Abstract
This study evaluated the effects of 2 levels of intake of high-amylose maize type 2 resistant starch (HAM-RS2) on insulin sensitivity (SI) in participants with waist circumference ≥89 (women) or ≥102 cm (men). Participants received 0 (control starch), 15, or 30 g/d (double-blind) of HAM-RS2 in random order for 4-wk periods separated by 3-wk washouts. Minimal model SI was assessed at the end of each period using the insulin-modified i.v. glucose tolerance test. The efficacy evaluable sample included 11 men and 22 women (mean ± SEM) age 49.5 ± 1.6 y, with a BMI of 30.6 ± 0.5 kg/m2 and waist circumference 105.3 ± 1.3 cm. A treatment main effect (P = 0.018) and a treatment × sex interaction (P = 0.033) were present. In men, least squares geometric mean analysis for SI did not differ after intake of 15 g/d HAM-RS2 (6.90 × 10−5 pmol−1 · L−1 × min−1) and 30 g/d HAM-RS2 (7.13 × 10−5 pmol−1 · L−1 × min−1), but both were higher than after the control treatment (4.66 × 10−5 pmol−1 · L−1 × min−1) (P < 0.05). In women, there was no difference among the treatments (overall least squares ln-transformed mean ± pooled SEM = 1.80 ± 0.08; geometric mean = 6.05 × 10−5 pmol−1 · L−1 × min−1). These results suggest that consumption of 15–30 g/d of HAM-RS2 improves SI in men. Additional research is needed to understand the mechanisms that might account for the treatment × sex interaction observed.
Introduction
RS6 is defined as the fraction of starch resistant to pancreatic α-amylase hydrolysis in the small intestine that therefore passes undigested to the large bowel, where it can act as a substrate for microbial fermentation (1, 2). The digestibility of starch is influenced by processing, how it is cooked and stored, as well as its inherent physiochemical properties, such as variations in granular structure and the ratio of starch types present (amylose and amylopectin). Uncooked high-amylose starches are more resistant to enzymatic hydrolysis than high-amylopectin starches; however, cooking can increase the digestibility of amylose (3, 4).
The main sources of RS in the diet include breads, cereals, pastas, and vegetables (5). Recent estimates indicate Americans consume ~4.9 g/d of RS (5), whereas estimated intakes in 10 European countries ranged from 3.2 to 5.7 g/d (6). However, such levels are far below intakes previously demonstrated to confer health benefits (>20 g/d), including improved bowel health, increased nutrient absorption, and improved glycemic and insulinemic responses (7, 8). The metabolic effects of commercially available sources of RS have been studied in animals and also investigated in humans at intakes of 10–60 g RS/d (9). Results from studies with a granular, type 2 RS from HAM-RS2 made from corn with an amylose content >50% suggest beneficial effects of consumption on outcomes related to large bowel health, such as changes in colonic cellular events and fecal variables such as reduced pH, bulking, and microbial flora shifts as well as systemic metabolic effects on glycemia and insulinemia (10–13).
More recent work has demonstrated improved SI with consumption of HAM-RS2 (14–16). For example, Robertson et al. (16) showed that insulin sensitivity assessed by mathematical modeling of data from a meal tolerance test improved by 33% relative to control following consumption of 30 g/d HAM-RS2 for 4 wk in healthy men and women. Similar results have been shown in insulin-resistant men and women following 40 g/d HAM-RS2 consumption over a 12-wk period (14). These results have important implications for human health, because insulin resistance (i.e., impaired SI) is a central pathophysiologic feature of metabolic syndrome, a cluster of risk factors for the development of atherosclerotic cardiovascular disease and diabetes mellitus.
The mechanisms underlying the effects of HAM-RS2 on SI are not well understood. One hypothesis is that fermentation end products, particularly SCFA, are involved in a cascade of events that may lead to improved SI (15–17). SCFA (acetate, propionate, and butyrate) are absorbed from the colon and appear to suppress the activity of hormone-sensitive lipase, reducing release of FFA and glycerol from adipose depots, although the exact cellular processes through which this occurs have not been fully described (18). Metabolic studies have shown that raising the circulating FFA level for several hours will reduce SI and that lowering the FFA concentration will have the opposite effect, providing a possible mechanistic link between consumption of HAM-RS2 that undergoes fermentation in the colon and improved SI (17, 19, 20).
In the present study, the effects of two doses of HAM-RS2 on SI were evaluated in overweight and obese participants with increased waist circumference [as defined by the U.S. National Cholesterol Education Program Adult Treatment Panel III in its definition of metabolic syndrome (21)], a group that would be expected to contain a high proportion of insulin-resistant individuals (22).
Materials and Methods
Study design.
This was a double-blind, randomized crossover study with three 4-wk treatment periods separated by 3-wk washout periods. The study was conducted at a clinical research center (Provident Clinical Research in Addison, IL) according to Good Clinical Practice Guidelines, the Declaration of Helsinki (2000) and the United States 21 Code of Federal Regulations. An institutional review board (Quorum Review IRB) approved the protocol before the study began and informed consent was obtained from all participants prior to the initiation of any study-related procedures.
