Abstract
As prevention of mother-to-child HIV-1 transmission (PMTCT) programs decrease the numbers of HIV-1–infected infants, it remains important to improve growth in HIV-1–exposed, uninfected (EU) infants. To determine the growth rate and predictors of growth faltering in breast-fed and formula-fed EU infants, growth analyses [weight-for-age (WAZ), weight-for-length (WLZ), and length-for-age (LAZ) Z-scores] were conducted by using data from a randomized feeding trial in HIV-1–infected women in Kenya. Growth faltering in EU infants was compared based on randomization to breastfeeding (BF) or formula feeding (FF) using Cox proportional hazards regression models. Linear mixed-effects models determined rate and cofactors of length growth. Among 338 EU infants, 164 (49%) were breast-fed and 174 (51%) formula-fed. In both arms, growth declined steadily during follow-up. By 2 y, 29% of children were underweight (WAZ < −2), 18% were wasted (WLZ < −2), and 58% were stunted (LAZ < −2), with no differences by feeding arm. Higher maternal education (y) and taller stature (cm) were associated with a decreased risk of underweight and stunting [underweight: adjusted HR (aHR) = 0.90 (95% CI: 0.83, 0.99), P = 0.03, and aHR = 0.92 (95% CI: 0.87, 0.97), P = 0.002; and stunting: aHR = 0.91 (95% CI: 0.85, 0.97), P = 0.003, and aHR = 0.96 (95% CI: 0.92, 0.99), P = 0.02, respectively]. Diarrhea was associated with an increased risk of wasting [aHR = 2.26 (95% CI: 1.11, 4.62), P = 0.03]. In multivariate analyses, FF was associated with slower declines in length velocity [0.24 LAZ/y (95% CI: 0.06, 0.43), P = 0.009]. Despite being uninfected, HIV-1–exposed infants showed frequent growth faltering, suggesting the need for vigilance in recognizing stunting within PMTCT programs. The slower rate of decline in length growth with FF may reflect benefits of micronutrients. Because BF is the best option for HIV-1–infected mothers in resource-limited settings, nutritional interventions should be examined for their impact on growth in EU breast-fed infants.
Introduction
Accompanying the tremendous strides in PMTCT12 is a growing population of EU children. Determining how to optimize growth of uninfected infants born to HIV-1–infected women has become an area of public health importance. Lower birth weight has been described in EU infants compared with HIV-1–unexposed infants (1, 2). However, there are conflicting data regarding whether HIV-1 exposure confers growth risk postnatally. Some studies have noted minimal differences between HIV-1–exposed and HIV-1–unexposed uninfected infants, whereas others observed increased growth compromise in HIV-1–exposed infants (1–4). Maternal HIV-1 reduces passive immunity, which, in turn, may increase the risk of infections and subsequent poor growth in EU infants (5).
BF has been associated with reductions in infant morbidity and mortality, particularly in the developing world (6–8). Exclusive or prolonged BF may benefit growth in EU infants (2, 9, 10). A shorter duration of BF was associated with declines in weight (9, 10) and length growth (9) in EU infants in studies from Malawi and Zambia. There are fewer studies comparing growth among breast-fed vs. never-breast-fed EU infants in Africa. In a South African study, weight growth was considered good in both breast-fed and non-breast-fed EU infants (2).
There is need for a comprehensive understanding of growth faltering in EU infants. We previously conducted a randomized clinical trial of BF vs. FF in HIV-1–infected women in Nairobi, Kenya. In previous analyses comparing growth among all infants (both HIV-1 infected and EU), growth at 2 y was comparable, although breast-fed infants had better WLZ at 6 mo (11). We sought to determine growth rate and incidence and cofactors of growth faltering in EU infants and to compare growth among those infants in the BF and FF arms.
Participants and Methods
Study population.
We conducted secondary analysis of data collected from the Breastfeeding and HIV Transmission Study in Nairobi, Kenya, from November 1992 to July 1998, as described elsewhere (11, 12). Pregnant HIV-1–positive women were eligible to participate if they were Nairobi residents, had access to municipal-treated water, and were willing to be randomly assigned to BF or FF. This trial was conducted prior to the availability of antiretroviral prophylaxis for PMTCT in Africa; thus, none of the women received ARV.
Of 425 women enrolled in the study, 212 were randomly assigned to the BF arm and 213 to the FF arm. There were 401 live-born singletons and firstborn infants, 197 in the BF arm and 204 in the FF arm. Follow-up information and a confirmed HIV PCR negative result at the first test were available for 164 infants in the BF arm and 174 in the FF arm (Supplemental Fig. 1). The study was approved by the institutional review boards of the University of Washington and the University of Nairobi, and all women provided informed consent.
Randomization and study procedures.
