccharides were detected, of which 45 were well defined. Fucosylated oligosaccharides accounted for 56.1% of total HMO. Sialylated or both fucosylated and sialylated oligosaccharides comprised 31.6 and 12.4%, respectively. Since lacto-N-tetraose and LNnT are the most abundant HMO, accounting for 16.1% of total HMO, LNnT was used as a representative of HMO in this study.
Gas production.
Gas production from the 4 substrates did not differ at 2 h fermentation (Table 1). LNnT fermentation resulted in a greater (P < 0.05) volume of gas at 8 and 12 h in the FF9 and SR17 groups than did the other substrates. In the other donor groups (FF17, SR9), LNnT was the higher gas producer at 8 and 12 h compared with other substrates, but the values were not significantly different from that of scFOS. PDX/GOS produced less gas compared with the other substrates after 12 h fermentation (P < 0.05), with the exception of the SR17 group in which PDX/GOS was slightly lower than the HMO treatment without a significant difference. Gas production resulting from scFOS and HMO fermentation was intermediate, but scFOS was higher than HMO at 12 h in SR groups (P < 0.05).
TABLE 1.
Gas production after 2, 4, 8, and 12 h in vitro fermentation of oligosaccharides with pooled ascending colonic contents from 9- and 17-d-old formula-fed and sow-reared piglets1
| Fermentation |
||||
| Substrate | 2h | 4h | 8h | 12h |
| mL/mg substrate DM | ||||
| FF9 | ||||
| scFOS | 0.00 | 34.0b | 94.8b | 142b |
| PDX/GOS | 3.45 | 56.3a | 89.6b | 115c |
| LNnT | 5.33 | 70.7a | 129a | 157a |
| HMO | 0.00 | 27.9b | 96.0b | 136b |
| SR9 | ||||
| scFOS | 10.4 | 35.8b | 115a | 204a |
| PDX/GOS | 5.40 | 58.8a | 85.5b | 137c |
| LNnT | 9.97 | 45.7ab | 126a | 191a |
| HMO | 0.00 | 34.2b | 107a | 160b |
| FF17 | ||||
| scFOS | 0.70 | 13.5b | 99.6a | 143a |
| PDX/GOS | 17.6 | 44.2a | 76.2b | 116b |
| LNnT | 11.8 | 41.3a | 101a | 149a |
| HMO | 10.9 | 23.7ab | 90.8ab | 131ab |
| SR17 | ||||
| scFOS | 0.00 | 2.09ab | 45.2b | 140b |
| PDX/GOS | 0.00 | 16.1ab | 65.0b | 114c |
| LNnT | 0.00 | 21.3a | 86.8a | 174a |
| HMO | 0.00 | 0.84b | 58.7b | 119c |
| SEM | 3.39 | |||
Values are least-squares means and pooled SEM, = 3. Within donor group, labeled means at a time without a common letter differ, P < 0.05.The effects of substrate, donor, and time of fermentation and their interactions (substrate × time, substrate × donor, donor × time, and substrate × time × donor) on fermentation were significant (P < 0.0001) for gas production. DM, dry matter; FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; GOS, galactooligosaccharides; HMO, human milk oligosaccharides; LNnT, lacto-N-neotetraose; PDX, polydextrose; scFOS, short-chain fructooligosaccharides; SR9, 9-d-old sow-reared piglets; SR17, 17-d-old sow-reared piglets.
Gas production did not differ among donor groups at 2 h fermentation. The pooled microbiota of the SR17 group produced the lower volume of gas across substrates at 4 and 8 h fermentation compared with other donor groups (with scFOS at 8 h, PDX/GOS at 4 h, LNnT at 4 h, HMO at 4 and 8 h; P < 0.05). The SR9 group produced a higher amount of gas at 12 h (P < 0.05), regardless of substrate, whereas the other 3 donor groups were not different at 12 h.
pH change.
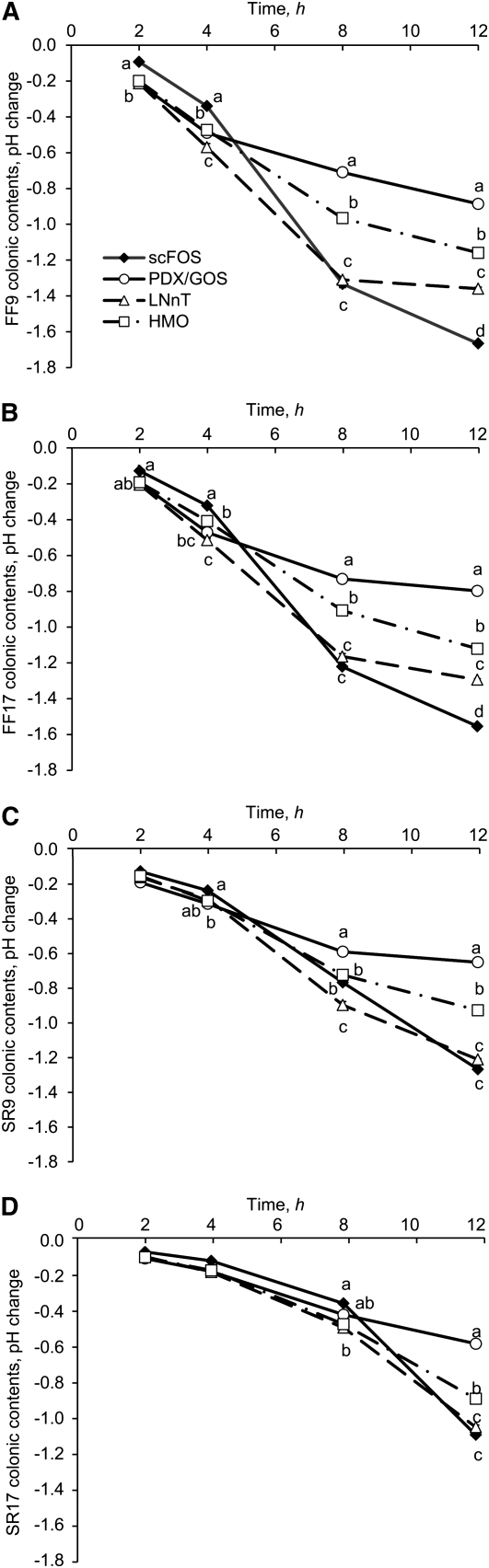
Before fermentation, the pH value among substrates did not differ (6.51 ± 0.01); however, at 12 h it decreased to 5.29 ± 0.04 (mean ± SEM). The scFOS substrate resulted in a greater decrease in pH compared with other substrates after 12 h fermentation by inocula from the FF groups (P < 0.05) (Figure 1). This was the case for the SR group, but the results for scFOS did not differ from those for LNnT. The change in pH was lower for PDX/GOS at 12 h (P < 0.05), followed by HMO and LNnT.
