Abstract
Hyperglycemia-induced vascular inflammation resulting in the enhanced monocyte-endothelial cell (EC) interaction is the key event in the pathogenesis of atherosclerosis in diabetes. Here, we investigated the effect of isoflavone genistein on hyperglycemia-stimulated vascular inflammation. Human aortic EC (HAEC) were pretreated with genistein before the addition of high glucose (HG; 25 mmol/L) for 48 h. Genistein at a physiological concentration (0.1 μmol/L) significantly inhibited HG-induced adhesion of monocytes to HAEC and suppressed endothelial production of monocyte chemotactic protein-1 (MCP-1) and IL-8. Inhibition of adenylate cyclase or protein kinase A (PKA) significantly attenuated the antiadhesion effect of genistein. Consistently, genistein improved HG-impaired intracellular cAMP production and PKA activity in HAEC. Six-week-old diabetic db/db mice were untreated (db/db) or treated with a diet containing 1 g genistein/kg diet (db/db+G) for 8 wk. Their nondiabetic db/+ mice were used as normal controls. Circulating concentrations of MCP-1/JE and KC were significantly greater, whereas IL-10 concentrations were lower in db/db mice than those in normal mice. Dietary supplementation of genistein did not normalize but significantly suppressed the elevated serum concentrations of MCP-1/JE from 286 ± 30 ng/L to 181 ± 35 ng/L and KC from 321 ± 21 ng/L to 232 ± 20 ng/L while increasing that of IL-10 from 35 ± 4 ng/L to 346 ± 35 ng/L in db/db+G mice. Further, genistein treatment suppressed diabetes-induced adhesion of monocytes to EC by 87% and endothelial secretion of adhesion molecules. We conclude that genistein improves diabetes-caused vascular inflammation, which may be mediated through promoting the cAMP/PKA pathway.
Introduction
Diabetes is a major risk factor for cardiovascular disease such as atherosclerosis, which accounts for the largest number of all deaths among American diabetic patients (1). One of the key early events in the pathogenesis of atherosclerosis is inflammation-triggered endothelial activation, which leads to the adhesion of monocytes to the endothelium followed by their transmigration into the subendothelial space (2, 3). In diabetes, hyperglycemia-induced vascular inflammation and the subsequent endothelial dysfunction play a pivotal role in the development of atherosclerosis (1). Indeed, enhanced monocyte-EC6 interactions are reported in diabetic animal models (4), diabetic patients (5), and in EC exposed to HG in vitro (4). HG can trigger several intracellular signaling events that ultimately upregulate the expression of a number of proinflammatory chemokines such as IL-8 and MCP-1 and adhesion molecules, including VCAM-1, ICAM-1, and endothelial-leukocyte adhesion molecule-1, which are key factors for inducing adhesion of monocytes to EC, and their subsequent transendothelial migration in the vessels (3, 4, 6, 7). These results clearly indicate that increased monocyte-EC interactions induced by hyperglycemia and subsequent vascular inflammation may play a central role in diabetic endothelial dysfunction and atherosclerosis. As such, the development of method for the prevention and treatment of vascular inflammation is increasingly important in the management of diabetic vascular complications.
Recently, the bioactive compound genistein, a major isoflavone in soy and red clover, has drawn wide attention due to its potential beneficial effects on some of the degenerative diseases such as cardiovascular disease, osteoporosis, and hormone-related cancers (8, 9). Genistein has various biological actions, including a weak estrogenic effect, by binding to ER and the inhibition of PTK at pharmacological doses (9). Recent human studies have shown that dietary supplementation of genistein has a beneficial effect on atherosclerosis (10) and markers of cardiovascular risk (11). Further, proteosome analysis for the identification of target proteins of genistein in EC demonstrated that genistein affects the expression of atherosclerosis-relevant genes and prevents the expression of atherogenic factors (12). However, to our knowledge, the role of genistein in the prevention of diabetic vascular inflammation has not been examined. Further, the results from most of the reported studies reflect a pharmacological rather than physiological effect of genistein, because the effective doses used in most of the studies were well above achievable plasma genistein concentrations in both rodents and humans following the consumption of genistein (≤5 μmol/L) (13, 14). Therefore, although studies indicate a potential protective role of pharmacological doses of genistein in the vasculature, the physiological roles as well as the mechanisms underlying the beneficial effects of genistein are unknown. We recently found that genistein directly activates the cAMP/PKA cascade in primary human vascular EC (15). Emerging studies indicate that activation of the cAMP/PKA pathway can effectively inhibit the expression of chemokines and adhesion molecules in EC (16, 17). In the present study, we tested the hypothesis that genistein prevents hyperglycemia-induced vascular inflammation.
Materials and Methods
Materials.