Per Kenyan guidelines at the time, mothers who were randomly assigned to BF were counseled to continue BF up to 2 y and beyond. Demonstrations of safe formula preparation in addition to free infant formula were given to the formula arm. Women were counseled to introduce complementary feeding at 6 mo of age. Study staff provided guidance on the optimal use and preparation of readily available household foods as complementary foods. Infants showing early signs of growth faltering were provided with formula as an adjunct to complementary foods.
A complete physical examination was conducted in infants within 48 h of birth, at wk 2 and 6, then monthly until 12 mo, and quarterly until 24 mo to detect signs of HIV-1 infection and monitor growth and development. Weight was measured to the nearest 0.05 kg by using a beam balance scale, and recumbent length was measured to the nearest cm by using a length board. At each visit, information on duration and amount of breast-milk exposure was ascertained through maternal self-report. Infant blood samples were collected, and an HIV-1 DNA PCR assay was conducted to test for HIV-1 status at birth, at wk 6 and 14, and every 3 mo thereafter, as previously described (12). Maternal sociodemographic and behavioral characteristics were evaluated at enrollment through questionnaires. At 32 wk gestation, maternal plasma HIV-1 RNA viral load was measured by using a Gen-Probe HIV-1 RNA assay, and absolute CD4 cell count was obtained by using monoclonal antibodies and flow cytometry. Mortality and cause of death were determined by hospital record or verbal autopsy.
Statistical analysis.
Z-scores were used to standardize anthropometric measurements. The Z-score quantifies how many SD a child’s anthropometric value varies from the mean (Z-score = 0 or 50th percentile) value of a child of the same age and sex in a reference population. The 2006 WHO Child Growth Standards (13) were used to calculate WAZ, LAZ, and WLZ.
This analysis included EU infants defined as HIV-1 antibody negative by PCR assay. Excluded infants included the following: 1) those testing HIV-1 PCR positive at birth or infants who never had a negative HIV-1 test result and 2) infants with no follow-up information after birth. Duration of follow-up was defined as age at last HIV-1–negative test result or age at last regular follow-up visit, whichever was less.
Two analytical approaches were used. First, we conducted comparisons based on randomization to BF and FF by using intent-to-treat analysis. Second, because the randomized clinical trial had substantial noncompliance with the assigned feeding modality, we compared feeding as practiced: infants who ever breast-fed were compared with those who never breast-fed. Pearson’s chi-square test and Fisher’s exact test were used to compare categorical variables, and the Mann-Whitney U test was used to compare continuous variables.
We considered confounding factors based on a priori knowledge of causal influences with growth. Birth weight, birth length, and feeding modality are known to influence growth and thus were included in all multivariate models. In addition, the following variables were considered potential confounding factors: maternal age, maternal height, education, gravidity, marital status, maternal HIV-1 viral load and CD4 count, diarrheal episode in the month preceding the study visit, and early introduction of other foods. Confounding factors were included in the final multivariate model if they were associated with growth (P < 0.10) in bivariate analysis and/or influenced the other coefficients upon inclusion into the model if P ≥ 0.10.
Kaplan-Meier survival curves were used to compare time to growth faltering (Z-score of < −2) within the first 2 y of life. Specifically, growth faltering was subdivided and defined by the following parameters: underweight as a WAZ of < −2; wasting as a WLZ of < −2; and stunting as an LAZ of < −2. The log-rank test was used to compare differences between time-to-event curves. Cox proportional hazards regression models were used to estimate the risk of growth faltering for each of 3 anthropometric indicators independently, and RRs and 95% CI were calculated for covariates of interest including BF vs. FF arm. Infants who were lost to follow-up or who died were censored at the last known visit date. Infants who seroconverted to HIV-1 were censored at the last HIV-1–negative PCR test. We explored interactions between maternal height and infant birth size, modeling maternal height and birth weight and length as continuous variables, on risk of growth faltering.
Loess curves were used to plot growth profiles based on feeding modality. Linear mixed-effects models with unstructured correlation and random intercepts and slopes for time were used to assess the rate of change in length growth and to determine the influence of BF on growth profiles. We modeled length as a continuous variable and calculated change in yearly LAZ in each level of the potential confounding factor by using an interaction term between the covariate and time since birth. The multivariate model included the main effect and interaction term with time for each covariate. Details of the confounding factors were considered, and model construction was similar to those described above. Statistical analyses were conducted by using SPSS 18 (SPSS, Inc.) and STATA 11.0 (StataCorp).
Results
Characteristics of the cohort according to feeding modality are shown in Table 1. The two groups had similar enrollment characteristics, and infants in each group were comparable with regard to sex and anthropometric indexes at birth. The median follow-up time was 17.0 mo (IQR: 5.9, 23.9), and mortality did not significantly differ by feeding arm (28 deaths in the BF arm and 18 deaths in the FF arm).
TABLE 1.