Participants.
Generally, healthy men and women 18–69 y of age, each with waist circumference ≥89.0 cm for females or ≥102.0 cm for males (23), were eligible for enrollment. Participants were excluded if they had a BMI ≥35.0 kg/m2; clinically important abnormal laboratory test results at screening; a history of cardiac, renal, hepatic, endocrine, pulmonary, biliary, pancreatic, gastrointestinal, or neurologic disorders; recent history of cancer; known sensitivity to any of the ingredients in the study foods; or active infections. The use of systemic corticosteroids, antibiotics, medications (other than hormonal contraceptives or postmenopausal sex hormones) or dietary supplements known to influence carbohydrate metabolism were not permitted during the study. Women of childbearing potential who were not pregnant or lactating and not planning to become pregnant during the study period were eligible for enrollment if they were using a medically acceptable contraceptive. All participants agreed to maintain a stable body weight during the trial and follow their usual dietary, smoking, dietary supplement, and physical activity habits, except for consumption of the study products. Participants were queried at each visit to confirm compliance with these instructions.
Test products and study procedures.
After screening (wk −1), eligible participants were randomly assigned to 1 of 6 treatment sequences. Participants ingested 1 of 3 study products during each 4-wk intervention period. This duration was based on previous studies that demonstrated changes in SI after RS feeding at higher levels of intake for periods ranging from 24 h to 12 wk. The study product was high-amylose corn starch (Hi-maize 260) containing ~60% RS or a control starch (Amioca) containing no RS, both supplied by National Starch. Two intake levels of the high-amylose starch were tested, providing 15 or 30 g/d of HAM-RS2 (as measured by AOAC total dietary fiber method 991.43). The control was designed to match the content of digestible starch provided in 15 g/d HAM-RS2 (~11.6 g/d). Using the Atwater factor of 16.8 kJ/g for the digestible portion of starch, the approximate energy value of the control and low-dose products was matched (194 kJ/d), whereas the energy content in the high-dose condition was 388 kJ/d (24). The study product was provided in individually packaged, ready-to-use sachets that could be mixed into cold or room-temperature beverages or foods. Participants were instructed to consume 2 servings daily of their assigned study product at separate eating occasions during the day. During the middle of each treatment period, staff contacted participants via telephone or e-mail to encourage compliance with study product consumption. Participants were asked to consume the last dose of their assigned study product during the evening prior to test visits and to avoid vigorous physical activity the day prior to the test visit. Compliance was assessed by counting unopened sachets at the end of each treatment period in conjunction with a query regarding whether any servings were missed. At baseline and at the end of each treatment phase, participants completed a GI symptom survey that included a series of questions regarding the presence and severity on a 6-point scale from 0 (none) to 5 (much more than usual) of bloating, flatulence, diarrhea/loose stools, constipation, abdominal cramping, and nausea over the previous 7 d (25). Adverse events were assessed at each visit using a nonleading question to assess changes in health status since the previous query.
i.v. glucose tolerance tests.
After an overnight fast (9–15 h, only water allowed), the insulin-modified i.v. glucose tolerance test was performed to assess SI and secretion on the last day of each treatment period. Briefly, an i.v. catheter was placed in the antecubital space of each arm, one for collecting blood samples and the other for injecting glucose and insulin. At t = 0 min, a 300-mg/kg body weight i.v. glucose (50% dextrose solution) injection was administered over ~1.5 min. At t = 20 min, an i.v. injection of regular human insulin (0.03 U/kg, diluted to 10 mL with normal saline) was administered over ~1 min. Blood samples were collected at the following pre- and postglucose administration time points: t = −10, −5, 3, 5, 7, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 75, 90, 120, 150, and 180 min.
Plasma glucose and insulin concentrations were measured and the values were entered into the MINMOD MILLENNIUM computer program (version 6.02; RN Bergman, USC) for determination of SI and SG (15–18, 26). Valid fits were obtained for all tests with the model converging in all cases. AIRG was calculated as previously described (15, 19). HOMA%S and HOMA%B were determined from fasting glucose and insulin values using the HOMA calculator (27).
Laboratory measurements.
Clinical laboratory measurements were conducted by Medpace Laboratories. Plasma glucose was measured by photometry using a hexokinase reaction (28) and plasma insulin was determined via an electrochemiluminescence immunoassay (29). Serum hsCRP was measured by particle-enhanced immunonephelometry (Cardiophase hsCRP; Dade Behring) on a BN* Systems Nephelometer (Dade Behring). Serum FFA were assessed by an enzymatic colorimetric assay (HR Series NEFA-HR, Wako) according to the manufacturer’s instructions and fructosamine was measured by an end point colorimetric assay (30). Plasma adiponectin was measured by ELISA kit (Quantikine, R&D Systems) according to the manufacturer’s instructions. SCFA were measured by GC in the laboratory of Dr. Papasani Subbaiah at the University of Illinois, Chicago, IL (31).