HAEC (human aortic EC) and endothelial growth factors were purchased from Lonza. M199 media, FBS, cell culture supplements, and calcein-AM were from Invitrogen; protein assay kits were from Bio-Rad; ELISA kits for the determination of human IL-8 and MCP-1, mouse chemokines MCP-1/JE and KC, mouse IL-10, and soluble mouse adhesion molecules VCAM-1 and ICAM-1 were from R&D Systems; cAMP enzyme immunoassay kits were from Assay Design Inc.; PKA assay kits were from Promega; 3,3′-dioctadecyloxacarbocyanine perchlorate labeled acetylated LDL was from Biomedical Technologies Inc.; dispase was from BD Biosciences; ICI 182,780 and SQ22536 were from Tocris Cookson; PKI (PKA inhibitor), H89, forskolin, isobutylmethylxanthine, genistein, protease and phosphatase inhibitor cocktails, and other general chemicals were obtained from Sigma-Aldrich. Human monocytic U937 cells were from ATCC and mouse monocytic cells WEHI78/24 were a kind gift from Dr. Judith A. Berliner (UCLA). Stock solution of 20 mmol/L genistein in DMSO was stored at –80°C before use.
Cell culture.
HAEC were cultured in M199 medium containing 2% heat-inactivated FBS and endothelial growth supplements at 37°C in a 5% CO2/95% air environment. U937 cells were cultured in RPMI-1640 medium with 10% FBS and WEHI78/24 monocytic cells were cultured in DMEM medium plus 10% FBS.
Monocyte adhesion assay.
The adhesion of monocytes to EC was determined by using U937 human monocytic cells. For assessing time- and dose-dependent effects of glucose on the EC–monocyte interaction, HAEC were cultured with 25 mmol/L glucose for 24–72 h or with various concentrations of glucose (5.5–25 mmol/L) or mannitol (25 mmol/L) for 48 h. For testing the effect of genistein on monocyte adhesion to EC, HAEC were grown to confluence and treated with genistein (0.01–10 μmol/L) for 30 min before the addition of HG (25 mmol/L) for 48 h. In some experiments, HAEC were pretreated with ER blocker ICI 182,780 (1 μmol/L), adenylate cyclase inhibitor SQ22536 (10 μmol/L), or PKA inhibitor PKI (2 μmol/L) for 30 min before the addition of genistein (5 μmol/L) or 17 β-estradiol (10 nmol/L). Cells were then incubated with medium containing either 5.5 mmol/L or HG in the continued presence or absence of these agents for 48 h. HAEC were gently washed with serum free medium and Calcein-AM–labeled U937 cells (105 in 100 μL DMEM medium containing 1% FBS) were then added to HAEC. After 20 min of incubation, the HAEC monolayer was gently washed with PBS to remove unbound monocytes. The fluorescence was measured to determine the bound monocytes using a FLX800 multi-detection microplate reader (Bio-Tek Instruments) at excitation and emission wavelengths of 496 and 520 nm, respectively.
Measurements of MCP-1 and IL-8 in cell culture supernatants.
HAECs were pretreated with or without genistein (0.01–10 μmol/L) for 30 min before the addition of HG. Cells were then incubated with medium containing either 5.5 mmol/L or HG in the continued presence or absence of genistein for 48 h. The cell culture supernatants were collected and the production of MCP-1 and IL-8 by HAEC were measured by using ELISA kits.
Cyclic AMP and PKA activity assays.
HAECs were incubated with 5.5 mmol/L glucose or HG in the presence or absence of genistein (1 and 5 μmol/L) for 48 h. Cells were then washed and incubated in HBSS, followed by stimulation with forskolin (10 μmol/L) in the presence of isobutylmethylxanthine (0.2 mmol/L) or vehicle. The intracellular cAMP content was measured by Enzyme immunoassay and PKA activity in cell extracts were assessed by measuring PKA-induced phosphorylation of kemptide as we previously described (15).
Mice and genistein treatment.
Five-week-old male diabetic mice (db/db; B6.Cg-m+/+Leprdb) were obtained from Jackson Laboratory (stock no. 000642). This is a widely used type 2 diabetic animal model that spontaneously develops vascular complications. Age-matched db/+ mice (stock no. 000642) were used as normal controls (Normal). Mice were housed in micro-isolator cages in a pathogen-free facility and were provided free access to a rodent diet (AIN 93G diet, Dyet) with corn oil substituted for soybean oil (18). After 1 wk of environmental acclimation, diabetic mice were divided into 2 groups (n = 20 mice/group) and given either 0% (diabetic control, db/db) or 0.1% genistein (db/db+G) in the diet. Recently, we showed that the plasma genistein concentrations in spontaneously hypertensive rats fed 0, 0.2, 0.5, and 2.0 g/kg diet of genistein were 0, 1.20 ± 0.03, 1.90 ± 0.20, and 5.05 ± 0.49 μmol/L, respectively (19). Thus, the dose used in the present study may overlap the attainable plasma concentration of genistein (4.09 μmol/L) in humans following consumption of a soy meal (13). Body weight and food intake were recorded weekly throughout the study. After 8 wk of treatment, the mice were killed using CO2 after overnight food deprivation, and serum samples were frozen at −80°C for the analysis. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University and it conforms to the Guide for the Care and Use of Laboratory Animals published by the US NIH.