FIGURE 1.
Changes in pH after 2, 4, 8, and 12 h in vitro fermentation of oligosaccharides with pooled ascending colonic contents from FF9 (A), FF17 (B), SR9 (C), and SR17 (D) donor groups. The effects of substrate, donor, and time of fermentation and their interactions (substrate × time, substrate × donor, donor × time, and substrate × time × donor) on fermentation were significant (P < 0.0001) for pH change. Values are least-squares means (pooled SEM = 0.012); n = 3. For each donor group, labeled means at a time without a common letter differ, P < 0.05. FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; GOS, galactooligosaccharides; HMO, human milk oligosaccharides; LNnT, lacto-N-neotetraose; PDX, polydextrose; scFOS, short-chain fructooligosaccharides; SR9, 9-d-old sow-reared piglets; SR17, 17-d-old sow-reared piglets.
With the exception of results at 2 h fermentation, the decrease in pH for all substrates was greater for the pooled inoculum from FF than that from SR groups (P < 0.0001). The decrease in pH was greater in the 9-d-old piglets than in the 17-d-old piglets for the SR groups (SR9 > SR17; P < 0.0001). With the exception of results for PDX/GOS, the pH change in the FF9 group also was greater than in the FF17 group (P < 0.001).
SCFA and lactate production.
The substrate fermentation by ascending colonic microbiota resulted in SCFA and lactate production (Table 2). LNnT fermentation produced a higher amount of total SCFA followed by HMO and scFOS at most time points in the FF groups (P < 0.05). LNnT was also the higher total SCFA producer in the SR groups but was not significantly different from HMO at most time points. PDX/GOS fermentation resulted in a lower amount of total SCFA production at 8 and 12 h than did other substrates (P < 0.05), with the exception of the SR17 group in which values were not different from those with scFOS.
TABLE 2.
SCFA and lactate production after 2, 4, 8, and 12 h in vitro fermentation of oligosaccharides with pooled ascending colonic contents from 9 and 17 d-old formula-fed and sow-reared piglets1
| Acetate |
Propionate |
Butyrate |
Total SCFA |
Lactate |
||||||||||||||||
| Substrate | 2 h | 4 h | 8 h | 12 h | 2 h | 4 h | 8 h | 12 h | 2 h | 4 h | 8 h | 12 h | 2 h | 4 h | 8 h | 12 h | 2 h | 4 h | 8 h | 12 h |
| mmol/g substrate DM | ||||||||||||||||||||
| FF9 | ||||||||||||||||||||
| scFOS | 0.43c | 1.25d | 3.61c | 5.01b | 0.11 | 0.35 | 1.08a | 1.53a | 0.02 | 0.05 | 0.18a | 0.27a | 0.57b | 1.67c | 4.93c | 6.89b | 0.04 | 0.08 | 0.23a | 0.29a |
| PDX/GOS | 0.74b | 1.85c | 2.95d | 3.73c | 0.18 | 0.43 | 0.74c | 0.91c | 0.01 | 0.06 | 0.14ab | 0.22ab | 0.94ab | 2.36b | 3.87d | 4.91c | 0.06 | 0.08 | 0.02d | 0.00c |
| LNnT | 1.15a | 2.78a | 5.50a | 6.08a | 0.18 | 0.39 | 0.84b | 1.02b | 0.03 | 0.06 | 0.20a | 0.28a | 1.36a | 3.27a | 6.57a | 7.44a | 0.06 | 0.12 | 0.16b | 0.11b |
| HMO | 1.04a | 2.33b | 4.73b | 5.82a | 0.14 | 0.41 | 1.02a | 1.53a | 0.00 | 0.03 | 0.11b | 0.17b | 1.19a | 2.78b | 5.90b | 7.60a | 0.06 | 0.10 | 0.09c | 0.03c |
| SR9 | ||||||||||||||||||||
| scFOS | 0.06 | 0.24b | 1.29c | 2.34c | 0.06 | 0.17b | 0.89a | 1.57a | 0.01 | 0.03 | 0.18c | 0.67b | 0.14 | 0.45c | 2.41c | 4.67b | 0.13 | 0.26b | 0.44a | 0.32a |
| PDX/GOS | 0.12 | 0.41b | 1.09c | 1.74d | 0.05 | 0.27a | 0.70b | 0.94d | 0.00 | 0.06 | 0.18c | 0.44c | 0.17 | 0.75bc | 2.00d | 3.18c | 0.15 | 0.35a | 0.28c | 0.02c |
| LNnT | 0.36 | 0.89a | 2.58a | 3.28a | 0.04 | 0.22ab | 0.86a | 1.11c | 0.01 | 0.08 | 0.37a | 0.88a | 0.41 | 1.20a | 3.87a | 5.34a | 0.12 | 0.24b | 0.37b | 0.26b |
| HMO | 0.30 | 0.77a | 2.18b | 2.89b | 0.06 | 0.25ab | 0.90a | 1.33b | 0.01 | 0.07 | 0.29b | 0.69b | 0.37 | 1.10ab | 3.42b | 4.99ab | 0.12 | 0.28b | 0.31c | 0.02c |
| FF17 | ||||||||||||||||||||