Fasting lipid profiles were drawn at screening and at the end of each treatment period. TC and TG in serum were measured by photometry on a Beckman Coulter AU2700/AU5400 Analyzer using Beckman Coulter reagents OSR 6216 and D8787–5G, respectively. HDL-C was isolated by a 2-step precipitation method with Mg-dextran sulfate (Sigma-Aldrich reagents) and measured by photometry after an enzymatic reaction on a Beckman Coulter AU2700/AU5400 Analyzer. The LDL-C concentration in mg/dL was calculated according to the Friedewald formula (32) as follows: LDL-C = TC – HDL-C – TG/5 and converted to mmol/L using a conversion factor of 0.0258. Because this equation is not valid when TG are >4.5 mmol/L, LDL-C was not calculated in those circumstances. Non-HDL-C was calculated as the difference between TC and HDL-C.
Statistical analyses.
Statistical analyses were conducted using SAS version 9.2 (SAS Institute). An evaluable sample of 27 participants was required to provide 80% power to detect an effect of 0.67 SD between treatment conditions with a 2-sided α = 0.05 after adjustment for 2 comparisons to the control condition. The assumption of normality of residuals from the final model for each outcome variable was investigated using the Shapiro-Wilk test. If it was determined that the distribution could not be approximated by a normal curve (P ≤ 0.01), then the analysis was completed after applying a natural logarithm transformation to improve kurtosis and/or skew.
The analysis of the primary and secondary outcome variables was completed on an efficacy evaluable and a per protocol sample. The efficacy evaluable sample included all participants who were randomized and completed the control and at least one active test condition. The per protocol sample was a subset of the efficacy evaluable sample that excluded participants who had protocol violations that could have influenced responses (e.g., poor compliance, not completing all test conditions, violations of inclusion/exclusion criteria, illness, use of an excluded medication during the treatment period). All decisions regarding exclusion of participants from the per protocol sample were finalized and documented prior to locking the study database and breaking the treatment code. The safety sample included all participants who were randomized and consumed at least one sachet of study product. Because there were no material differences apparent between the efficacy evaluable and per protocol samples, only data from the efficacy evaluable sample are presented.
Possible differences between treatment conditions for adverse events and other nominal variables were evaluated using Cochran’s chi-square test. Differences between conditions in responses for continuous variables were assessed using SAS PROC MIXED repeated-measures ANOVA, including subject as a random variable and terms for treatment condition, sex, sequence, period, and treatment condition by sex interaction. Models for glucose homeostasis variables derived from the i.v. glucose tolerance test also included the HOMA%S value from the control condition as a covariate based on the a priori expectation that response would be related to pretreatment SI. Models were reduced in a stepwise manner until only treatment condition and any significant (P < 0.05) terms remained.
Sensitivity analyses were completed to assess the influence of age and menopausal status and to evaluate possible treatment × sequence and treatment × period interactions. Some significant period × sequence interactions were observed, but further investigation indicated there was not a clinically significant pattern, nor were there a greater number than would be expected given the number of tests being run, thus pooling of the data across treatment sequences was judged to be appropriate. A significant treatment × sex interaction was observed for the primary outcome variable, SI, so results for all variables were analyzed and presented separately for men and women. Pairwise comparisons between treatment conditions were conducted and analyzed using Tukey’s procedure to adjust P values for multiple comparisons for each dependent variable. Values presented are least squares means ± SEM (between-subjects) unless otherwise noted. For variables where the analysis was based on loge-transformed values, the geometric least squares mean is also presented.
Results
Of the 50 participants screened, 41 were randomized. One withdrew due to an adverse event (constipation) during the first treatment period (30 g/d HAM-RS2). Seven additional participants withdrew consent without completing at least the control and one other test condition; therefore, 33 participants were included in the efficacy evaluable population (Table 1).
TABLE 1.