Measurements of physiological variables.
Blood pressure was determined in conscious mice using a computerized, noninvasive blood pressure system (Kent Scientific) as we previously described (19). A F90 Minispec Time Domain Nuclear Magnetic Resonance Spectrometer (Bruker Optics) was used to determine body composition. Blood glucose concentrations in tail vein blood samples were measured using a glucometer (Kroger). Serum cholesterol, HDL-cholesterol, and TG concentrations were measured using PTS CardioChek blood analysis meters (Maria Stein). For IPGTT (i.p. glucose tolerance test), mice were deprived of food for 8 h and were then injected with a single bolus of glucose (2 g/kg body weight) followed by measurements of blood glucose concentrations at 0, 15, 30, 60, and 120 min after glucose administration. For IPITT (i.p. insulin tolerance test), mice were deprived of food for 8 h and injected with insulin (0.75 units/kg body weight) and blood glucose concentrations were measured at 0, 15, 30, 60, and 120 min after insulin administration.
Isolation of mouse aortic EC.
MAEC from normal, db/db, and db/db+G mice were harvested from mouse aorta under sterile conditions as described (4). The purity of EC was tested by using 3,3′-dioctadecyloxacarbocyanine perchlorate labeled acetylated LDL and passages 2–3 were used for the experiments.
Ex vivo monocyte adhesion assay.
Monocyte adhesion to MAEC was determined by a fluorochrome adhesion assay using WEHI78/24 cells, a well-characterized mouse monocytic cell line. MAECs from normal, db/db, and db/db+G mice were cultured to confluence in 96-well plates. As a positive control, MAEC were incubated with 5 μg/L TNFα for 6 h. The medium was aspirated and MAEC were washed with serum-free medium. WEHI78/24 cells labeled with calcein-AM (105 in 100 μL) were then added to MAEC and incubated for 1 h at 37°C. Nonadherent cells were removed by gentle washing and monocytes adhered to EC were determined as describe above.
Measurements of chemokines, cytokines, and adhesion molecules.
JE/MCP-1, KC, IL-10, ICAM-1, and VCAM-1 in serum and/or cell culture supernatants were measured by ELISA kits according to the manufacturer’s instructions.
Statistical analysis.
Data were analyzed with one-way ANOVA using SPSS/10 software and are expressed as mean ± SEM. Data from in vitro studies were derived from at least three independent experiments performed in duplicate and data from animal studies were obtained from at least six mice in each group. IPGTT and IPITT data were analyzed with repeated-measures ANOVA. The total AUCs were calculated using the Trapezoidal rule method. Significant treatment differences were identified using Tukey’s multiple comparison tests. P < 0.05 was considered different.
Results
Genistein attenuates HG-induced inflammation in EC.
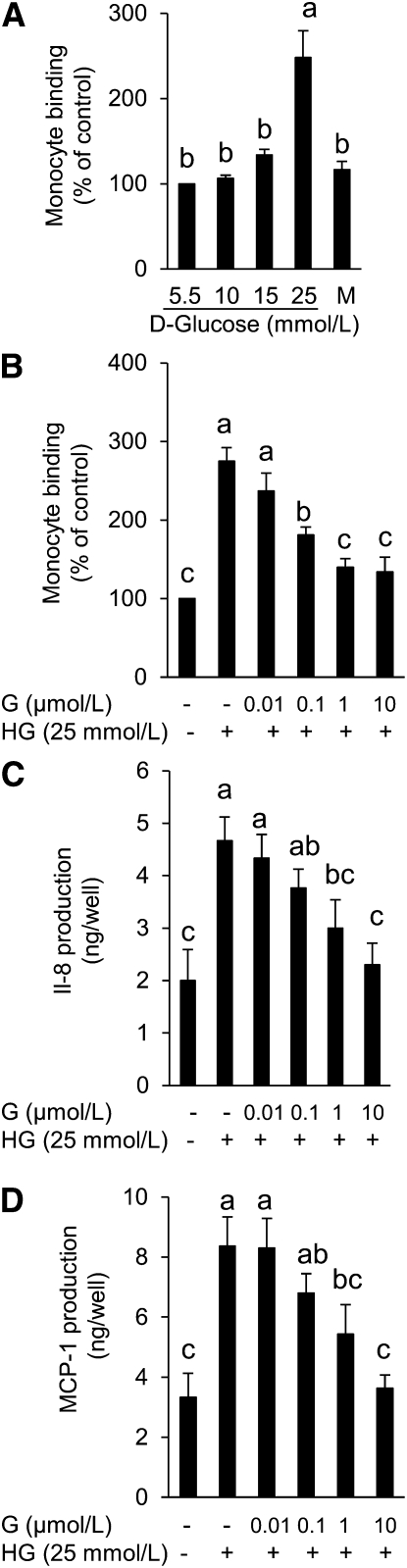
Our preliminary study demonstrated that HG-stimulated adhesion of monocytes to EC is time dependent with a significant increase in leukocyte adherence beginning at 48-h exposure of EC to HG (data not shown). We therefore chose 48 h as the incubation period for all further experiments. Exposure of HAEC to 25 mmol/L glucose (HG) but not mannitol for 48 h significantly stimulated the adhesion of monocytes to EC (Fig. 1A). However, the addition of genistein as low as 0.1 μmol/L suppressed HG-induced adhesion of U937 cells to HAEC, with 1 or 10 μmol/L genistein ablating adhesion by 75% (Fig. 1B). HG greatly increased IL-8 and MCP-1 production in ECs over that from normal glucose-incubated cells. However, the addition of genistein significantly inhibited HG-induced IL-8 (Fig. 1C) and MCP-1 (Fig. 1D) production in a concentration-dependent manner, with a significant inhibition observed at a concentration of genistein as low as 1 μmol/L, a pattern consistent with its effect on monocyte adhesion.