| scFOS | 0.34b | 0.98c | 2.95c | 3.95c | 0.08 | 0.26b | 0.96c | 1.48b | 0.01 | 0.04 | 0.18a | 0.29a | 0.43 | 1.30c | 4.14c | 5.81c | 0.02 | 0.04 | 0.16a | 0.23a |
| PDX/GOS | 0.49ab | 1.32b | 2.16d | 2.79d | 0.10 | 0.42a | 0.79d | 0.98d | 0.01 | 0.05 | 0.14a | 0.21b | 0.60 | 1.81b | 3.13d | 4.04d | 0.03 | 0.05 | 0.02c | 0.00c |
| LNnT | 0.70a | 1.85a | 4.33a | 5.15a | 0.09 | 0.38a | 1.14b | 1.36c | 0.01 | 0.05 | 0.18a | 0.32a | 0.80 | 2.30a | 5.72a | 6.92a | 0.02 | 0.04 | 0.08b | 0.09b |
| HMO | 0.67a | 1.42b | 3.48b | 4.24b | 0.09 | 0.41a | 1.42a | 1.93a | 0.00 | 0.00 | 0.05b | 0.10c | 0.77 | 1.85b | 5.02b | 6.37b | 0.02 | 0.04 | 0.04bc | 0.01c |
| SR17 | ||||||||||||||||||||
| scFOS | 0.04 | 0.13b | 0.69c | 1.60c | 0.02 | 0.07ab | 0.29b | 0.65b | 0.00 | 0.02 | 0.11c | 0.36c | 0.06 | 0.23 | 1.10b | 2.64b | 0.04 | 0.06 | 0.13b | 0.34a |
| PDX/GOS | 0.07 | 0.25b | 0.84c | 1.47c | 0.04 | 0.13a | 0.43a | 0.66b | 0.00 | 0.03 | 0.15bc | 0.42c | 0.11 | 0.42 | 1.44b | 2.58b | 0.05 | 0.10 | 0.17ab | 0.05c |
| LNnT | 0.22 | 0.60a | 1.75a | 3.11a | 0.00 | 0.01b | 0.33b | 0.67b | 0.01 | 0.06 | 0.22a | 0.77a | 0.24 | 0.66 | 2.32a | 4.60a | 0.04 | 0.10 | 0.19a | 0.23b |
| HMO | 0.11 | 0.39ab | 1.42b | 2.74b | 0.03 | 0.13a | 0.46a | 0.89a | 0.00 | 0.01 | 0.18ab | 0.61b | 0.14 | 0.54 | 2.08a | 4.29a | 0.04 | 0.07 | 0.11b | 0.03c |
| SEM | 0.05 | 0.02 | 0.01 | 0.07 | 0.01 | |||||||||||||||
Values are least-squares means and pooled SEM, = 3. Within donor group, labeled means at a time without a common letter differ, P < 0.05. The effects of substrate, donor, and time of fermentation and their interactions (substrate × time, substrate × donor, donor × time, and substrate × time × donor) on fermentation were significant (P < 0.0001) for SCFA and lactate production. DM, dry matter; FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; GOS, galactooligosaccharides; HMO, human milk oligosaccharides; LNnT, lacto-N-neotetraose; PDX, polydextrose; scFOS, short-chain fructooligosaccharides; SR9, 9-d-old sow-reared piglets; SR17, 17-d-old sow-reared piglets.
Acetate was the major SCFA product from all substrate fermentations. Acetate production was greater for LNnT fermentation at all time points (P < 0.05), followed by HMO. The least acetate production occurred with PDX/GOS at 8 and 12 h (P < 0.05) except in the SR17 group. HMO and scFOS produced greater amounts of propionate at 12 h; HMO was higher yet in 17-d-old pigs (FF17, SR17; P < 0.05). PDX/GOS produced less propionate at 8 and 12 h for most donors than did the other substrates (FF9, FF17, and SR9; P < 0.05). Butyrate production was variable among substrates and donors. In FF donors, LNnT and scFOS fermentations produced a higher amount of butyrate (P < 0.05), whereas HMO fermentation resulted in a lower amount at all time points (P < 0.05). In SR donors, LNnT was the highest producer of butyrate (P < 0.05), followed by HMO, whereas the lowest producer of butyrate was PDX/GOS in the SR9 group and scFOS in the SR17 group. Lactate production decreased after 8 h fermentation of most substrates. scFOS was the higher (P < 0.05) producer at 8- and 12-h time points, followed by LNnT. HMO and PDX/GOS were the lower producers (P < 0.05) at 8 and 12 h.
Pooled digesta derived from FF piglets produced greater amounts of total SCFA and acetate than that from SR pigs after 2 h fermentation (P < 0.05), whereas SR groups produced more butyrate and lactate at 8 and 12 h (P < 0.05). Pooled inoculum of 9-d-old piglets produced more total SCFA, acetate, and lactate than did that of 17-d-old piglets, except for propionate production, as a result of LNnT and HMO fermentation, which was higher in FF17 than in FF9 piglets (P < 0.05).
Microbial population changes.