Characteristics of HIV-1–infected mothers and their EU infants by feeding as randomized and as practiced1
|
Feeding as randomized |
Feeding as practiced |
|||
| BF(n = 164) | FF(n = 174) | BF(n = 196) | Never BF(n = 142) | |
| Maternal characteristics | ||||
| Age, y | 24 (21, 27) | 24 (21, 26) | 23 (21, 27) | 24 (21, 27) |
| Height, cm | 158 (154, 163) | 158 (155, 163) | 158 (154, 163) | 159 (155, 163) |
| CD4 cell count, cells/ μL | 392 (262, 552) | 419 (275, 541) | 410 (285, 559) | 390 (261, 524) |
| HIV RNA viral load, log10 | 4.7 (4.1, 5.2) | 4.6 (3.9, 5.1) | 4.6 (4.0, 5.1) | 4.7 (4.0, 5.2) |
| Gravidity ≥2, % (n) | 64 (105) | 71 (123) | 64 (126) | 72 (102) |
| Married, % (n) | 74 (121) | 79 (137) | 76 (148) | 78 (110) |
| ≤8 y of education, % (n) | 50 (82) | 50 (87) | 51 (99) | 49 (70) |
| Randomization arm, % (n) | ||||
| Breastfeeding | — | — | 79 (155) | 6 (9) |
| Formula feeding | — | — | 21 (41) | 94 (133) |
| Infant characteristics | ||||
| Female, % (n) | 50 (82) | 43 (75) | 49 (95) | 44 (62) |
| Birth WAZ | −0.28 (−0.96, 0.34) | −0.29 (−1.16, 0.51) | −0.10 (−0.96, 0.36) | −0.30 (−1.18, 0.36) |
| Birth WLZ | 0.48 (−0.34, 1.22) | 0.48 (−0.50, 1.22) | 0.47 (−0.46, 1.34) | 0.50 (−0.44, 1.22) |
| Birth LAZ | −0.67 (−1.69, 0.06) | −1.00 (−1.69, 0.06) | −0.63 (−1.69, 0.06) | −1.00 (−1.75, −0.08) |
Values are median (IQR) or percentage ( ). BF, breastfeeding; EU, HIV-1–exposed, uninfected; FF, formula feeding; LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
The median age at introduction of other foods was 4.5 mo (IQR: 3.4, 5.5), and there were no differences in median age at introduction of other foods between groups (data not shown). By 3 mo, 15% of breast-fed and 10% of formula-fed infants were receiving other foods (P = 0.13). Among infants in the BF arm who received other foods during the first 2 mo, no mothers reported difficulty feeding as a reason for introduction of complementary foods. Furthermore, maternal report of difficulty feeding and median age of introduction of other foods did not differ by maternal age, HIV-1 viral load, or CD4 count.
By 2 y, 29% of formula-fed and 29% of breast-fed infants were underweight (Supplemental Fig. 2A). Using Cox regression to examine correlates of underweight, greater WAZ at birth and greater maternal height were associated with a reduced risk of underweight (P < 0.001 for each) (Table 2). After adjustment for cofactors potentially associated with risk of underweight, greater birth WAZ (P < 0.001) and higher maternal education (P = 0.03) remained significantly associated with a reduced risk of underweight (Table 3, model 1). Because maternal height and education were colinear, a separate multivariate model was constructed including the covariates in model 1 except for education. The association between increased maternal height and lower risk of underweight remained (P = 0.002). Similar results were found when we examined the influence of BF as practiced (Table 3, model 2).
TABLE 2.
Cox regression analysis of associations between growth faltering in EU infants and maternal and infant characteristics1
|
Underweight (WAZ < −2) |
Wasting (WLZ < −2) |
Stunting (LAZ < −2) |
||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Infant characteristics | ||||||
| Feeding as randomized | ||||||
| BF arm | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| FF arm | 1.01 (0.60, 1.68) | 0.98 | 1.27 (0.64, 2.52) | 0.49 | 0.82 (0.56, 1.19) | 0.30 |
| Feeding as practiced | ||||||
| Ever breast-fed | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| Never breast-fed | 1.21 (0.73, 2.02) | 0.46 | 1.36 (0.69, 2.67) | 0.37 | 0.93 (0.64, 1.36) | 0.71 |
| WAZ, WLZ, or LAZ at birth | 0.37 (0.27, 0.52) | <0.001 | 0.76 (0.58, 1.00) | 0.05 | 0.48 (0.41, 0.56) | <0.001 |
| Diarrheal episode in past month2 | ||||||
| No | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| Yes | 1.52 (0.84, 2.73) | 0.16 | 2.37 (1.17, 4.80) | 0.02 | 0.99 (0.61, 1.63) | 0.98 |
| Maternal characteristics | ||||||
| Age, per 10 y | 1.12 (0.62, 2.00) | 0.72 | 0.61 (0.27, 1.38) | 0.23 | 1.24 (0.80, 1.93) | 0.33 |
| Plasma HIV RNA viral load, log10 | 1.31 (0.94, 1.83) | 0.11 | 0.91 (0.60, 1.39) | 0.66 | 1.16 (0.91, 1.47) | 0.24 |
| CD4 count, cells/μL | ||||||
| >200 | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| <200 | 1.03 (0.44, 2.40) | 0.95 | 0.91 (0.28, 3.02) | 0.88 | 1.12 (0.62, 2.05) | 0.71 |
| Height, cm | 0.90 (0.86, 0.95) | <0.001 | 1.02 (0.97, 1.08) | 0.39 | 0.94 (0.90, 0.97) | <0.001 |
| Education, y | 0.93 (0.85, 1.01) | 0.08 | 0.94 (0.84, 1.05) | 0.30 | 0.90 (0.85, 0.96) | 0.001 |
Values are HR (95% CI). BF, breastfeeding; EU, HIV-1–exposed, uninfected; FF, formula feeding; LAZ, length-for-age Z-score; ref, reference; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
Episode of diarrhea occurring in the month prior to Z-score < −2.