Participant characteristics1
| Characteristic2 | Total | Men | Women |
| n | 33 | 11 | 22 |
| n (%) | |||
| Race | |||
| Non-Hispanic white | 27 (82) | 9 (82) | 18 (82) |
| African American | 2 (6) | 1 (9) | 1 (5) |
| Other | 4 (12) | 1 (9) | 3 (14) |
| Smoking status | |||
| Nonsmoker | 17 (52) | 5 (46) | 12 (55) |
| Current smoker | 5 (15) | 3 (27) | 2 (9) |
| Past smoker | 11 (33) | 3 (27) | 8 (36) |
| Menopausal status | |||
| Premenopausal | N/A | N/A | 9 (41) |
| Oral contraceptive use | N/A | N/A | 1 (5) |
| Postmenopausal | N/A | N/A | 13 (59) |
| Hormone therapy | N/A | N/A | 3 (14) |
| Fasting glucose status | |||
| Normal | 22 (67) | 7 (64) | 15 (68) |
| Impaired | 11 (33) | 4 (36) | 7 (32) |
| Metabolic syndrome | 18 (55) | 7 (64) | 11 (50) |
| Age, y | 49.5 ± 1.6 | 48.1 ± 3.3 | 50.2 ± 1.7 |
| BMI, kg/m2 | 30.6 ± 0.5 | 30.7 ± 0.9 | 30.6 ± 0.5 |
| Waist circumference, cm | 105.3 ± 1.3 | 108.8 ± 1.5 | 103.6 ± 1.8 |
| Fasting glucose, mmol/L | 5.29 ± 0.08 | 5.30 ± 0.15 | 5.29 ± 0.10 |
| TC, mmol/L | 5.26 ± 0.14 | 5.05 ± 0.33 | 5.37 ± 0.13 |
| LDL-C, mmol/L | 3.17 ± 0.10 | 3.10 ± 0.23 | 3.20 ± 0.11 |
| HDL-C, mmol/L | 1.34 ± 0.08 | 0.98 ± 0.06 | 1.52 ± 0.09 |
| Non-HDL-C, mmol/L | 3.92 ± 0.14 | 4.07 ± 0.30 | 3.84 ± 0.14 |
| TC:HDL-C | 4.24 ± 0.22 | 5.21 ± 0.31 | 3.75 ± 0.23 |
| TG, mmol/L | 1.64 ± 0.15 | 2.12 ± 0.33 | 1.39 ± 0.13 |
| Alcoholic drinks, n/wk | 1.8 ± 0.5 | 3.2 ± 1.3 | 1.1 ± 0.3 |
Data are (%) or mean ± SEM. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
Laboratory values determined in plasma for glucose and serum for lipids.
Compliance based on interview and measurement of the unused study product was 98.3 ± 0.6% of expected servings of study product for the control condition, 96.7 ± 1.1% for the 15 g/d HAM-RS2 condition, and 96.4 ± 0.9% for the 30-g/d HAM-RS2 condition. There were no significant or clinically meaningful differences in body weight, waist circumference, or blood lipids at the end of each treatment condition (data not shown).
Glucose homeostasis.
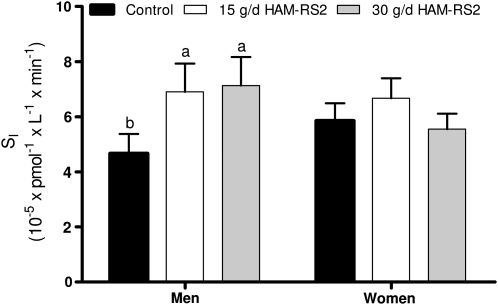
For SI, the primary outcome variable, there were effects for HOMA%S (P = 0.0008), treatment condition (P = 0.018), and a treatment × sex interaction (P = 0.033). Accordingly, values for men and women are presented separately (Fig. 1; Table 2).
FIGURE 1.
SI following 4-wk feeding periods for control (0 g/d HAM-RS2), 15 g/d HAM-RS2, and 30 g/d HAM-RS2 in men (n = 11) and women (n = 22). Bars represent least squares geometric means and error bars extend to the value of the loge least squares mean + 1 SEM, back transformed to the original units. Labeled means without a common letter differ, P < 0.05. Least squares mean and SEM values for loge SI were generated from repeated-measures ANOVA models containing terms for participant as a random variable, treatment condition, treatment sequence, and HOMA%S. HAM-RS2, high-amylose maize type 2 resistant starch; HOMA%S, homeostasis model assessment of insulin sensitivity; SI, insulin sensitivity.
TABLE 2.
Glucose homeostasis and SCFA variables in men and women following 4-wk feeding periods for control (0 g/d HAM-RS2), 15 g/d HAM-RS2, and 30 g/d HAM-RS21
| Variable2 | Control | 15 g/d HAM-RS2 | 30 g/d HAM-RS2 | P |
| Men | ||||
| n | 11 | 11 | 11 | |
| ln SG,100 x min− | 0.85 ± 0.14 (2.35) | 0.79 ± 0.14 (2.19) | 0.84 ± 0.14 (2.31) | 0.88 |
| ln AIRG, pmol x L x min− | 7.98 ± 0.28 (2918) | 7.52 ± 0.28 (1844) | 7.88 ± 0.28 (2637) | 0.06 |
| Total FFA, mmol/L | 0.46 ± 0.05 | 0.50 ± 0.05 | 0.47 ± 0.05 | 0.81 |
| ln Acetate, μmol/L | 4.3 ± 0.2 (75.2) | 4.5 ± 0.2 (90.5) | 4.6 ± 0.2 (96.2) | 0.61 |
| ln Butyrate, μmol/L | −0.2 ± 0.4 (0.8) | 0.5 ± 0.4 (1.6) | 0.9 ± 0.4 (2.4) | 0.21 |
| ln Propionate, μmol/L | 2.0 ± 0.2 (7.1) | 1.9 ± 0.2 (6.5) | 2.0 ± 0.2 (7.4) | 0.80 |
| Women | ||||
| n | 22 | 21 | 22 | |
| SG, x 100 min− | 2.54 ± 0.17 | 2.33 ± 0.18 | 2.34 ± 0.18 | 0.41 |
| AIRG, pmol x L x min− | 2540 ± 340 | 2120 ± 344 | 2720 ± 349 | 0.14 |
| Total FFA, mmol/L | 0.56 ± 0.04 | 0.67 ± 0.05 | 0.59 ± 0.04 | 0.07 |
| ln Acetate, μmol/L | 4.2 ± 0.1a (68.9) | 4.2 ± 0.1ab (69.5) | 4.4 ± 0.1b (84.5) | 0.03 |
| ln Butyrate, μmol/L | −0.4 ± 0.3 (0.7) | −0.1 ± 0.3 (0.9) | −0.1 ± 0.3 (0.9) | 0.73 |
| ln Propionate, μmol/L | 1.8 ± 0.1 (6.0) | 1.7 ± 0.1 (5.4) | 1.8 ± 0.1 (6.1) | 0.69 |
Data are least squares mean ± SEM (geometric least squares mean of the transformed data). Means in a row with superscripts without a common letter differ, < 0.05. AIRG, acute insulin response to i.v. glucose; HAM-RS2, high-amylose maize type 2 resistant starch; SG, glucose effectiveness.