FIGURE 1.
Adhesion of monocytes to HAEC cultured with mannitol or various concentrations of glucose for 48 h (A) and monocyte adhesion (B) and IL-8 (C) and MCP-1 (D) production in HAEC cultured with normal glucose (5.5 mmol/L) or HG (25 mmol/L) in the presence or absence of genistein for 48 h. Values are mean ± SEM, n = 3 (means of duplicates). Means without a common letter differ, P < 0.05. G, genistein; HAEC, human aortic endothelial cell; HG, high glucose.
The inhibitory effect of genistein on HG-induced monocyte adhesion to EC is independent of ER.
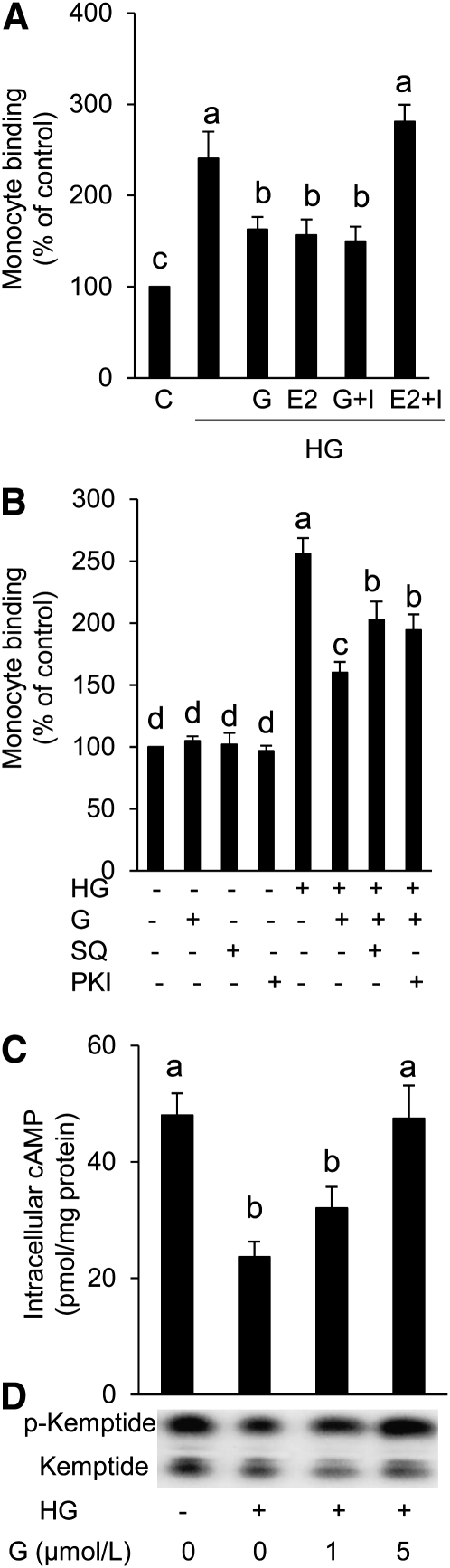
The ER antagonist ICI 182,780 (I), which successfully inhibited the estrogen effect in HAEC, did not inhibit the effect of genistein on the HG-induced EC-monocyte interaction (Fig. 2A), suggesting that genistein activity is not mediated through ER.
FIGURE 2.
Adhesion of monocytes to HAEC cultured with normal glucose or HG with or without ICI 182,780 in the presence or absence of genistein or 17β-estradiol (A). Monocyte adhesion to HAEC cultured with normal glucose or HG with or without SQ22536 or PKI in the presence or absence of genistein (B). Intracellular cAMP production (C) and PKA activity (D) in HAEC cultured with normal glucose or HG in the presence or absence of genistein for 48 h. Values are mean ± SEM, n = 3 (means of duplicates). Means without a common letter differ, P < 0.05. E2, 17β-estradiol (10 nmol/L); G, genistein (5 μmol/L); HAEC, human aortic endothelial cell; HG, high glucose (25 mmol/L); I, ICI 182,780 (1 μmol/L); PKI, protein kinase A inhibitor (2 μmol/L); SQ, adenylate cyclase inhibitor SQ22536 (10 μmol/L).
