Abstract
The integration of nutrition support for infants of HIV-infected mothers is a recognized need; however, the evidence for effective programmatic solutions is weak. The objective of our study was to implement and evaluate a new infant feeding support intervention for HIV-exposed, uninfected, non–breast-fed infants 6–12 mo of age attending the Groupe Haïtien d’Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) pediatric clinic in Port-au-Prince, Haiti. The 24-wk intervention included a lipid-based nutrient supplement, education, promotion of existing clinical services, and social support. We compared growth outcomes among intervention participants (n = 73) at start (wk 0) and end (wk 24) of intervention to a historical control group of HIV-exposed infants seen at the GHESKIO in the year prior to the intervention who would have met the intervention entrance criteria (n = 294). The intervention and historical control groups did not differ significantly at age 6 mo (wk 0). At age 12 mo (wk 24), the intervention group had a lower prevalence of underweight and stunting than the historical control group (weight-for-age Z-score < −2 SD: 6.8 vs. 20.8%, P = 0.007; length-for-age Z-score < −2 SD: 9.6 vs. 21.2%, P = 0.029). Wasting tended to be lower in the intervention group than the historical control (weight-for-length Z-score < −2 SD: 2.9 vs. 8.9%, P = 0.11). Implementation of the intervention was associated with reduced risk of growth faltering in HIV-exposed uninfected children from 6 to 12 mo of age. This is a promising intervention model that can be adapted and scaled-up to other HIV care contexts.
Introduction
The need for better nutrition during infancy to improve growth and child survival outcomes is well recognized (1–5) and is especially acute in the context of maternal HIV infection. The WHO Guidelines on Infant Feeding and HIV call for support of HIV-infected caregivers during infant feeding transitions for the first year of life (6). Integration of nutrition care and support for clinical PMTCT7 services is a global health priority (7), but there is a weak evidence base for effective intervention models to support infant feeding after 6 mo of age.
In 2007, >85% of HIV-exposed infants at the GHESKIO (Groupe Haïtien d’Etude du Sarcome de Kaposi et des Infections Opportunistes) centers in Port-au-Prince, Haiti, were replacement-fed from birth (8). The clinic provided a 6-mo supply of no-cost infant formula to HIV-infected caregivers who chose to feed breast milk substitutes and who were enrolled in the PMTCT program. A review of pediatric clinic medical records confirmed observations made by GHESKIO clinicians that the risk of growth faltering in HIV-exposed uninfected infants increased from age 6 mo onwards (9). No infant feeding support protocols existed at GHESKIO after 6 mo of age.
Recent advances in the production of fortified food products have invigorated support for infant feeding programs globally. The use of specially designed infant food products combined with behavior change education is efficacious and is a recommended approach for reducing the risk of stunting and the burden of disease in children 6–24 mo of age (10, 11). However, the impact of infant feeding interventions has been variable and evidence is weak on how to adapt such interventions to the needs of HIV-affected households (12–14).
The objective of our study was to implement and evaluate a new clinic-based, infant feeding support intervention at GHESKIO targeting HIV-exposed, non-breast-fed infants between 6 and 12 mo of age. The intervention was designed to increase infant nutrient intake through the provision of a ready-to-use fortified food supplement. Behavior change education about infant feeding, hygiene, and diarrhea treatment was designed to improve overall dietary nutrient intake and decrease nutrient losses due to diarrheal illness.
Participants and Methods
Study population.
The intervention study was conducted from July 2008 to October 2009 at the GHESKIO Centers, a Haitian nongovernmental organization dedicated to clinical care, research, and training in HIV/AIDS and related infectious diseases. GHESKIO provides free medical care to HIV-affected clients from across the Port-au-Prince metro region, most of whom live on <$1/d.
HIV-infected women who received prenatal care through GHESKIO’s PMTCT program were given perinatal ARV (antiretroviral) prophylaxis per Ministry of Health and Population guidelines and counseled about options for feeding during early infancy. GHESKIO provided pediatric clinical care to all HIV-exposed children for the first 24 mo of life. All HIV-exposed infants received cotrimoxazole prophylaxis from their first clinic visit until negative HIV status was confirmed. Pediatric highly active ARV therapy was provided to all confirmed HIV-infected infants.
HIV-exposed uninfected children aged 5.5–6.5 mo without severe malnutrition [WLZ (weight-for-length Z-score) > −3 SD) and no known allergy to peanuts who visited the pediatric clinic between 15 June 2008 and 5 January 2009 were eligible for recruitment into the intervention group. Potential participants were identified by pediatric clinic staff from the daily clinic visit registry. Infants were screened for enrollment based on birth date and maternal HIV test result as recorded in the GHESKIO enrollment database. Oral and written informed consents were obtained from caregivers prior to study enrollment.