Glucose homeostasis variables and SCFA concentrations are from measurements in plasma; FFA concentrations were measured in serum.
In men, values were higher during both the 15-g/d (P = 0.031) and 30-g/d HAM-RS2 (P = 0.019) conditions compared with the control condition (Fig. 1). When expressed as percent differences from control, the increases were 56.5% for the 15-g/d treatment (P = 0.006) and 72.8% (P = 0.017) for the 30-g/d treatment. HOMA%S was a significant covariate (P = 0.005), but differences between the 2 active conditions and control remained significant when HOMA%S was not included as a covariate in the model (data not shown). Values for SG and AIRG did not differ significantly across treatment conditions (Table 2). Fasting concentrations of insulin and glucose, HOMA%S, and HOMA%B did not significantly differ across treatment conditions (Table 3). In women (Fig. 1; Tables 2 and 3), no significant differences across treatment conditions were observed for any of the indicators of glucose homeostasis. Results from sensitivity analyses did not suggest that the results varied by age in either sex (data not shown).
TABLE 3.
Selected laboratory values in men and women following 4-wk feeding periods for control (0 g/d HAM-RS2), 15 g/d HAM-RS2, and 30 g/d HAM-RS21
| Variable2 | Control | 15 g/d HAM-RS2 | 30 g/d HAM-RS2 | P |
| Men | ||||
| n | 11 | 11 | 11 | |
| ln hsCRP, mg/L | 0.4 ± 0.2 (1.4) | 0.2 ± 0.2 (1.2) | 0.4 ± 0.2 (1.4) | 0.33 |
| Adiponectin, mg/L | 5.9 ± 0.6 | 5.8 ± 0.6 | 5.6 ± 0.6 | 0.70 |
| Fructosamine, μmol/L | 198 ± 6 | 198 ± 6 | 197 ± 6 | 0.98 |
| ln HOMA%B | 4.4 ± 0.1 (78.1) | 4.1 ± 0.1 (61.5) | 4.3 ± 0.1 (70.3) | 0.18 |
| ln HOMA%S | 4.6 ± 0.1 (97.7) | 4.7 ± 0.1 (115) | 4.6 ± 0.1 (96.4) | 0.38 |
| Fasting insulin, pmol/L | 62.5 ± 4.7 | 50.1 ± 4.7 | 58.5 ± 4.7 | 0.15 |
| ln Fasting glucose, mmol/L | 1.7 ± 0.1 (5.4) | 1.8 ± 0.8 (5.8) | 1.8 ± 0.22 (5.7) | 0.40 |
| Women | ||||
| n | 22 | 21 | 22 | |
| ln hsCRP, mg/L | 0.8 ± 0.2 (2.1) | 0.5 ± 0.2 (1.6) | 0.7 ± 0.2 (2.1) | 0.32 |
| ln Adiponectin, mg/L | 2.3 ± 0.1 (10.4) | 2.4 ± 0.1 (10.9) | 2.4 ± 0.1 (10.6) | 0.30 |
| Fructosamine, μmol/L | 207 ± 4 | 205 ± 4 | 206 ± 4 | 0.70 |
| HOMA%B | 89.3 ± 6.1 | 84.2 ± 6.2 | 80.7 ± 6.1 | 0.38 |
| ln HOMA%S | 4.5 ± 0.1 (91.1) | 4.6 ± 0.1 (97.6) | 4.7 ± 0.1 (113) | 0.10 |
| Fasting insulin, pmol/L | 56.2 ± 4.9 | 51.6 ± 5.0 | 47.5 ± 4.9 | 0.25 |
| Fasting glucose, mmol/L | 5.5 ± 0.1 | 5.5 ± 0.1 | 5.4 ± 0.1 | 0.29 |
Data presented as least squares means ± SEM, (geometric least squares means). Means in a row with superscripts without a common letter differ, < 0.05. AIRG, acute insulin response to i.v. glucose; HAM-RS2, high-amylose maize type 2 resistant starch; HOMA%B, homeostasis model assessment of β-cell function; HOMA%S, homeostasis model assessment of insulin sensitivity; hsCRP, high-sensitivity CRP; SG, glucose effectiveness;
All outcomes were measured in plasma, except for hsCRP which was measured in serum.