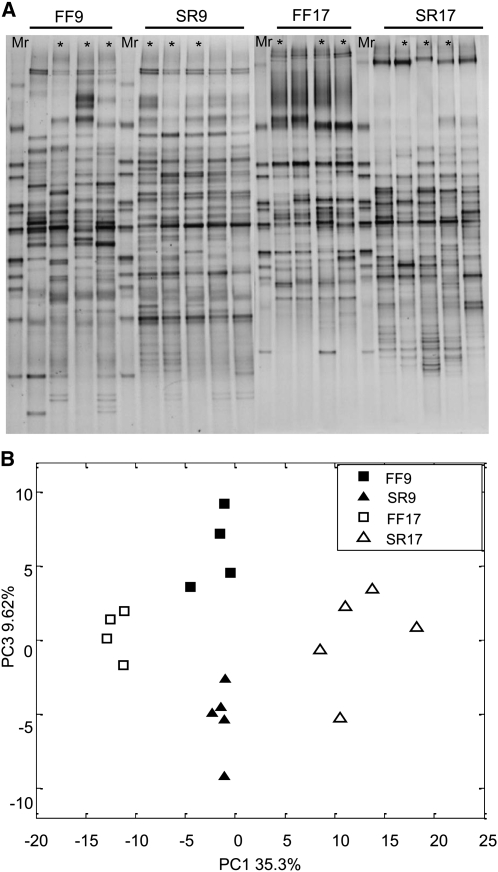
The differences in ascending colonic microbiota among the four piglets groups before fermentation were assessed by 16S rRNA V3 gene DGGE and qPCR. The PCA score plot of DGGE patterns showed that piglets fed the same diet and of the same age were clustered together and were significantly separated from the other groups (Figure 2), indicating that gut microbial communities varied by diet and age of piglets. qPCR results showed that the pooled microbiota of FF piglets had higher amounts of Clostridium cluster IV (P < 0.05) and XIVa (P < 0.05) and B. vulgatus (P < 0.05) than did SR piglets at 9 d of age. This was the case for 17-d-old piglets in which the amounts of 3 bacterial groups were higher in the FF groups (P < 0.05), whereas SR pigs had more bifidobacteria (P < 0.05) (Table 3). The amount of total bacteria was higher in FF than in SR piglets at 9 d of age (P < 0.05), although this was not the case in 17-d-old piglets. The concentration of Lactobacillus spp. was comparable in FF and SR groups. Compared with d 17, 9-d-old pigs had more Clostridium cluster IV and XIVa in the FF group (P < 0.05), whereas SR piglets had more Bifidobacterium spp. and B. vulgatus (P < 0.05). The concentration of Lactobacillus spp. was higher in 9-d-old piglets than in 17-d-old piglets, regardless of diet (P < 0.05).
FIGURE 2.
16S rRNA V3 gene DGGE profile (A) and PCA (B) of ascending colonic microbiota from 9- and 17-d-old formula-fed and sow-reared piglets before in vitro fermentation. The first principal component (PC1) explained 35.3% of the variation and shows the difference between FF17 and SR17 groups; the third principal component (PC3) explained 9.62% of the variation and shows the difference between FF9 and SR9. *Indicates piglets used for in vitro fermentation. DGGE, denaturing gradient gel electrophoresis; FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; Mr, DGGE marker that was made by the mixture of randomly selected bands; PCA, principal components analysis; SR9, 9-d-old sow-reared piglets; SR17, 17-d-old sow-reared piglets.
TABLE 3.
Bacterial densities before (0 h) and after 8 and 12 h in vitro fermentation of oligosaccharides with pooled ascending colonic contents from 9- and 17-d-old formula-fed and sow-reared piglets1
| 16S total bacteria |
Lactobacillus spp. |
Bifidobacterium spp. |
Clostridium cluster IV |
Clostridium cluster XIVa |
Bacteroidesvulgatus |
|||||||||||||
| Substrate | 0 h | 8 h | 12 h | 0 h | 8 h | 12 h | 0 h | 8 h | 12 h | 0 h | 8 h | 12 h | 0 h | 8 h | 12 h | 0 h | 8 h | 12 h |
| log10 copies/g content | ||||||||||||||||||
| FF9 | ||||||||||||||||||
| Baseline | 10.9a | 9.56a | 4.59c | 10.3a | 10.8a | 9.84a | ||||||||||||
| scFOS | 11.2* | 11.1 | 9.75 | 9.62 | 5.27* | 4.97 | 10.6 | 10.2 | 11.5* | 11.0 | 10.7* | 10.7* | ||||||
| PDX/GOS | 11.3* | 11.2* | 9.81 | 9.46 | 5.18* | 5.07 | 10.5 | 10.2 | 11.4* | 11.1 | 10.6* | 10.7* | ||||||
| LNnT | 11.2* | 11.5* | 9.48 | 9.92 | 5.25* | 5.30* | 10.3 | 10.8* | 11.3* | 11.9* | 10.6* | 10.8* | ||||||
| HMO | 11.2* | 11.2* | 9.75 | 9.79 | 5.22* | 5.18* | 10.5 | 10.5 | 11.2* | 11.5* | 10.6* | 10.6* | ||||||
| SR9 | ||||||||||||||||||
| Baseline | 10.6b | 9.77a | 8.12a | 9.47c | 9.98b | 9.28b | ||||||||||||
| scFOS | 11.0* | 11.1* | 10.1 | 9.76 | 8.59 | 8.96* | 9.82* | 9.63 | 10.5* | 10.8* | 9.83* | 10.1* | ||||||
| PDX/GOS | 11.0* | 11.1* | 10.2* | 9.70 | 8.57 | 8.61 | 10.1* | 9.65 | 10.7* | 10.5* | 9.76* | 10.5* | ||||||
| LNnT | 11.0* | 10.8 | 9.73 | 9.67 | 9.06* | 9.27* | 9.95* | 9.79 | 10.8* | 10.8* | 9.81* | 9.84* | ||||||
| HMO | 10.9 | 10.9* | 9.73 | 9.81 | 8.77* | 8.69* | 9.77 | 9.75 | 10.7* | 10.9* | 9.94b | 9.88* | ||||||
| FF17 | ||||||||||||||||||
| Baseline | 10.5b | 8.79b | 4.60c | 9.93b | 10.2b | 10.0a | ||||||||||||
| scFOS | 10.5 | 10.0* | 9.05 | 8.46 | 4.85 | 4.81 | 9.91 | 9.34* | 10.8* | 9.96 | 10.5* | 9.86 | ||||||
| PDX/GOS | 10.6 | 10.6 | 8.78 | 8.96 | 4.95 | 5.00 | 10.0 | 10.1 | 10.6* | 10.7* | 10.4* | 10.3 | ||||||
| LNnT | 10.9* | 10.5 | 8.91 | 8.58 | 5.24* | 5.04 | 10.2 | 9.90 | 10.9* | 10.4 | 10.6* | 10.2 | ||||||
| HMO | 11.0* | 11.1* | 8.91 | 9.25* | 5.11 | 5.16* | 10.1 | 10.4* | 10.7* | 11.0* | 10.5* | 10.9* | ||||||
| SR17 | ||||||||||||||||||
| Baseline | 10.5b | 8.95b | 7.57b | 9.50c | 9.94b | 8.63c | ||||||||||||
| scFOS | 11.0* | 11.1* | 9.32 | 9.39* | 8.52* | 8.85* | 9.86* | 9.94* | 10.9* | 11.3* | 9.96* | 10.0* | ||||||