TABLE 3.
Multivariate Cox proportional hazards regression models assessing risk of growth faltering in EU infants by feeding as randomized and as practiced1
|
Underweight (WAZ < −2) |
Wasting (WLZ < −2) |
Stunting (LAZ < −2) |
||||
| aHR (95% CI) | P-value | aHR (95% CI) | P-value | aHR (95% CI) | P-value | |
| Model 1 (as randomized)2 | ||||||
| Infant feeding as randomized | ||||||
| BF arm | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| FF arm | 1.05 (0.63, 1.77) | 0.84 | 1.34 (0.67, 2.68) | 0.40 | 1.01 (0.69, 1.50) | 0.94 |
| Infant WAZ, WLZ, or LAZ at birth | 0.38 (0.27, 0.53) | <0.001 | 0.78 (0.59, 1.02) | 0.07 | 0.48 (0.40, 0.57) | <0.001 |
| Diarrheal episode in past month3 | ||||||
| No | — | — | 1.00 (ref) | — | — | — |
| Yes | — | — | 2.26 (1.11, 4.62) | 0.03 | — | — |
| Maternal HIV RNA viral load, log10 | 1.25 (0.91, 1.72) | 0.18 | 0.87 (0.57, 1.34) | 0.53 | 1.30 (1.01, 1.66) | 0.04 |
| Maternal education, y | 0.90 (0.83, 0.99) | 0.03 | — | — | 0.91 (0.85, 0.97) | 0.004 |
| Model 2 (as practiced)4 | ||||||
| Infant feeding as practiced | ||||||
| Ever breast-fed | 1.00 (ref) | — | 1.00 (ref) | — | 1.00 (ref) | — |
| Never breast-fed | 1.14 (0.67, 1.92) | 0.64 | 1.40 (0.71, 2.73) | 0.33 | 0.94 (0.64, 1.38) | 0.74 |
| Infant WAZ, WLZ, or LAZ at birth | 0.38 (0.28, 0.53) | <0.001 | 0.78 (0.59, 1.02) | 0.07 | 0.48 (0.41, 0.57) | <0.001 |
| Diarrheal episode in past month3 | ||||||
| No | — | — | 1.00 (ref) | — | — | — |
| Yes | — | — | 2.21 (1.09, 4.50) | 0.03 | — | — |
| Maternal HIV RNA viral load, log10 | 1.23 (0.89, 1.71) | 0.20 | 0.86 (0.56, 1.31) | 0.48 | 1.30 (1.02, 1.67) | 0.04 |
| Maternal education, y | 0.90 (0.82, 0.99) | 0.03 | — | — | 0.91 (0.85, 0.97) | 0.003 |
Values are aHR (95% CI); = 285 for underweight, n = 280 for wasting, n = 264 for stunting. aHR, adjusted HR; BF, breastfeeding; EU, HIV-1-exposed, uninfected; FF, formula feeding; LAZ, length-for-age Z-score; ref, reference; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
Model 1 covariates: infant feeding as randomized, infant birth Z-score, diarrheal episode, maternal HIV viral load, and maternal education. Due to colinearity, the impact of maternal education and maternal height was evaluated in separate models. When all variables in model 1 except for education were adjusted for, greater maternal height was associated with lower risk of underweight and stunting [multivariate model: aHR = 0.92 (95% CI: 0.87, 0.97), = 0.002, and aHR = 0.96 (95% CI: 0.92, 0.99), P = 0.02, respectively].
Episode of diarrhea occurring in the month prior to Z-score < −2.
Model 2 covariates: infant feeding as practiced, infant birth Z-score, diarrheal episode, maternal HIV viral load, and maternal education.