Other metabolic variables.
There were no significant differences between conditions in fasting concentrations of hsCRP, adiponectin, or fructosamine in men or women assessed at the end of each treatment condition (Table 3). In women, the plasma acetate concentration was greater in the 30-g/d HAM-RS2 condition than in the control (P = 0.047) (Table 2). A pooled analysis for acetate showed a main effect for treatment condition (P = 0.0007), but no treatment × sex interaction (P = 0.93). There were no differences in circulating FFA concentrations in men or women across treatment conditions (Table 2).
Tolerability and adverse events.
There were no differences between conditions in the frequencies of reported adverse events. Most adverse events were mild and not related to consumption of the study product. There were no differences in mean scores between conditions for the individual symptom components of the GI tolerability questionnaire (data not shown). Scores ≥4.0 indicating that the frequency of each symptom occurred “more than usual” or “much more than usual” for flatulence was 9.1% for the control, 9.1% for the 15-g/d HAM-RS2 condition, and 33.3% for the 30-g/d HAM-RS2 treatment (P = 0.014 vs. control). There were no differences between conditions in scores ≥4.0 for gas/bloating, nausea, loose stools, constipation, or GI cramping.
Discussion
The results of this study indicate that consumption of 15–30 g/d of HAM-RS2 improved SI in overweight and obese men. The improvement in SI relative to control is consistent with data from other studies of HAM-RS2 conducted in men and women in short-term and longer-term feeding trials (14–16). However, the present study is the first to our knowledge to show such improvements in SI at a level of intake as low as 15 g/d HAM-RS2. A previous study did not show an effect following intakes of 12 g/d HAM-RS2 for 6 wk; however, HOMA%S was used as an indicator of SI, which is less sensitive compared to the method used in the present study (33). The magnitude of the increase in SI in men is similar to that observed with weight loss of ~10% of body weight in obese individuals (34–36).
It is unclear why SI increased in men but not women in the present trial. One of the potential mechanisms for the insulin-sensitizing effects of fermentable fiber is that absorption of SCFA generated in the colon due to fermentation may trigger a reduction in release of FFA and glycerol from adipose depots, presumably through direct or indirect inhibition of hormone sensitive lipase (18). Numerous studies have demonstrated that maintenance of a lower FFA level for several hours enhances SI and elevation for several hours has the opposite effect (37–40). Hoeg et al. (41) recently showed that increasing FFA concentrations via intralipid infusion resulted in a decrease in whole-body SI by 38% in men but only 26% in matched women (P < 0.05). These results suggest that women are less sensitive to changes in circulating concentrations of FFA. Thus, if HAM-RS2 affects SI via alterations in FFA levels, the effect might be more readily detectable in men than in women. In the present study, there were no differences in pretest FFA levels in men or women across treatment conditions. However, fasting FFA concentrations were obtained in the morning and may not reflect those that prevail overnight, because sympathetic activation in the morning hours may raise levels more in participants with lower values than in those with higher values (42, 43). It has been suggested that FFA concentrations in the late evening and overnight periods may be important determinants of SI and secretion (22, 42).
Acetate, the predominant SCFA in plasma, was greater in women after HAM-RS2 consumption compared to control. Differences in the timing and fraction of HAM-RS2 reaching the colon and capacity for colonic fermentation may influence the timing and quantity of SCFA absorption differently in men and women. Although there is considerable individual variability, men generally have accelerated transit times compared with women, which may influence substrate availability for fermentation in the large bowel (44). Studies in which transit time is accelerated by a pharmacological agent (cisapride) have shown that this produced an increase in fermentation, as measured by breath hydrogen and concentrations of fecal SCFA (45). Weickert et al. (46) showed a modest but significant 8% improvement in SI assessed by euglycemic-hyperinsulinemic clamp in overweight and obese women consuming a fermentable cereal fiber for 72 h, and the effect appeared somewhat larger (13%) in the subset of women with evidence of colonic fermentation as demonstrated by increased breath hydrogen. There is also some evidence of changes in starch fermentation over the course of the menstrual cycle (47). However, results from other studies have not supported the thesis that there are differences in fermentation between men and women (48). Because breath hydrogen was not assessed in the present study, it is not possible to determine to what extent differences in HAM-RS2 fermentation in the colon, if any, may have influenced the results.
There is evidence that SI varies across phases of the menstrual cycle, with observed values higher in the follicular phase and lower in the luteal phase (49–51). In the present study, there was some qualitative evidence that menstrual cycle may have confounded the results in premenopausal women, because there was a greater tendency to show improvement among women for whom tests were conducted during the same phase of the menstrual cycle than for women whose tests were not phase concordant. The sample of premenopausal women was too small to provide a meaningful statistical analysis of this effect. However, postmenopausal women also had a lower response than men, suggesting that confounding due to menstrual cycle phase cannot entirely explain the difference in SI responses between men and women. Exclusion of women using contraceptive or postmenopausal sex hormones from the analysis did not alter the results.