Genistein#x2019s suppression of monocyte-EC interaction is partially mediated through the cAMP/PKA pathway.
The addition of adenylate cyclase inhibitor SQ22536 or PKA inhibitor significantly attenuated the inhibitory effect of genistein on HG-induced monocyte adhesion to HAEC (Fig. 2B), suggesting that this genistein action may be at least partially mediated through the cAMP/PKA pathway. Furthermore, HG impaired cAMP production (Fig. 2C) and diminished PKA activity (Fig. 2D) in response to adenylate cyclase activator forskolin in HAEC, suggesting the adverse effects of HG on the cAMP signaling system. However, co-incubation with genistein significantly improved these detrimental effects of HG on HAEC (Fig. 2C,D).
Effect of dietary supplementation of genistein on physiological variables in db/db mice.
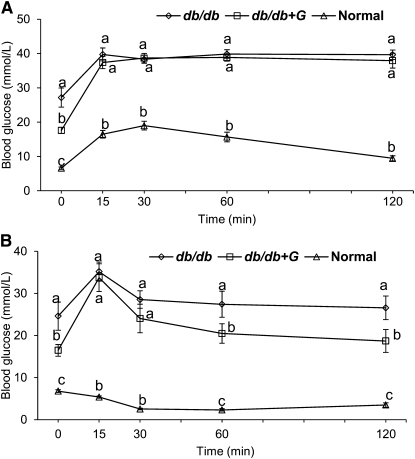
Body weight, food intake, serum cholesterol and TG, and systolic and diastolic blood pressures were greater in db/db mice compared with those in normal mice. Fat mass and fluid volume was greater, whereas lean mass was less in db/db mice than those in normal mice (Supplemental Table 1). These variables did not differ between db/db and db/db+G mice (Supplemental Table 1). However, dietary intake of genistein significantly reduced the concentrations of blood glucose in db/db+G mice (Supplemental Table 1). Although genistein treatment did not affect glucose tolerance in db/db+G mice (Fig. 3A), it improved insulin tolerance after 1 h of glucose administration (Fig. 3B). The total AUC for IPGTT was consistently greater in db/db mice (1090 ± 50 mmol·min/L) compared with that in normal mice (439 ± 27 mmol·min/L), which did not differ significantly from db/db+G mice (983 ± 39 mmol·min/L). The total AUC for IPITT was greater in db/db mice (926 ± 46 mmol·min/L) than that in normal mice (151 ± 5 mmol·min/L), which did not differ significantly from db/db+G mice (810 ± 70 mmol·min/L). Therefore, the improvement in insulin sensitivity due to genistein largely may have been due to the lower blood glucose concentrations in food-deprived db/db+G mice than those in db/db mice.
FIGURE 3.
Blood glucose concentration during a glucose tolerance test (A) and an insulin tolerance test (B) in normal, db/db, and db/db+G mice treated for 8 wk. Values are mean ± SEM, n = 10. Means at time without a common letter differ, P < 0.05.
Dietary genistein reduces vascular inflammation in db/db mice.
There was a greater binding of WEHI 78/24 cells to MAEC isolated from db/db mice as compared with normal mice (Table 1). However, supplementation of genistein for 8 wk normalized the adverse effect of diabetes on vascular EC (Table 1). The serum concentrations of MCP-1/JE and KC, the mouse homologs of human MCP-1 and IL-8, respectively, were greater in db/db mice than those in the normal group (Table 2). However, dietary intake of genistein greatly reduced but did not normalize the circulating MCP-1/JE and KC concentrations in db/db+G mice (Table 2), suggesting that genistein indeed has an antiinflammatory effect in vivo. The serum IL-10 concentrations were lower in db/db mice than those in normal mice, but this effect was completely reversed by genistein treatment (Table 2). The serum concentrations of ICAM-1 and VCAM-1 did not differ between normal and db/db mice. But genistein treatment significantly reduced the serum concentrations of ICAM-1 and VCAM-1 in db/db+G mice (Table 2). The concentrations of VCAM-1 and ICAM-1 released into culture medium from db/db MAEC were greater compared to those from normal mice (Table 3). However, the secretion of these adhesion molecules from MAEC isolated from db/db+G mice was significantly lower than that from db/db mice (Table 3).
TABLE 1.
Adhesion of monocytes to MAECs isolated from normal, db/db, and db/db+G mice treated for 8 wk1
| Groups | Monocyte binding |
| % of control | |
| Normal | 106 ± 4c |
| db/db | 145 ± 12b |
| db/db+G | 106 ± 11c |
| TNFα2 | 287 ± 5a |
Values are mean ± SEM, = 6 (means of quadruplicates). Means without a common letter differ, P < 0.05. MAEC, mouse aortic endothelial cell.
Positive control: MAEC incubated with 5 g/L TNFα for 6 h.
TABLE 2.