The 2-y probability of wasting did not differ significantly between the FF (22%) and the BF (13%) arms (P = 0.35) (Supplemental Fig. 2B). Greater birth WLZ was associated with a reduction in risk of wasting (P = 0.05) (Table 2). A diarrheal episode in the last month was associated with a 2-fold increased risk of wasting (P = 0.02), and this association remained in multivariate analysis (P = 0.03) (Table 3, model 1). There was a trend toward a 22% reduction in risk of wasting, per increase in WLZ at birth (P = 0.07). Models to assess feeding as practiced produced similar results (Table 3, model 2).
Among infants in the FF and BF groups, 56% and 60%, respectively, experienced stunting by 2 y (Supplemental Fig. 2C). Greater birth LAZ, greater maternal height, and a higher level of educational attainment were associated with a decreased risk of stunting (P < 0.01 for each) (Table 2). In multivariate analyses, greater birth LAZ and higher maternal education remained independently associated with reduced risk of stunting during the first 2 y of life (P < 0.001 and P = 0.004, respectively) (Table 3, model 1). Furthermore, there was a 30% increased risk of stunting per log increase in maternal viral load (P = 0.04). In a separate multivariate model that excluded maternal education, greater maternal height remained associated with a reduced risk of stunting [aHR = 0.96 per cm (95% CI: 0.92, 0.99), P = 0.02]. Findings were similar in a multivariate model that examined feeding as practiced (Table 3, model 2). In the secondary analysis we found no significant interaction between maternal height and infant birth size.
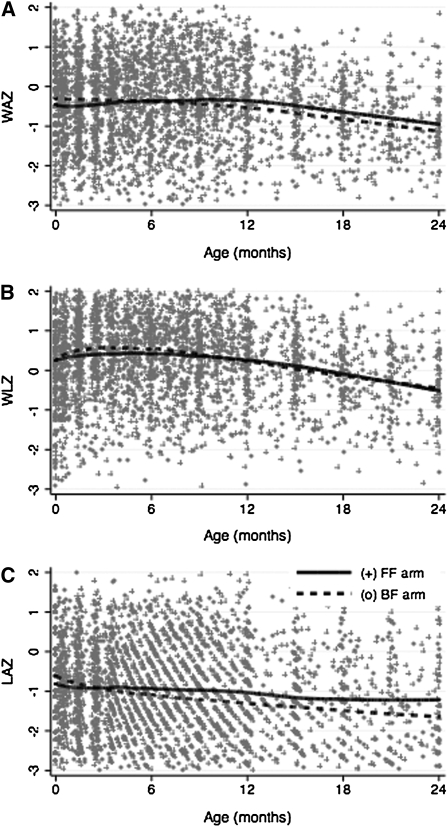
Growth patterns showed a modest, nonsignificant increase in WAZ and WLZ during the first 6 mo, after which weight growth declined in both groups (Fig. 1A, B). There were no differences in the rate of change in WAZ or WLZ by feeding modality in intent-to-treat or as-practiced analyses. However, there was a significantly slower rate of decline in LAZ growth among infants in the FF arm compared with those in the BF arm (P = 0.03) (Fig. 1C, Table 4). The overall rate of change in length during the first 2 y of life was −0.37 LAZ/y (95% CI: −0.47, −0.27; P < 0.001). Greater LAZ at birth was associated with more rapid declines in the rate of yearly length growth (P < 0.001), whereas slower declines in length growth were noted among infants with more educated mothers (P = 0.02). There was no difference in the rate of decline in length growth on the basis of sex [mean change = −0.11 LAZ/y (95% CI: −0.31, 0.10), P = 0.31]. In addition, diarrhea was not associated with length velocity [mean change = 0.04 LAZ/y (95% CI: −0.09, 0.17), P = 0.54].
FIGURE 1.
Change in WAZ (A), WLZ (B), and LAZ (C) in EU infants by randomization to the BF or FF study arm. Curves represent locally weighted smoothed curves of growth over time, by feeding modality. BF, breastfeeding; EU, HIV-1–exposed, uninfected; FF, formula feeding; LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
TABLE 4.