The study was approved by the GHESKIO Centers Institutional Review Board in Port-au-Prince, Haiti, the Cornell University Committee on Human Subjects in Ithaca, NY, and the Weill Cornell Medical College Institutional Review Board in New York, NY.
Study design.
This study assessed of the effectiveness of a 24-wk intervention for the prevention of growth faltering in HIV-exposed children. Due to ethical concerns and logistical constraints, it was not feasible to randomize treatment or follow a concurrent control group. Growth outcomes in intervention participants were compared with children of the same age who were seen at GHESKIO in the year prior to the intervention. This historical control group was constructed from medical records from all HIV-exposed uninfected children aged 5.5–12.5 mo who were born between 1 November 2005 and 15 December 2006, had a least one clinic visit after July 2007 when the clinic obtained length measuring equipment, and were not enrolled in a small pediatric study protocol conducted at GHESKIO during part of that period. The number and timing of visits available in the medical records of historical control individuals varied, as control children were seen during routine and sick child visits scheduled according to individual needs. Less than one-half of the historical control children had growth outcomes at both 6 and 12 mo of age. Therefore, achieved growth was compared cross-sectionally at the two time points. Models of longitudinal growth from 5.5 to 12.5 mo of age used all available data points for the two groups of children and accounted for repeated measures on individual children.
Intervention.
The intervention included three activities: 1) provision of a daily ration of lipid-based supplement; 2) infant feeding education delivered through biweekly group and individualized counseling sessions; and 3) promotion of existing clinical services, including immunizations, high-dose vitamin A supplementation, oral rehydration solution, chlorine water purification solution, and World Food Program household food rations. Participants continued routine and sick child visits with GHESKIO pediatricians.
Caregivers and infants came to the clinic every 2 wk across the 24-wk intervention period. Children were enrolled in the intervention at age 5.5–6.5 mo and were 11.0–12.35 mo of age at intervention end. All participants were assigned to a “caregiver club” for group education and social support that included 8–12 infants born within 4 wk of one another and their caregivers. The analysis includes children who completed the full 24-wk intervention period.
A team of two nutrition counselors and two study assistants delivered all intervention activities at the GHESKIO site. Each visit included a brief interview about illness and supplement use since last visit, infant feeding education, and distribution of supplement. For the education component, visits alternated between one-on-one visits with a counselor for growth monitoring and individualized counseling and club visits for interactive group-based infant feeding education and social support.
To help ensure delivery of the supplement, missed visits were followed up by study staff through phone calls and/or home visits as feasible. Educational messages from missed group lessons were reviewed during subsequent individual visits.
Education sessions.
Topics for individual counseling and group education were based on key practices identified in WHO guidance (15). Based on formative interviews, context-specific messages were developed about food choice, quantity, and frequency of feeding, responsive feeding behaviors, hygiene, and diarrhea care. Each 45- to 60-min group education session was delivered by a staff counselor who followed a structured curriculum. Key messages were reinforced during the individual visits.
Supplementary food.
The daily supplement ration provided ~50% of daily energy needs, approximating the usual energy intake from breast milk in fully breast-fed children in this age group (13, 15) plus one RDA or Adequate Intake of key micronutrients for the target age group. The lipid-based nutrient supplement used in the study was produced by Meds and Foods for Kids. The ingredient composition by weight was 25% peanuts, 30% milk powder, 28% sugar, 15% oil, and 14% micronutrient pre-mix. The targeted daily ration of 65 g provided 345 kcal and an RDA/Adequate Intake of problem micronutrients for children age 6–12 mo (Table 1). Each caregiver was given two 500-g plastic, screw-top containers of supplement at each biweekly visit and a separate small glass measuring jar. The number of spoonfuls needed to achieve the 65-g daily ration was determined for each caregiver based on their measuring style, which we asked them to demonstrate. Caregivers were advised to feed the supplement over single or multiple feeding episodes by mixing the supplement into a small portion of soft food or feeding directly from a spoon depending on child preferences. Participants were advised against sharing the supplement with other children in the household.
TABLE 1.
Energy and micronutrient content of the supplement1
| Nutrient | unit/100 g | unit/65 g |
| Energy, kcal | 529 | 344 |
| Protein, g | 15.9 | 10.3 |
| Fat, g | 33.5 | 21.8 |
| Calcium, mg | 415 | 270 |
| Iron, mg | 17.0 | 11.0 |
| Zinc,2mg | 7.7 | 5.0 |
| Iodine, μg | 200 | 130 |
| Folate, DFE | 123 | 80 |
| Vitamin A, μg RAE | 693 | 500 |
DFE, dietary folate equivalent; RAE, retinol activity equivalent.