| PDX/GOS | 10.7* | 10.9* | 9.17 | 9.21 | 8.27* | 8.12* | 9.98* | 9.97* | 10.7* | 10.8* | 9.90* | 10.1* | ||||||
| LNnT | 10.8* | 10.8 | 9.04 | 8.95 | 8.35* | 8.63* | 9.89* | 9.83 | 10.7* | 10.7* | 9.91* | 9.85* | ||||||
| HMO | 10.8 | 10.5 | 9.31 | 8.93 | 8.43* | 8.38* | 9.65 | 9.31 | 10.8* | 10.5* | 9.81* | 10.4* | ||||||
| SEM | 0.03 | 0.07 | 0.04 | 0.08 | 0.05 | 0.11 | 0.04 | 0.07 | 0.03 | 0.08 | 0.03 | 0.07 | ||||||
Values are least-squares means and pooled SEM, = 3 or 15 (baseline, mean of all groups). *Different from baseline, P < 0.05. Baseline means in a column without a common letter differ, P < 0.05.The effects of substrate, donor, and time of fermentation and their interactions (substrate × time, substrate × donor, donor × time, and substrate × time × donor) on fermentation were significant (P < 0.02) for each bacterial group density, except for the interaction of substrate × time in Bifidobacterium spp. qPCR data (P = 0.11). FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; GOS, galactooligosaccharides; HMO, human milk oligosaccharides; LNnT, lacto-N-neotetraose; PDX, polydextrose; scFOS, short-chain fructooligosaccharides; SR9, 9-d-old sow-reared piglets; SR17, 17-d-old sow-reared piglets.
The microbial population changed during fermentation. The global microbial profiles assessed by DGGE were not dramatically shifted by 12 h of fermentation; however, the intensities of several bands changed for most substrates. Several bands that increased in density were identified by sequencing (Table 4). In the FF9 group, 2 bands increased in density with LNnT, HMO, and scFOS fermentation, which are related to uncultured Anaerovibrio sp. and Collinsella aerofaciens with 100 and 98% sequence similarity, respectively. Three bands homologous with C. aerofaciens (98% similarity), Clostridium ramosum (100% similarity), and Ruminococcus obeum (100% similarity) increased in the FF17 group during PDX/GOS, LNnT, and scFOS fermentation. Only 1 band increased in intensity with HMO fermentation in the SR9 group, and was related to C. aerofaciens (98% similarity). In the SR17 group, the intensity of 2 bands identified as B. vulgatus (100% similarity) and Clostridium clostridioforme (100% similarity) increased for all 4 substrate fermentations, and 2 bands related to Escherichia coli (100% similarity) and uncultured Oscillibacter sp. (100% similarity) changed only with HMO fermentation.
TABLE 4.
Selected 16S ribosomal RNA V3 DGGE bands that increased in density during in vitro fermentation and their nearest relatives as determined by sequence analysis1
| Band no. | Donors (substrate)2 | Closest relatives (Genbank accession no.) | Similarity | Taxonomic group |
| % | ||||
| 1 | FF9 (scFOS, LNnT, HMO) | Uncultured Anaerovibrio sp. (FJ683186) | 100 | Firmicutes/ Clostridia |
| 2 | FF9,FF17 (all substrates) SR9 (HMO) | Collinsella aerofaciens (T) (AB011816) | 98 | Actinobacteria |
| 3 | FF17 (scFOS, PDX/GOS, LNnT) | Clostridium ramosum (EU869233) | 100 | Clostridium cluster XVIII |
| 4 | FF17 (scFOS) | Ruminococcus obeum A2–162 (FP929054) | 100 | Clostridium cluster XIVa |
| 53 | SR17 (all substrates) | Bacteroides vulgatus (AB510712) | 100 | Bacteroidetes |
| 6 | SR17 (all substrates) | Clostridium clostridioforme (HM008264)/Clostridium sp. MLG080-1(AY653233) | 100 | Clostridium cluster XIVa |
| 7 | SR17 (HMO) | Escherichia coli (X80728) | 100 | Proteobacteria |
| 8 | SR17 (HMO) | Uncultured Oscillibacter sp. (DQ326974) | 100 | Clostridium cluster IV |
DGGE, denaturing gradient gel electrophoresis; FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; GOS, galactooligosaccharides; HMO, human milk oligosaccharides; LNnT, lacto- -neotetraose; PDX, polydextrose; scFOS, short-chain fructooligosaccharides; SR9, 9-d-old sow-reared piglets; SR17, 17 d-old sow-reared piglets.
Represents donor groups and substrates in which DGGE band density increased during the fermentation.
This band was quantitatively verified by qPCR.
The density changes in several bacterial groups of pooled microbiota during fermentation were measured by qPCR. The densities of total bacteria, Bifidobacterium spp., Clostridium cluster XIVa, and B. vulgatus were greater (P < 0.05) at 8 and/or 12 h fermentation for most substrates compared with baseline (Table 3). The abundance of Clostridium cluster IV increased in SR pigs, except during HMO fermentation. Lactobacillus spp. concentration did not change during fermentation.