Bivariate and multivariate linear mixed-effects models assessing predictors of yearly change in length growth in EU infants by feeding as randomized and as practiced1
|
Bivariate |
Multivariate model 12Feeding as randomized |
Multivariate model 23Feeding as practiced |
||||
| Mean change,units/y (95% CI) | P-value | Mean change,units/y (95% CI) | AdjustedP-value4 | Mean change,units/y (95% CI) | AdjustedP-value4 | |
| Infant characteristics | ||||||
| Feeding as randomized | ||||||
| BF arm | Ref | — | Ref | — | — | — |
| FF arm | 0.24 (0.03, 0.44) | 0.03 | 0.24 (0.06, 0.43) | 0.009 | — | — |
| Feeding as practiced | ||||||
| Ever breast-fed | Ref | — | — | — | Ref. | — |
| Never breast-fed | 0.30 (0.10, 0.51) | 0.004 | — | — | 0.22 (0.04, 0.41) | 0.02 |
| LAZ at birth | −0.24 (−0.31, −0.18) | <0.001 | −0.25 (−0.32, −0.18) | <0.001 | −0.24 (−0.31, −0.18) | <0.001 |
| Maternal characteristics | ||||||
| HIV viral load, log10 | 0.10 (−0.03, 0.23) | 0.13 | 0.02 (−0.10, 0.13) | 0.79 | 0.003 (−0.11, 0.12) | 0.96 |
| Education, y | 0.04 (0.01, 0.08) | 0.02 | 0.04 (0.01, 0.07) | 0.01 | 0.04 (0.01, 0.07) | 0.02 |
| Height, cm | 0.01 (−0.01, 0.03) | 0.15 | — | — | — | — |
Values are means (95% CI); = 2862 observations across 269 individuals. Values represent the difference between the mean yearly change in Z-score for children with the characteristic compared with the mean yearly change in Z-score for children in the reference group. Interpretation: The rate of decline in the FF arm is 0.24 LAZ/y lower than the rate of decline in the BF arm. BF, breastfeeding; EU, HIV-1–exposed, uninfected; FF, formula feeding; LAZ, length-for-age Z-score; Ref, reference.
Model 1 covariates: infant feeding as randomized, infant LAZ at birth, maternal HIV viral load, and maternal education. Due to colinearity, the impact of maternal education and maternal height were evaluated in separate models. When all variables in model 1 except for education were adjusted for, greater maternal height was associated with lower declines in length growth (mean change/y = 0.02; 95% CI: 0.005, 0.04; = 0.01).
Model 2 covariates: infant feeding as practiced, infant LAZ at birth, maternal HIV viral load, and maternal education. When all variables in model 2 except for education were adjusted for, greater maternal height was associated with lower declines in length growth (mean change/y = 0.02; 95% CI: 0.003, 0.04; = 0.02).
The -value represents the result of a statistical test of the difference in adjusted average rates of change in growth between children with and without the characteristic.
In multivariate analysis, the FF arm had significantly slower decline in length growth as compared with the BF arm (P = 0.009) (Table 4, multivariate model 1). The adjusted mean rate of decline was −0.91 LAZ/y (95% CI: −1.48, −0.34; P = 0.002) in the FF arm and −1.15 LAZ/y (95% CI: −1.73, 0.57; P < 0.001) in the BF arm. Greater birth LAZ remained associated with faster rate of decline in length growth (P < 0.001), and higher maternal education remained associated with slower decline in rate of length growth (P = 0.01). Findings were similar in the multivariate model that examined feeding as practiced (Table 4, multivariate model 2). The adjusted mean rate of decline was −0.80 LAZ/y (95% CI: −1.39, −0.21; P = 0.008) in never-breast-fed infants and −1.03 LAZ/y (95% CI: −1.60, −0.45; P < 0.001) in ever-breast-fed infants.
Discussion
In this cohort of EU infants we made several important observations. Despite being uninfected, infants born to HIV-1–infected mothers experienced steady declines in growth, and >50% experienced stunting by 2 y of age. Stunting and underweight were associated with maternal education and stature, and the risk of wasting increased with history of diarrhea. Infants with greater weight and length at birth were less likely to experience growth faltering during the first 2 y of life. Although WAZ and WLZ did not decline until after 6 mo of age, declines in length growth started from birth. The rate of decline in length growth was significantly slower among infants in the FF arm, which suggests a potential role of nutritional supplementation to improve length growth during later BF in resource-limited settings.
Overall, the 2-y probability of stunting, underweight, and wasting in our cohort was 58%, 29%, and 18%, respectively. Although our cohort was not HIV-1 infected, these numbers are higher than the proportion of growth faltering at age 2 y in the Kenyan general population: 52% stunted, 20% underweight, and 7% wasted (14). Rates of growth faltering vary widely in studies of EU children and range from 5 to 40% in African cohorts (4, 10, 15). Furthermore, the use of different growth curves (CDC and WHO) makes it difficult to directly compare results of growth faltering (13, 16). Given extreme variability in poverty and malnutrition in developing country settings, these findings underscore the importance of monitoring growth and promoting early detection and prevention of growth faltering in EU children.