Zinc fortification levels were based on recommendations from the International Zinc Nutrition Consultative Group.
Measurement of outcomes.
The primary outcomes were weight-for-age, length-for-age, and weight-for-length at age 12 mo. These were analyzed using the WHO 2006 Growth Standard (16) as both Z-scores and prevalence of underweight [WAZ (weight-for-age Z-score) < −2], stunting [LAZ (length-for-age Z-score) < −2], and wasting (WLZ < −2).
The length and weight of intervention participants were measured every 4 wk. Length was measured in the recumbent position using an infantometer (Easy-Glide Bearing Infantometer, Perspective Enterprises) to the nearest 0.1 cm and weight was measured on a research-quality digital scale (Tanita Model 1583) to 0.01 kg. Growth data for the historical control group was collected using the same equipment and protocols as the intervention group. The staff was trained to take measures in duplicate with a third measure added if there was a discrepancy. Clinic staff was standardized on these measures when the equipment was first introduced and received refresher training during the intervention period.
The use of the supplement was assessed every 2 wk in the intervention group by weighing all jars delivered to and returned by caregivers. Caregivers were asked directly about supplement consumption by the child since the previous distribution and feeding of supplement was assessed in 24-h dietary recalls at wk 12 and 24. Attendance was documented at all visits.
Infant feeding-, hygiene-, and diarrhea-related knowledge and practices were assessed in the intervention group using a survey at intervention wk 0 and 24. Results for knowledge outcomes are presented in the paper.
HIV status, immunization, and vitamin A records and a limited number of demographic variables were collected for all study children from GHESKIO clinical records. Additional data on socioeconomic status and access to GHESKIO services were collected for the intervention group using surveys at study enrollment and final intervention visit.
Sample size.
A sample size of 82 intervention children was estimated to be sufficient to detect a 0.5 LAZ difference between the intervention and historical control groups with 95% sensitivity and 80% power. This was calculated based on effect sizes observed in moderately malnourished Malawian children receiving a lower dosage of a similar lipid-based nutrient supplement without education targeted to the mother (17). Our sample size calculation assumed that 10% of the intervention children would not complete the 24-wk intervention, 10% might be excluded due to HIV infection, and up to 25% might be excluded if the mother did not receive prenatal care at GHESKIO.
Statistical analysis.
Data management was completed using Microsoft Access version 2000 and all statistical analyses were performed using SPSS software version 19. Growth Z-scores were calculated using the WHO Anthro macro for SPSS.
To assess cross-sectional growth outcomes at age 6 mo (wk 0) and 12 mo (wk 24), WAZ, LAZ, and WLZ were compared between intervention and historical control group using a t test and prevalence of underweight, stunting, and wasting were compared by Pearson’s chi-square or Fisher’s Exact test. Multilevel models for change controlling for group and child age at visit were used to test for a treatment effect on the slopes of growth trajectories across the 6- to 12-mo period. The SPSS 19 MIXED procedure with random intercept and random slopes accounts for repeated measures on individuals and allows for irregularly spaced measurements and missing values across individuals. The adjusted models included infant birth weight and maternal education as covariates. Covariates were selected based on a previous analysis of growth patterns among GHESKIO infants (9).
For all other outcomes including adherence-related and knowledge-related variables, means were assessed by t test and categorical variables by Pearson’s chi-square or Fisher’s exact test. Significance was accepted at a P < 0.05 level and all tests were two-sided. Values presented in the text are means ± SD or percentages.
Results
Eighty-two children were enrolled in the intervention group. Seventy-three HIV-negative infants completed the intervention and were included in the analyses of 24-wk outcomes. There was a total of 294 HIV-exposed, uninfected children included in the historical control group, contributing 944 observations to the longitudinal analysis between 5.5 and 12.5 mo of age (Supplemental Fig. 1).
At age 6 mo (Table 2), there were no differences between intervention and historical control groups in gender, proportion enrollment in the GHESKIO PMTCT program, bacille Calmette-Guerin vaccine coverage, or other maternal variables. Several variables suggested that the intervention group was slightly worse off at age 6 mo than the historical controls: birth weight tended to be lower (P = 0.07) and fewer children in the intervention group had completed perinatal ARV prophylaxis (P = 0.016) (Table 2).
TABLE 2.