Chemokines, cytokine, and adhesion molecules in the serum of normal, db/db, and db/db+G mice treated for 8 wk1
| Groups | MCP-1 | KC | IL-10 | sICAM-1 | sVCAM-1 |
| ng/L | μg/L | ||||
| Normal | 53 ± 6c | 108 ± 13c | 116 ± 40b | 357 ± 19ab | 546 ± 37ab |
| db/db | 286 ± 30a | 321 ± 21a | 35 ± 4c | 396 ± 22a | 568 ± 7a |
| db/db+G | 181 ± 35b | 232 ± 20b | 346 ± 35a | 310 ± 17b | 497 ± 16b |
Values are mean ± SEM, = 8 (means of duplicates). Means in a column without a common letter differ, P < 0.05. sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular adhesion molecule-1.
TABLE 3.
Cell adhesion molecules secreted by MAEC from normal, db/db, and db/db+G mice treated for 8 wk1
| Groups | sICAM-1 | sVCAM-1 |
| ng/L medium | ||
| Normal | 64 ± 12b | 197 ± 25b |
| db/db | 113 ± 10a | 300 ± 24a |
| db/db+G | 68 ± 11b | 196 ± 54b |
Values are mean ± SEM, = 6 (means of duplicates). Means in a column without a common letter differ, P < 0.05. sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular adhesion molecule-1.
Discussion
Diabetes is an independent risk factor for atherosclerosis-associated morbidity and mortality (20). In diabetes, hyperglycemia-enhanced monocyte-EC interaction triggers vascular inflammation, which then contributes to atherosclerosis (4). The vasculoprotective effects of genistein were reported in previous studies, but the underlying mechanism remains elusive and whether genistein offers a protective role in diabetic vascular inflammation is unknown. In the present study, we showed for the first time, to our knowledge, that genistein at physiologically relevant concentrations suppresses the hyperglycemia-triggered EC-monocyte interaction, which is associated with reduced production of chemokines and adhesion molecules from EC. This action of genistein is partially dependent on the cAMP/PKA signaling pathway but is independent of the ER-mediated signaling machinery, which is often used to explain the vasculoprotective effects of genistein. These data thereby provide evidence suggesting that genistein may be a novel agent to protect vasculature from diabetes-caused inflammation and dysfunction.
In the present study, genistein at concentrations as low as 0.1 μmol/L exerted a significant antiinflammatory effect by inhibiting HG-induced adhesion of monocytes to HAEC, although the maximal effect of genistein was achieved at 5 μmol/L. Genistein is a widely used dietary supplement. The reported plasma concentrations of genistein in both humans (13) and rodents (21) through dietary supplementation are usually within the range of 1–5 μmol/L. Therefore, the effective doses of genistein observed in this study overlap the concentration range of circulating genistein in humans or in experimental animals following dietary intake of soy products or genistein supplements, which suggests that this finding may have important physiological relevance. Indeed, data from our animal study showed that aortic vessels in diabetes are activated and inflammatory as demonstrated by increased binding of monocytes to EC isolated from db/db mice, which was significantly reduced by dietary intake of genistein.
The activated EC secrete MCP-1 and IL-8 (4, 7), which play a key role in the firm adhesion of monocytes to activated ECs and subsequent monocyte recruitment into subendothelial lesion (22). In this study, there was a significant increase in the secretion of these chemokines in HAEC exposed to HG. Consistently, the serum concentrations of MCP-1/JE and KC were greater in db/db mice than those in the normal group. These results suggest that hyperglycemia may play an important role in the initiation of vascular inflammation mediated by MCP-1 and IL-8, which were shown to be major factors involved in the initiation and development of atherosclerosis (23, 24). Mice treated with genistein for 8 wk abolished diabetes-caused increases in circulating MCP-1/JE and KC, which is consistent with its suppressive effect on monocyte adhesion to MAECs. Although the major sources from where these chemokines are released are still unclear, the results from ex vivo study suggest that genistein directly acts on vascular EC to inhibit HG-induced MCP-1 and IL-8 production, which therefore may at least partially contribute to the reduced serum chemokine concentrations by genistein treatment in diabetic mice. Nevertheless, these results indicate that genistein has an antiinflammatory effect in vivo by inhibiting monocyte binding to the vascular wall. IL-10 is a well-established antiinflammatory cytokine primarily secreted by Th2 cells and regulatory T-cells and its deficiency induces the pathogenesis of atherosclerotic lesion (25), suggesting its role in the prevention of atherosclerosis. Consistently, it was reported that IL-10 can inhibit leukocyte-EC interaction in vivo, thereby exerting an antiinflammatory effect on the vascular system (26). In the present study, we observed that the circulating concentrations of IL-10 were significantly reduced in db/db mice, which were restored by genistein treatment. Although it is unclear how genistein altered plasma IL-10 concentrations, it is intriguing to speculate that the effect of genistein on vascular inflammation in vivo may be partially mediated by stimulating IL-10 production, which warrants further investigation.