This study was conducted before PMTCT antiretroviral use, which enabled assessment of HIV-1 exposure that was not confounded by antiretroviral therapy. There was an association between stunting and HIV-1 RNA viral levels, which suggests that antiretroviral therapy may decrease stunting. We did not find an association between growth or growth faltering and maternal immunosuppression. This is consistent with a study from the Democratic Republic of Congo (1) but differs from a South African study in which maternal immunosuppression was associated with stunting and underweight (17). In addition, because in utero exposure to specific ARV has been associated with low birth weight it is possible that rather than ameliorating growth faltering, maternal highly active antiretroviral therapy (HAART) or ARV may increase susceptibility to growth faltering (18, 19). EU infants in Botswana exposed to HAART in utero had lower length at birth and at age 6 mo compared with zidovudine-exposed infants (20). In our study, all birth growth indicators were significantly associated with later infant growth. Further studies to determine predictors of birth growth may be useful.
Malnutrition was high; 58% of children in our cohort were stunted by 2 y of age. Stunting is associated with poverty, poor nutrition, and coinfections and may be associated with impaired cognitive development and school attainment (21–25). Both maternal education and stature influenced the likelihood of underweight and stunting, which is consistent with other studies in similar settings (1, 25, 26). Higher levels of maternal education may influence feeding, hygiene, and use of health services. Whereas height is partly heritable, early stunting is more so an indicator of poverty, and poor socioeconomic conditions can lead to an intergenerational cycle of stunting (1, 26–29).
In our study, feeding modality was not associated with growth faltering. We did, however, observe a significantly slower rate of decline in length growth during the 2-y follow-up in formula-fed infants compared with breast-fed infants. It is plausible that the micronutrient or macronutrient content of infant formula may be responsible for this observation. Maternal nutritional status during lactation directly influences infant health (30, 33). Maternal micronutrient deficiencies may affect breast-milk composition and the development and nutritional status of the infant (33). Furthermore, high prevalence of concurrent micronutrient deficiencies, including iron, zinc, and vitamins A, B-12, and D, may contribute to growth faltering and poor height growth among children in Africa (34, 35). The Millennium Developmental Goals do not specifically address micronutrient deficiencies; however, their reduction may be essential to achieving goals related to childhood growth (30).
An inherent strength of this study was the randomization of feeding modality. This provided a unique and unbiased opportunity to evaluate the influence of BF and FF on infant growth. Whereas noncompliance was 24% in the FF arm and 5% in the BF arm, our results were similar for intent-to-treat and as-practiced analyses.
Our study does have several limitations. The close monitoring of mothers and infants throughout follow-up, nutritional counseling, and the free provision of infant formula and counseling on its preparation may have limited generalizability. In programmatic settings it is possible that growth compromise in formula-fed infants may occur if infants receive inadequate amounts of formula or if mothers are not counseled on proper preparation. Whereas the WHO recommends exclusive BF through 6 mo of age, only 32% of Kenyan children <6 mo of age are exclusively breast-fed (14), and <5% of infants in our study were exclusively breastfed at 6 mo.
Despite being uninfected, EU infants are at risk for growth compromise. In resource-limited settings, EU infants may benefit from multidimensional nutritional interventions designed to improve maternal health, BF, and complementary feeding in early childhood. Emphasis should be placed on the monitoring of length growth and early identification of high-risk children, including EU infants and young children with poor length (LAZ < −1), because these children can be expected to be at greatest risk of stunting.
Supplementary Material
Acknowledgments
C.J.M., G.C.J.-S., A.R.K., C.F., B.A.R., and R.N. conceived and designed the study; G.C.J.-S., R.N., and D.M.-N. collected data; C.J.M., A.R.K., and B.A.R. performed statistical analysis; and C.J.M. and G.C.J.-S. drafted the manuscript. All authors contributed to data interpretation and critical revision of the manuscript for intellectual content. All authors read and approved the final manuscript.
Footnotes
Supported by grant NICHD-23412 from the NIH. Other funding sources include NIH TL1 (RR025016 to C.J.M.), NIH K24 grant (HD054314 to G.C.J.-S.), NIH K24 grant (AI087399 to C.F.), the University of Washington Center for AIDS Research (NIH P30 AI027757), and the International AIDS Research and Training Program, supported by the Fogarty International Center (D43-TW00007 to G.C.J.-S., R.N., and D.M.-N.).