General characteristics of children in the intervention and historical control groups1
| Characteristic | n | Intervention | n | Historical control | P-value |
| Infant characteristics | |||||
| Female gender, % | 73 | 49.3 | 294 | 49.0 | 0.96 |
| PMTCT participant, % | 73 | 89.0 | 294 | 85.0 | 0.36 |
| Birth weight, kg | 62 | 2.82 ± 0.55 | 231 | 2.95 ± 0.49 | 0.07 |
| Birth weight <2500 g, % | 62 | 22.6 | 231 | 15.6 | 0.19 |
| Completed short course ARV prophylaxis, % | 63 | 60.3 | 250 | 76.4 | 0.016 |
| Received bacille Calmette-Guerin vaccine, % | 72 | 91.7 | 275 | 93.8 | 0.43 |
| Ever breast-fed before age 6 mo, % | 73 | 12.3 | n.a. | n.a. | n.a. |
| Still breast-fed at age 6 mo, % | 73 | 0.0 | n.a. | n.a. | n.a. |
| Received free Enfamil before age 6 mo, % | 73 | 90.4 | n.a. | n.a. | n.a. |
| Started semi-solids before age 6 mo, % | 73 | 79.5 | n.a. | n.a. | n.a. |
| Maternal characteristics | |||||
| Education ≥ any secondary school, % | 67 | 50.7 | 269 | 50.6 | 0.98 |
| On ARV during 0- to 24-mo period, % | 64 | 37.5 | 258 | 31.8 | 0.38 |
| Employed in previous 12 mo, % | 73 | 30.1 | n.a. | n.a. | n.a. |
| Household characteristics | |||||
| Household asset index2 | 73 | 10.2 ± 2.6 | n.a. | n.a. | n.a. |
| Has a water source in home, % | 73 | 49.3 | n.a. | n.a. | n.a. |
| Has an improved latrine or toilet, % | 73 | 64.4 | n.a. | n.a. | n.a. |
Values are mean ± SD or percentage, of children with available data for each characteristic varies as indicated. The maximum number of children included in the analysis was n = 73 (intervention group) and n = 294 (historical control). ARV, antiretroviral; n.a., data not available; PMTCT, prevention of mother-to-child transmission.
Household asset index is a measure of how many total assets are owned by the participant's household of a list of 16 items appropriate to the urban Haiti context.
Provision and utilization of intervention.
There were no documented interruptions in the provision of the intervention by the GHESKIO staff team. The disappearance of supplement across the 24-wk intervention was 97.0% ± 3.51 based on weighing of jars. A total of 96.4% of participants used at least 90% of all supplements provided. There was good agreement between caregiver responses to direct questions about whether supplement was fed to the child in the previous day (80% at wk 12, 73.6% at wk 24) compared to supplement use in the previous day reported in the 24-h recall (80.8% at wk 12, 63.0% at wk 24).
The attendance on caregiver club visit days was 87.4 ± 15.7%. Using a stricter measure requiring the caregiver to be present for at least 50% of the club teaching time, the attendance rate at caregivers clubs was 73.3 ± 22.2%.
Participation in the intervention was associated with increased utilization of other clinical services. A total of 97.3% of the intervention group received at least one dose of vitamin A compared to 28.7% of the historical control. Within the intervention group, 95.9% reported ever receiving a household food ration from GHESKIO at wk 24 compared to 52.1% at wk 0.