Endothelium-derived adhesion molecules play a pivotal role in vascular inflammation. Circulating adhesion molecules ICAM-1, VCAM-1, and endothelial-leukocyte adhesion molecule-1 are also considered atherosclerotic inflammatory markers that are elevated and involved in diabetic vascular dysfunction in monkeys (27). They play a key role in attracting, binding, and transmigrating of leukocytes into sites of inflammation (28, 29). In this study, the secretion of ICAM-1 and VCAM-1 from aortic EC of diabetic mice were greater than that from normal mice, suggesting that the vascular wall in diabetic mice is inflammatory. However, genistein treatment normalized the secretion of these adhesion molecules from MAEC isolated from db/db+G mice. This genistein effect could be partially due to the secondary action whereby it modulates IL-8 and MCP-1 expression in the cells. Collectively, our studies of circulating and EC-derived proinflammatory molecules, including chemokines, cytokines, and adhesion molecules, show that genistein treatment appears to skew the inflammatory environment triggered by hyperglycemia toward a more regulatory profile.
Although genistein had no effect on food intake, body weight gain, and plasma lipid profiles in diabetic mice, it improved fasting blood glucose concentrations. Therefore, genistein protection of vascular inflammation in vivo may be partially ascribed to its beneficial effect on glucose homeostasis, given that chronic hyperglycemia can directly trigger vascular EC inflammation, as observed in our ex vivo study using primary HAEC. Genistein is an inhibitor of PTK (30) and is often used to study PTK-mediated signaling events. However, the antiinflammatory effect of genistein in EC may not be related to PTK inhibition, because our recent study (30) showed that the effective genistein concentrations observed in this study (0.1–10 μmol/L) had no effect on basal or agonist-stimulated PTK activity. Genistein inhibits PTK only at the 100-μmol/L concentration (30), which is 1000-fold higher than the threshold for genistein protection of inflammation in EC ex vivo and ~30-fold of the possible plasma genistein concentration in mice fed 0.1% dietary genistein. Genistein has weak estrogenic effects in some tissues and it binds to ERβ with an affinity comparable to 17β-estradiol (31) but has a considerably lower affinity for ERα. Both ER are present in vascular EC. Estrogen such as 17β-estradiol is known to inhibit adhesion of EC toward monocytes (32). We therefore evaluated whether the antiinflammatory effect of genistein in EC was mediated through ER. Our data indicate that genistein action on glucose-triggered inflammation was independent of these classical ER, because the specific ER antagonist ICI 182,780, which is considered a pure antagonist of both ERα- and ERβ-mediated estrogen action by inhibiting receptor dimerization and inducing their degradation (33–35), did not inhibit the effect of genistein but completely blocked the suppressive effect of estrogen on EC activation. Thus, this genistein effect on EC is different from the ER-mediated mechanism.
Cyclic AMP is a central signaling molecule in a variety of cellular systems. In the vasculature, activation of cAMP signaling can protect EC from inflammation by depressing leukocyte adhesion to EC (17) and maintaining normal endothelial barrier function (36), which are implicated in diabetic vascular disease. Recently, we reported that genistein, at physiologically attainable concentrations, directly activates the cAMP signaling system in primary aortic EC (15). In the present study, we further showed that the antiinflammatory effect of genistein in EC is at least partially dependent on PKA, which is downstream of cAMP. Consistently, in parallel to increased inflammation of HAECs, hyperglycemia impaired cAMP production and the subsequent PKA activation in HAEC, which were reversed by treatment with genistein.
In conclusion, genistein improves hyperglycemia-caused human vascular endothelial inflammation ex vivo, which is at least partially mediated through promoting the cAMP/PKA signaling pathway. However, whether protection or activation of this pathway plays an essential role in mediating the antiinflammatory effect of genistein in vivo needs further investigation.
Supplementary Material
Table 1 and Figure 1
Acknowledgments
P.V.A.B., H.S., and D.L. designed research; P.V.A.B., H.S., Z.F., W.Z., and D.L. conducted research; P.V.A.B. and D.L. analyzed data and wrote the paper; and D.L. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the National Center for Complimentary and Alternative Medicine in the NIH (grants R21AT004694, 3R21AT004694-02S1 to D. Liu), the Diabetes Action Research and Education Foundation (grant to D. Liu), and the AHA Mid-Atlantic Affiliate (postdoctoral fellowship award to P.V.A. Babu and a predoctoral fellowship to Z. Fu).
Supplemental Table 1 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: db/db, untreated db/db mice; db/db+G, db/db mice treated with a diet containing 1 g genistein/kg diet; EC, endothelial cell; ER, estrogen receptor; HAEC, human aortic endothelial cell; HG, high glucose; ICAM-1, intercellular adhesion molecule-1; IPGTT, i.p. glucose tolerance test; IPITT, i.p. insulin tolerance test; MAEC, mouse aortic endothelial cell; PKA, protein kinase A; PKI, protein kinase A inhibitor, PTK, protein tyrosine kinase; VCAM-1, vascular adhesion molecule-1.