Another possible explanation may relate to differences between men and women in baseline SI. During the control condition, SI was ~26% higher for women than for men. It is possible that RS-induced improvements in SI are more likely in individuals with lower baseline levels. However, a post hoc analysis of responses in participants higher and lower than the median SI value within each sex did not provide clear evidence to support this possibility. Future research to better understand sex differences in SI and relationships to fermentation capacity, SCFA, FFA, and circulating hormones is warranted.
The present study has several limitations. Dietary intake, including dietary fiber, was not assessed; therefore, we cannot rule out that changes in dietary composition influenced the study results or a treatment × fiber intake interaction. However, each participant acted as his or her own control and maintained his or her usual dietary habits except for consumption of the study product, reducing the likelihood of such effects. Neither breath hydrogen nor fecal SCFA were measured, and levels of FFA, SCFA, adiponectin, hsCRP, and other blood analytes were measured only once at the end of each treatment condition and in the fasting state. Nevertheless, the improvement in SI observed in men at an intake as low as 15 g/d extends the results reported in previous studies after feeding 30–60 g/d of HAM-RS2.
In conclusion, the present results showed that consumption of 15 and 30 g/d of HAM-RS2 improved SI in overweight and obese men. No significant change in SI was observed in women for reasons that remain to be determined. Additional investigation will be required to further delineate the mechanisms responsible for improved SI during HAM-RS2 consumption.
Acknowledgments
K.C.M., C.L.P., E.T.F., and T.M.R. designed the research; K.C.M., K.M.K., and A.L.L. conducted the research; K.C.M. and A.L.S. analyzed the data; K.C.M., C.L.P., E.T.F., A.L.S., and T.M.R. wrote the paper; and K.C.M. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by National Starch, LLC.
This trial was registered at clinicaltrials.gov as NCT01058135.
Abbreviations used: AIRG, acute insulin response to i.v. glucose; HAM-RS2, high-amylose maize type 2 resistant starch; HDL-C, HDL cholesterol; HOMA%B, homeostasis model assessment of β-cell function; HOMA%S, homeostasis model assessment of insulin sensitivity; hsCRP, high-sensitivity CRP; LDL-C, LDL cholesterol; RS, resistant starch; SG, glucose effectiveness; SI, insulin sensitivity; TC, total cholesterol.
Literature Cited
- 1.Englyst H, Wiggins HS, Cummings JH. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982;107:307–18 [DOI] [PubMed] [Google Scholar]
- 2.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46 Suppl 2:S33–50 [PubMed] [Google Scholar]
- 3.Finocchiaro ET, Birkett AM, Okoniewska M. Resistant starch. In: S Cho, P Samuel, editors. Fiber ingredients: food applications and health benefits. Boca Raton (FL): CRC Press; 2000 [Google Scholar]
- 4.Brown MA, Storlien LH, Brown IL, Higgins JA. Cooking attenuates the ability of high-amylose meals to reduce plasma insulin concentrations in rats. Br J Nutr. 2003;90:823–7 [DOI] [PubMed] [Google Scholar]
- 5.Murphy MM, Douglass JS, Birkett A. Resistant starch intakes in the United States. J Am Diet Assoc. 2008;108:67–78 [DOI] [PubMed] [Google Scholar]
- 6.Asp NG, van Amelsvoort JM, Hautvast JG. Nutritional implications of resistant starch. Nutr Res Rev. 1996;9:1–31 [DOI] [PubMed] [Google Scholar]
- 7.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64 [DOI] [PubMed] [Google Scholar]
- 8.Nugent AP. Health properties of resistant starch. Nutr Bull. 2005;30:27–54 [Google Scholar]
- 9.Miller Jones J, Witwer R, Birkett A. High amylose corn resistant starch monograph [N Starch, editor], 2005. Available from: www.resistantstarch.com. [Google Scholar]
- 10.Birkett A, Muir J, Phillips J, Jones G, O’Dea K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr. 1996;63:766–72 [DOI] [PubMed] [Google Scholar]
- 11.Muir JG, Yeow EG, Keogh J, Pizzey C, Bird AR, Sharpe K, O’Dea K, Macrae FA. Combining wheat bran with resistant starch has more beneficial effects on fecal indexes than does wheat bran alone. Am J Clin Nutr. 2004;79:1020–8 [DOI] [PubMed] [Google Scholar]
- 12.Noakes M, Clifton PM, Nestel PJ, Le Leu R, McIntosh G. Effect of high-amylose starch and oat bran on metabolic variables and bowel function in subjects with hypertriglyceridemia. Am J Clin Nutr. 1996;64:944–51 [DOI] [PubMed] [Google Scholar]
- 13.Phillips J, Muir JG, Birkett A, Lu ZX, Jones GP, O’Dea K, Young GP. Effect of resistant starch on fecal bulk and fermentation-dependent events in humans. Am J Clin Nutr. 1995;62:121–30 [DOI] [PubMed] [Google Scholar]
- 14.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med. 2010;27:391–7 [DOI] [PubMed] [Google Scholar]
- 15.Robertson MD, Currie JM, Morgan LM, Jewell DP, Frayn KN. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46:659–65 [DOI] [PubMed] [Google Scholar]
- 16.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–67 [DOI] [PubMed] [Google Scholar]