Discussion
Carbohydrate fermentation in human intestine principally occurred in the ascending colon (35). However, due to ethical issues and limitations regarding invasive procedures in humans, it is difficult to investigate the fermentation patterns of carbohydrates in the human large intestine. Thus, an appropriate animal model is essential. Piglets are considered an optimal model in pediatric nutritional research due to their remarkable similarities in gastrointestinal development, anatomy, physiology, and metabolism to human infants (14, 15). Most of the bacterial phylotypes identified in the pig gastrointestinal tract had high similarity (>97%) to known species isolated from human gut, indicating the resemblance of microbiota in humans and pigs (36). In addition, the oligosaccharide composition of porcine milk (37) is more similar to human milk than is bovine milk, which is the basis for most infant formulas. Therefore, these similarities render the neonatal piglet a more appropriate surrogate for human infants compared with other animal models, e.g., mice and rats (15). In the present study, we used a neonatal piglet model to investigate the fermentation properties of HMO and other prebiotics in vitro.
The potential for HMO to be fermented has been studied by using several isolated bacterial species (10, 11, 38); however, little data exist on the fermentation patterns of a complex mixture of HMO and/or a dominant component of HMO by a complex gut microbial community (12). In this study, the fermentation of HMO by the pooled ascending colonic microbiota of neonatal piglets resulted in a pH decrease and gas and SCFA production, indicating that they were fermentable. Among the 4 tested oligosaccharides, LNnT, a predominant HMO component consisting of 4 monosaccharides, was the highest gas, total SCFA, acetate, and butyrate producer for most donor groups. The mixture of long- and short-chain oligosaccharides (PDX/GOS) resulted in the smallest pH decline and the lowest gas and SCFA production during fermentation. These results are consistent with previous studies showing that short-chain oligosaccharides are more rapidly fermented and produce more gas and SCFA than do oligosaccharides of longer chain length or mixtures of short- and long-chain molecules (19, 21). However, it was surprising that the isolated HMO complex, which was composed of almost 100 carbohydrates varying from 3 to 32 monosaccharide units (10), was highly fermentable as reflected in greater total SCFA production than occurred for scFOS and PDX/GOS, and with the highest amount of propionate production among all substrates by 17-d-old piglets. These data are the first to show the high capacity for SCFA production from complex HMO, and a single HMO component, by neonatal gut microbial communities. Furthermore, these findings suggest the beneficial effect of HMO on neonatal intestinal health due to the well-known functions of SCFA, including serving as an energy source for epithelial cells and modulating colonocyte proliferation (39). Although the high gas production of LNnT fermentation seems to be an adverse effect in human infants, it could be attenuated by reducing the dose or blending with some long-chain oligosaccharides, e.g., PDX, or by replacing with a HMO mixture, which was the intermediate gas producer and a high propionate producer.
Selected oligosaccharides are considered prebiotics that can promote the growth and/or activities of beneficial gut bacteria. scFOS, PDX, and GOS are widely recognized prebiotics and have been added to infant formula (16, 19, 21). HMO also have been shown to stimulate Bifidobacterium spp. growth when fermented by individual bifidobacteria species (10) or by whole microbial communities (12). In our study, fermentations of all substrates increased the densities of Bifidobacterium spp. in both FF and SR donor groups, which confirms their bifidogenic effects. However, no DGGE band related to Bifidobacterium spp. was detected among the bands that increased in density during fermentation, which may be due to the differences in the specificity of the PCR primers (universal 16S rRNA V3 gene primers in DGGE vs. Bifidobacterium genus–specific primers in qPCR) and the detection limit of DGGE, because only those with >1% of total bacteria could be detected (40).
Bacteroides spp. and clostridia are 2 major commensal bacterial groups in mammalian intestine that have the capacity to degrade plant polysaccharides (41) and stimulate butyrate production (42), respectively. Marcobal et al. (11) and Shen et al. (12) showed that both of these bacterial groups were enriched during fermentation of HMO. Our results showed the increase in B. vulgatus and species belonging to Clostridium cluster IV and XIVa during HMO fermentation, which supports their conclusion that bifidobacteria are not the exclusive utilizer of HMO among the thousands of commensal bacteria in the gut (11). Within the clostridial species that increased during substrate fermentation, 1 DGGE band, which was related to uncultured Oscillibacter spp., was exclusively enriched during HMO fermentation in the SR17 donor group (Table 4). Oscillibacter spp. is a newly isolated, valerate-producing bacterial group (43), which is more dominant in SR than in FF piglet intestinal microbiota (44). We also observed more valerate production from HMO fermentation by the SR17 group (data not shown). Those results indicate that HMO may stimulate the growth of valerate-producing bacteria, such as Oscillibacter, in the gut. However, the relation between this genus and HMO remains to be studied due to the lack of physiologic and phylogenetic information on Oscillibacter.
Lactobacillus spp. also are beneficial bacteria in the infant gut; however, no significant change was detected using either DGGE or qPCR during fermentation of most substrates. A previous study reported that Lactobacillus spp. did not utilize complex HMO but could metabolize HMO components, such as lactose, glucose, and N-acetylglucosamine (38). The substrates tested in this study had ≥4 sugar units, suggesting that the carbohydrate hydrolysis ability of Lactobacillus spp. might be restricted to mono- and disaccharides (38).