Literature Cited
- 1.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81 [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26 [DOI] [PubMed] [Google Scholar]
- 3.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112 [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan S, Bolick DT, Hatley ME, Natarajan R, Reilly KB, Yeh M, Chrestensen C, Sturgill TW, Hedrick CC. Glucose regulates interleukin-8 production in aortic endothelial cells through activation of the p38 mitogen-activated protein kinase pathway in diabetes. J Biol Chem. 2004;279:31930–6 [DOI] [PubMed] [Google Scholar]
- 5.Kunt T, Forst T, Fruh B, Flohr T, Schneider S, Harzer O, Pfutzner A, Engelbach M, Lobig M, Beyer J. Binding of monocytes from normolipidemic hyperglycemic patients with type 1 diabetes to endothelial cells is increased in vitro. Exp Clin Endocrinol Diabetes. 1999;107:252–6 [DOI] [PubMed] [Google Scholar]
- 6.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan S, Yeh M, Danziger EC, Hatley ME, Riggan AE, Leitinger N, Berliner JA, Hedrick CC. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ Res. 2003;92:371–7 [DOI] [PubMed] [Google Scholar]
- 8.Nagarajan S. Mechanisms of anti-atherosclerotic functions of soy-based diets. J Nutr Biochem. 2010;21:255–60 [DOI] [PubMed] [Google Scholar]
- 9.Si H, Liu D. Phytochemical genistein in the regulation of vascular function: new insights. Curr Med Chem. 2007;14:2581–9 [DOI] [PubMed] [Google Scholar]
- 10.Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998;68:S1390–3 [DOI] [PubMed] [Google Scholar]
- 11.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:225–31 [DOI] [PubMed] [Google Scholar]
- 12.Fuchs D, Erhard P, Turner R, Rimbach G, Daniel H, Wenzel U. Genistein reverses changes of the proteome induced by oxidized-LDL in EA.hy 926 human endothelial cells. J Proteome Res. 2005;4:369–76 [DOI] [PubMed] [Google Scholar]
- 13.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867–72 [DOI] [PubMed] [Google Scholar]
- 14.Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr. 1997;127:263–9 [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Jiang H, Grange RW. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146:1312–20 [DOI] [PubMed] [Google Scholar]
- 16.Laudanna C, Campbell JJ, Butcher EC. Elevation of intracellular cAMP inhibits RhoA activation and integrin-dependent leukocyte adhesion induced by chemoattractants. J Biol Chem. 1997;272:24141–4 [DOI] [PubMed] [Google Scholar]
- 17.Morandini R, Ghanem G, Portier-Lemarie A, Robaye B, Renaud A, Boeynaems JM. Action of cAMP on expression and release of adhesion molecules in human endothelial cells. Am J Physiol. 1996;270:H807–16 [DOI] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51 [DOI] [PubMed] [Google Scholar]
- 19.Si H, Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr. 2008;138:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratmann B, Tschoepe D. Atherogenesis and atherothrombosis–focus on diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2009;23:291–303 [DOI] [PubMed] [Google Scholar]
- 21.Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR, Lubahn DB, Helferich WG, Cooke PS. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–20 [DOI] [PubMed] [Google Scholar]
- 22.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23 [DOI] [PubMed] [Google Scholar]
- 23.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–5 [DOI] [PubMed] [Google Scholar]
- 24.Lee YW, Hennig B, Toborek M. Redox-regulated mechanisms of IL-4-induced MCP-1 expression in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H185–92 [DOI] [PubMed] [Google Scholar]
- 25.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24 [DOI] [PubMed] [Google Scholar]
- 26.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88:877–87 [DOI] [PubMed] [Google Scholar]
- 27.Register TC, Cann JA, Kaplan JR, Williams JK, Adams MR, Morgan TM, Anthony MS, Blair RM, Wagner JD, Clarkson TB. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90:1734–40 [DOI] [PubMed] [Google Scholar]
- 28.Gerszten RE, Lim YC, Ding HT, Snapp K, Kansas G, Dichek DA, Cabanas C, Sanchez-Madrid F, Gimbrone MA, Jr, Rosenzweig A, et al. Adhesion of monocytes to vascular cell adhesion molecule-1-transduced human endothelial cells: implications for atherogenesis. Circ Res. 1998;82:871–8 [DOI] [PubMed] [Google Scholar]
- 29.Kevil CG, Patel RP, Bullard DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol. 2001;281:C1442–7 [DOI] [PubMed] [Google Scholar]
- 30.Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532–9 [DOI] [PubMed] [Google Scholar]
- 31.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70 [DOI] [PubMed] [Google Scholar]
- 32.Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, De Caterina R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ Res. 2000;87:19–25 [DOI] [PubMed] [Google Scholar]
- 33.Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, "pure" antiestrogen. Cancer. 2000;89:817–25 [DOI] [PubMed] [Google Scholar]
- 34.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–36 [DOI] [PubMed] [Google Scholar]
- 35.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180:727–38 [DOI] [PubMed] [Google Scholar]
- 36.Lum H, Jaffe HA, Schulz IT, Masood A. Ray Chaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol. 1999;277:C580–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 and Figure 1