- 17.Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int. 2004;87:761–8 [PubMed] [Google Scholar]
- 19.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production an utilization in man. J Clin Invest. 1983;72:1737–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E1775–81 [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunningshake DB, Pasternak RC, Smith SC, Jr, Stone NJ, National Heart Lung, and Blood Institute, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39 [DOI] [PubMed] [Google Scholar]
- 22.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120 (2 Suppl 1):S3–8; discussion S29–32 [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421 [PubMed] [Google Scholar]
- 24.Merrill AL, Watt BK. Energy value of foods: basis and derivation. Agriculture handbook 74. Washington, DC: USDA, Agricultural Research Service; 1973 [Google Scholar]
- 25.Maki KC, Carson ML, Miller MP, Turowski M, Bell M, Wilder DM, Reeves MS. High-viscosity hydroxypropylmethylcellulose blunts postprandial glucose and insulin responses. Diabetes Care. 2007;30:1039–43 [DOI] [PubMed] [Google Scholar]
- 26.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetes Trials Unit [Internet]. The Oxford Centre for Diabetes, Endocrinology and Metabolism [cited 2011 Dec 21]. Available from: http://www.dut.ox.ac.uk
- 28.Kadish AH, Hall DA. A new method for the continuous monitoring of blood glucose by measurement of dissolved oxygen. Clin Chem. 1965;11:869–75 [PubMed] [Google Scholar]
- 29.Allauzen S, Mani JC, Granier C, Pau B, Bouanani M. Epitope mapping and binding analysis of insulin-specific monoclonal antibodies using a biosensor approach. J Immunol Methods. 1995;183:27–32 [DOI] [PubMed] [Google Scholar]
- 30.Melzi d'Eril GV, Bosoni T, Solerte SB, Fioravanti M, Ferrari E. Performance and clinical significance of the new fructosamine assay in diabetic patients. Wien Klin Wochenschr Suppl. 1990;180:60–3 [PubMed] [Google Scholar]
- 31.Murase M, Kimura Y, Nagata Y. Determination of portal short-chain fatty acids in rats fed various dietary fibers by capillary gas chromatography. J Chromatogr B Biomed Appl. 1995;664:415–20 [DOI] [PubMed] [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL cholesterol in plasma without use of a preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 33.Penn-Marshall M, Holtzman GI, Barbeau WE. African Americans may have to consume more than 12 grams a day of resistant starch to lower their risk for type 2 diabetes. J Med Food. 2010;13:999–1004 [DOI] [PubMed] [Google Scholar]
- 34.Dubé JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, Coen P, Goodpaster BH. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54:1147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viljanen AP, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, Järvisalo M, Parkkola R, Rönnemaa T, Guiducci L, et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab. 2009;94:50–5 [DOI] [PubMed] [Google Scholar]
- 36.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–7 [DOI] [PubMed] [Google Scholar]
- 37.Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4649–55 [DOI] [PubMed] [Google Scholar]
- 38.Burns SF, Kelsey SF, Arslanian SA. Effects of an intravenous lipid challenge and free fatty acid elevation on in vivo insulin sensitivity in African American versus Caucasian adolescents. Diabetes Care. 2009;32:355–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18 [DOI] [PubMed] [Google Scholar]
- 40.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Høeg LD, Sjøberg KA, Jeppesen J, Jensen TE, Frøsig C, Birk JB, Bisiani B, Hiscock N, Pilegaard H, Wojtaszewski JF, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292:E1590–8 [DOI] [PubMed] [Google Scholar]
- 43.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–41 [DOI] [PubMed] [Google Scholar]
- 44.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Oufir L, Flourié B, Bruley des Varannes S, Barry JL, Cloarec D, Bornet F, Galmiche JP. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;38:870–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weickert MO, Möhlig M, Schöfl C, Arafat AM, Otto B, Viehoff H, Koebnick C, Kohl A, Spranger J, Pfeiffer AF. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care. 2006;29:775–80 [DOI] [PubMed] [Google Scholar]
- 47.McBurney MI. Starch malabsorption and stool excretion are influenced by the menstrual cycle in women consuming low-fibre Western diets. Scand J Gastroenterol. 1991;26:880–6 [DOI] [PubMed] [Google Scholar]
- 48.Hallfrisch J, Behall KM. Breath hydrogen and methane responses of men and women to breads made with white flour or whole wheat flours of different particle sizes. J Am Coll Nutr. 1999;18:296–302 [DOI] [PubMed] [Google Scholar]
- 49.Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19–22 [DOI] [PubMed] [Google Scholar]
- 50.Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95:5435–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bingley CA, Gitau R, Lovegrove JA. Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res. 2008;40:901–6 [DOI] [PubMed] [Google Scholar]