The composition of gut microbiota is influenced by various factors such as diet and age. In the present study, we showed that the FF and SR piglets had distinctive gut microbial profiles at 9 and 17 d of age. The pooled microbiota of SR piglets had more bifidobacteria, whereas FF piglets had more Clostridium cluster IV and XIVa and B. vulgatus counts, which supports previous reports of piglet gut microbiota enumeration by pyrosequencing (44). These results also agree with those that previously showed the predominance of bifidobacteria in the gut microbiota of human breast-fed infants (45) and clostridia and Bacteroides in FF infants (46, 47). Although diet may be a primary factor influencing the microbiota, the environment in which the SR piglets were constantly exposed to the microbes from the sow and her feces, and the early life stress associated with maternal separation of FF piglets (48), also should be considered when interpreting the microbial differences among the feeding groups. No significant difference in Lactobacillus spp. count existed between the pooled digesta of FF and SR piglets, although some studies showed the prevalence of Lactobacillus spp. in the breast-fed infant gut (47). It is interesting, however, that the distribution of Lactobacillus spp. was age-related: 9-d-old piglets had higher counts than did 17-d-old piglets, regardless of diet. A previous study reported that the number of Lactobacillus spp. decreased over time in the piglet intestine (49). Moreover, the age effect also was observed in the clostridia population, but only in pooled microbiota from FF piglets (FF9 > FF17). The interactions of diet, age, environment, and microbiota should be studied further due to the relatively small sample size and pooling samples in the present study and should be taken into account when making conclusions about the fermentability of various oligosaccharides by neonates.
The differences in gut microbiota associated with diet and age led to distinctive substrate fermentation patterns. In this study, fermentation by the pooled microbiota of FF piglets resulted in greater pH decreases and more total SCFA production than was the case for SR piglets, and as was the case for 9- compared with 17-d-old piglets. These differences might be due to the higher amount of total bacteria and/or more diverse microbiota populations in FF and 9-d-old piglets. The qPCR results showing that FF and 9-d-old piglets had higher amounts of universal bacterial 16S rRNA genes confirmed our speculation. Because the number of 16S rRNA gene copies varies among different bacterial species, we measured the number of Cpn60 genes, a universal single copy gene encoding 60 kDa chaperonin in all bacteria (50), before fermentation. The number of Cpn60 gene copies was higher in FF and 9-d-old piglets than in SR and 17-d-old piglets (P < 0.05, data not shown), suggesting that they had higher total bacterial counts. In addition, some researchers reported that FF infants had a higher diversity of gut microbiota than did breast-fed infants (47, 51). In contrast, SR piglets produced more lactate than did FF piglets, which was consistent with more Bifidobacterium spp. counts in SR groups because lactate is the end-product of bifidobacterial metabolism. However, the higher butyrate production of SR piglets seems to conflict with the lower concentrations of Clostridium cluster IV and XIVa. Although numerous butyrate-producing bacteria belong to these 2 clusters (42), the ability of other Clostridium clusters, such as I, XV, and XVI, to produce butyrate needs to be taken into account.
In conclusion, previous studies comparing in vitro fermentation of HMO have focused on pure cultures (10, 11) or feces from weaned FF infants (12). Our results showed that the composition of the gut microbiota was influenced by piglet diet and age, which led to different carbohydrate fermentation patterns. Therefore, it is important to consider that the potential prebiotic action of HMO is not constant because infant microbiota changes over the first year of life (13). This study also provided important data for comparative analysis with human infant gut microbiota. Furthermore, it is the first study, to our knowledge, to thoroughly assess the fermentation properties of HMO by mixed ascending colonic microbiota in a piglet model. In comparing 3 commonly used oligosaccharides with HMO, the latter produced more SCFA than did the prebiotics and stimulated beneficial gut bacteria, such as bifidobacteria and B. vulgatus, which supports their potential to promote neonatal intestinal health.
Supplementary Material
Acknowledgments
The authors thank Carlito Lebrilla and Shuai Wu of the Department of Chemistry at the University of California, Davis, for performing the HMO compositional analysis and Roderick Mackie of the Department of Animal Science at the University of Illinois for use of the DGGE equipment. We also thank Abbott Nutrition for providing the LNnT. S.M.D and G.C.F designed the study; M.L., L.L.B., X.C, M.W., and T.B.K. conducted the experiments; M.L. analyzed and interpreted the data; M.L. and S.M.D drafted the manuscript; M.S.K and G.C.F reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grant R01 HD061929 to S.M.D.
Supplemental Tables 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: BW, body weight; DGGE, denaturing gradient gel electrophoresis; DM, dry matter; FF, formula-fed; FF9, 9-d-old formula-fed piglets; FF17, 17-d-old formula-fed piglets; GOS, galactooligosaccharides; HMO, human milk oligosaccharides; LNnT, lacto-N-neotetraose; PCA, principal components analysis; PDX, polydextrose; rRNA, ribosomal RNA: scFOS, short-chain fructooligosaccharides; SR, sow-reared; SR9, 9-d-old sow-reared piglets; SR17, 17-d-old sow-reared piglets.