Supplemental Figures 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: ARV, antiretroviral drugs; BF, breastfeeding; EU, HIV-1–exposed, uninfected; FF, formula feeding; LAZ, length-for-age Z-score; PMTCT, prevention of mother-to-child transmission of HIV-1; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
Literature Cited
- 1.Bailey RC, Kamenga MC, Nsuami MJ, Nieburg P, St Louis ME. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol. 1999;28:532–40 [DOI] [PubMed] [Google Scholar]
- 2.Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, Newell ML. Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS. 2010;24:437–45 [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Borja MC, Peckham C. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111:e52–60 [DOI] [PubMed] [Google Scholar]
- 4.Bobat R, Coovadia H, Moodley D, Coutsoudis A, Gouws E. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21:203–10 [DOI] [PubMed] [Google Scholar]
- 5.Farquhar C, Nduati R, Haigwood N, Sutton W, Mbori-Ngacha D, Richardson B, John-Stewart G. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr. 1995;126:696–702 [DOI] [PubMed] [Google Scholar]
- 7.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–5 [PubMed] [Google Scholar]
- 8.Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol. 2010;37:843–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taha T, Nour S, Li Q, Kumwenda N, Kafulafula G, Nkhoma C, Broadhead R. The effect of human immunodeficiency virus and breastfeeding on the nutritional status of African children. Pediatr Infect Dis J. 2010;29:514–8 [DOI] [PubMed] [Google Scholar]
- 10.Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, Thea DM, Kuhn L. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha A, Ndinya-Achola J, Bwayo J, Kreiss J. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA. 2001;286:2413–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74 [DOI] [PubMed] [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization, 2006 [Google Scholar]
- 14.Kenya National Bureau of Statistics Kenya Demographic and Health Survey 2008–2009. Calverton (MD): KNBS and ICF Macro, 2010. p.455 [Google Scholar]
- 15.Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, Wester C, Moffat C, Arimi P, Ndase P, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007;196:562–9 [DOI] [PubMed] [Google Scholar]
- 16.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15 [PubMed] [Google Scholar]
- 17.Venkatesh KK, Lurie MN, Triche EW, De Bruyn G, Harwell JI, McGarvey ST, Gray GE. Growth of infants born to HIV-infected women in South Africa according to maternal and infant characteristics. Trop Med Int Health. 2010;15:1364–74 [DOI] [PubMed] [Google Scholar]
- 18.Machado ES, Hofer CB, Costa TT, Nogueira SA, Oliveira RH, Abreu TF, Evangelista LA, Farias IF, Mercadante RT, Garcia MF, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009;85:82–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekouevi DK, Coffie PA, Becquet R, Tonwe-Gold B, Horo A, Thiebaut R, Leroy V, Blanche S, Dabis F, Abrams EJ. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS. 2008;22:1815–20 [DOI] [PubMed] [Google Scholar]
- 20.Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden-Peterson S, van Widenfelt E, Leidner J, Makhema J, Essex M, Shapiro RL. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56:131–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129:1555–62 [DOI] [PubMed] [Google Scholar]
- 23.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–71 [DOI] [PubMed] [Google Scholar]
- 24.Walker SP, Chang SM, Powell CA, Grantham-McGregor SM. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet. 2005;366:1804–7 [DOI] [PubMed] [Google Scholar]
- 25.Adair LS, Guilkey DK. Age-specific determinants of stunting in Filipino children. J Nutr. 1997;127:314–20 [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan U, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. J Nutr. 1999;129:544S–9S [DOI] [PubMed] [Google Scholar]
- 27.Stein AD, Barnhart HX, Wang M, Hoshen MB, Ologoudou K, Ramakrishnan U, Grajeda R, Ramirez-Zea M, Martorell R. Comparison of linear growth patterns in the first three years of life across two generations in Guatemala. Pediatrics. 2004;113:e270–5 [DOI] [PubMed] [Google Scholar]
- 28.Ashworth A, Morris SS, Lira PI. Postnatal growth patterns of full-term low birth weight infants in Northeast Brazil are related to socioeconomic status. J Nutr. 1997;127:1950–6 [DOI] [PubMed] [Google Scholar]
- 29.Tanner JM, Whitehouse RH, Marshall WA, Carter BS. Prediction of adult height from height, bone age, and occurrence of menarche, at ages 4 to 16 with allowance for midparent height. Arch Dis Child. 1975;50:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60 [DOI] [PubMed] [Google Scholar]
- 31.Allen LH. Malnutrition and human function: a comparison of conclusions from the INCAP and nutrition CRSP studies. J Nutr. 1995;125:1119S–26S [DOI] [PubMed] [Google Scholar]
- 32.McCullough AL, Kirksey A, Wachs TD, McCabe GP, Bassily NS, Bishry Z, Galal OM, Harrison GG, Jerome NW. Vitamin B-6 status of Egyptian mothers: relation to infant behavior and maternal-infant interactions. Am J Clin Nutr. 1990;51:1067–74 [DOI] [PubMed] [Google Scholar]
- 33.Allen LH. Maternal micronutrient malnutrition: effects on breast milk and infant nutrition, and priorities for intervention. SCN News. 1994;11:21–4 [PubMed] [Google Scholar]
- 34.Chhagan MK, Van den Broeck J, Luabeya KK, Mpontshane N, Tomkins A, Bennish ML. Effect on longitudinal growth and anemia of zinc or multiple micronutrients added to vitamin A: a randomized controlled trial in children aged 6–24 months. BMC Public Health. 2010;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann CG, Bwibo NO, Murphy SP, Sigman M, Whaley S, Allen LH, Guthrie D, Weiss RE, Demment MW. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design and baseline findings. J Nutr. 2003;133:3941S–9S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.