Literature Cited
- 1.Coppa GV, Gabrielli O, Pierani P, Catassi C, Carlucci A, Giorgi PL. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics. 1993;91:637–41 [PubMed] [Google Scholar]
- 2.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 3.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–80 [DOI] [PubMed] [Google Scholar]
- 4.Niñonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, Freeman SL, Mills DA, German JB, Grimm R, Lebrilla CB. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J Agric Food Chem. 2008;56:618–26 [DOI] [PubMed] [Google Scholar]
- 5.Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–20 [DOI] [PubMed] [Google Scholar]
- 6.Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr. 2008;99:462–71 [DOI] [PubMed] [Google Scholar]
- 7.Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr. 2009;101:1306–15 [DOI] [PubMed] [Google Scholar]
- 8.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58 [DOI] [PubMed] [Google Scholar]
- 9.Bergner DW, Kuhlenschmidt TB, Hanafin WP, Firkins LD, Kuhlenschmidt MS. Inhibition of rotavirus infectivity by a neoglycolipid receptor mimetic. Nutrients. 2011;3:228–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol. 2006;72:4497–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58:5334–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Q, Tuohy KM, Gibson GR, Ward RE. In vitro measurement of the impact of human milk oligosaccharides on the faecal microbiota of weaned formula-fed infants compared to a mixture of prebiotic fructooligosaccharides and galactooligosaccharides. Lett Appl Microbiol. 2011;52:337–43 [DOI] [PubMed] [Google Scholar]
- 13.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herfel TM, Jacobi SK, Lin X, Fellner V, Walker DC, Jouni ZE, Odle J. Polydextrose enrichment of infant formula demonstrates prebiotic characteristics by altering intestinal microbiota, organic acid concentrations, and cytokine expression in suckling piglets. J Nutr. 2011;141:2139–45 [DOI] [PubMed] [Google Scholar]
- 15.Guilloteau P, Zabielski R, Hammon HM, Metges CC. Nutritional programming of gastrointestinal tract development: is the pig a good model for man? Nutr Res Rev. 2010;23:4–22 [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen LL, Knudsen K, Jensen B. In vitro fermentation of fructo-oligosaccharides and transgalacto-oligosaccharides by adapted and unadapted bacterial populations from the gastrointestinal tract of piglets. Anim Feed Sci Technol. 2004;116:225–8 [Google Scholar]
- 17.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smiricky-Tjardes MR, Flickinger EA, Grieshop CM, Bauer LL, Murphy MR, Fahey GC., Jr In vitro fermentation characteristics of selected oligosaccharides by swine fecal microflora. J Anim Sci. 2003;81:2505–14 [DOI] [PubMed] [Google Scholar]
- 19.Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS, Fahey GC., Jr In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J Agric Food Chem. 2009;57:1354–61 [DOI] [PubMed] [Google Scholar]
- 20.Hernot DC, Boileau TW, Bauer LL, Swanson KS, Fahey GC., Jr In vitro digestion characteristics of unprocessed and processed whole grains and their components. J Agric Food Chem. 2008;56:10721–6 [DOI] [PubMed] [Google Scholar]
- 21.Vester Boler BM, Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS, Fahey GC Jr. Carbohydrates blended with polydextrose lower gas production and short-chain fatty acid production in an in vitro system. Nutr Res. 2009;29:631–9 [DOI] [PubMed] [Google Scholar]
- 22.Bryant MP, Burkey LA. Cultural methods and some characteristics of some of the more numerous groups of bacteria in the bovine rumen. J Dairy Sci. 1953;36:205–17 [Google Scholar]
- 23.Campbell JM, Fahey GC., Jr Psyllium and methylcellulose fermentation properties in relation to insoluble and soluble fiber standards. Nutr Res. 1997;17:619–29 [Google Scholar]
- 24.Barker R, Summerson J. The colorimetric determination of lactic acid in biological meterials. Nutr Res. 1941;17:619–29 [Google Scholar]
- 25.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Zhang M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA. 2008;105:2117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson JR, Marcelino LA, Polz MF. Heteroduplexes in mixed-template amplifications: Formation, consequence and elimination by 'reconditioning PCR’. Nucleic Acids Res. 2002;30:2083–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyszynski G. RDPquery: a java program from the Sapelo Program Microbial Observatory for automatic classification of bacterial 16S rRNA sequences based on ribosomal database project taxonomy and Smith-Waterman alignment. 2006. [cited November 14, 2010]. Available from: http://simo.marsci.uga.edu/public_db/rdp_query.htm
- 28.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–66 [DOI] [PubMed] [Google Scholar]
- 29.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–77 [DOI] [PubMed] [Google Scholar]
- 30.Kok RG, De Wall A, Schut F, Welling GW, Weenk G, Hellingwerf KJ. Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl Environ Microbiol. 1996;62:3668–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004;70:7220–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang RF, Cao WW, Cerniglia CE. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl Environ Microbiol. 1996;62:1242–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott RL, Longnecker MT. An introduction to statistical methods and data analysis. 5th ed Pacific Grove, CA: Duxbury Press; 2001.. p. 758–85 [Google Scholar]
- 34.Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007;62:142–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Moller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol. 2002;68:673–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao N, Ochonicky KL, German JB, Donovan SM, Lebrilla CB. Structural determination and daily variations of porcine milk oligosaccharides. J Agric Food Chem. 2010;58:4653–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwab C, Ganzle M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides. FEMS Microbiol Lett. 2011;315:141–8 [DOI] [PubMed] [Google Scholar]
- 39.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43 [DOI] [PubMed] [Google Scholar]
- 40.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, Henrissat B, Coutinho PM, Minx P, Latreille P, et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007;5:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–9 [DOI] [PubMed] [Google Scholar]
- 43.Iino T, Mori K, Tanaka K, Suzuki K, Harayama S. Oscillibacter valericigenes gen. nov., sp. nov., a valerate-producing anaerobic bacterium isolated from the alimentary canal of a Japanese corbicula clam. Int J Syst Evol Microbiol. 2007;57:1840–5 [DOI] [PubMed] [Google Scholar]
- 44.Poroyko V, White JR, Wang M, Donovan S, Alverdy J, Liu DC, Morowitz MJ. Gut microbial gene expression in mother-fed and formula-fed piglets. PLoS ONE. 2010;5:e12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243:141–7 [DOI] [PubMed] [Google Scholar]
- 46.Balmer SE, Wharton BA. Diet and faecal flora in the newborn: breast milk and infant formula. Arch Dis Child. 1989;64:1672–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–7 [DOI] [PubMed] [Google Scholar]
- 48.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7 [DOI] [PubMed] [Google Scholar]
- 49.Konstantinov SR, Awati AA, Williams BA, Miller BG, Jones P, Stokes CR, Akkermans AD, Smidt H, de Vos WM. Post-natal development of the porcine microbiota composition and activities. Environ Microbiol. 2006;8:1191–9 [DOI] [PubMed] [Google Scholar]
- 50.Hill JE, Seipp RP, Betts M, Hawkins L, Van Kessel AG, Crosby WL, Hemmingsen SM. Extensive profiling of a complex microbial community by high-throughput sequencing. Appl Environ Microbiol. 2002;68:3055–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards CA, Parrett AM. Intestinal flora during the first months of life: new perspectives. Br J Nutr. 2002;88 Suppl 1:S11–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.