Impact on growth.
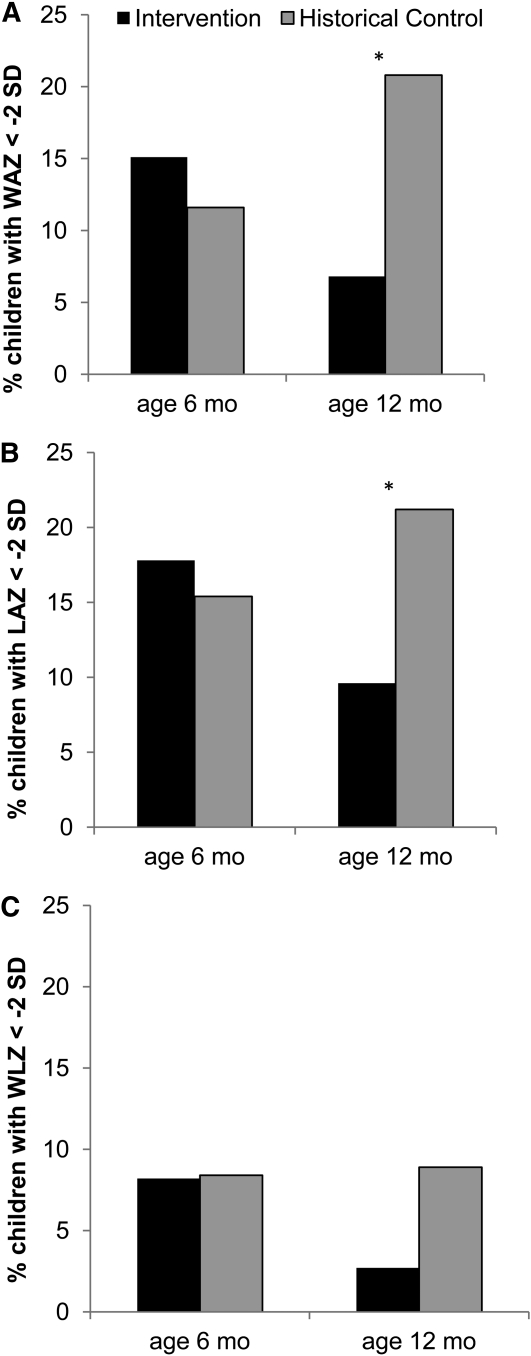
Cross-sectional comparisons of the intervention and historical control groups showed no significant differences in Z-scores at either age 6 or 12 mo (Table 3). There were no significant differences in prevalence of stunting, wasting, and underweight at 6 mo. In 12-mo-old infants, the prevalence of underweight was 67.3% lower (6.8 vs. 20.8%; P = 0.007) and stunting was 54.7% lower (9.6 vs. 21.2%; P = 0.029) in the intervention compared to historical control. There tended to be a 69.7% lower prevalence of wasting at age 12 mo in the intervention group (2.9 vs. 8.9%; P = 0.11) (Fig. 1).
TABLE 3.
Anthropometric outcomes of children in the intervention and historical control groups at age 6 mo (wk 0) and age 12 mo (wk 24)1
| Outcome | Group | n | 6 mo | n | 12 mo |
| WAZ | Intervention | 73 | −0.69 ± 1.09 | 73 | −0.58 ± 0.97 |
| Historical control | 138 | −0.49 ± 1.19 | 192 | −0.77 ± 1.39 | |
| LAZ | Intervention | 73 | −0.78 ± 1.31 | 73 | −0.74 ± 1.20 |
| Historical control | 130 | −0.64 ± 1.40 | 179 | −0.79 ± 1.41 | |
| WLZ | Intervention | 73 | −0.16 ± 1.19 | 73 | −0.28 ± 0.98 |
| Historical control | 131 | 0.01 ± 1.32 | 180 | −0.43 ± 1.21 |
Values are mean ± SD, varies by time point and group as indicated. Age range of intervention and historical control children was 5.5–6.76 mo at 6-mo and 11.0–12.25 mo at 12-mo time points. There were no significant differences between groups at either time point. LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
FIGURE 1.
Prevalence of HIV-exposed children being underweight (A), stunted (B), or wasted (C) in intervention and historical control groups at 6 and 12 mo of age. Intervention group, n = 73. Historical control at age 6 mo, n = 138 and at age 12 mo, n = 219. Age range of children at age 6 mo was 5.5–6.76 mo and at 12 mo was 11.0–12.25 mo. *Groups differ, P < 0.05.
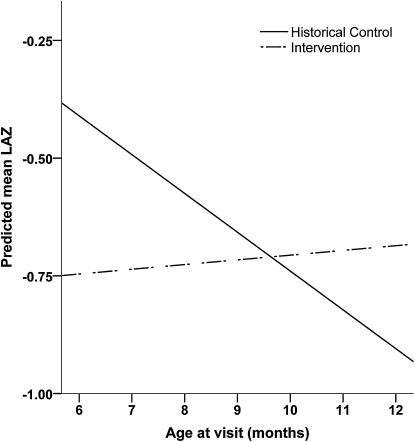
In further analysis using multilevel models for change to describe growth trajectories from age 6 to 12 mo, there was a significant group × age treatment effect in the WAZ- and LAZ-unadjusted and -adjusted models, indicating that WAZ and LAZ declined more steeply with age in the control group than in the intervention group (Table 4). This intervention effect was not observed for WHZ (Table 4). This significant treatment effect on LAZ is plotted in Figure 2, showing that the control infants tended to be better off than intervention infants at 6 mo of age but thereafter experienced much more profound stunting than the intervention infants.
TABLE 4.
Fixed effects from multilevel models for change for WAZ, LAZ, and WLZ in intervention and historical control groups
| WAZ |
LAZ |
WLZ |
||||||||||||||||
| Unadjusted |
Adjusted1 |
Unadjusted |
Adjusted1 |
Unadjusted |
Adjusted1 |
|||||||||||||
| β | SE | P value | β | SE | P value | β | SE | P value | β | SE | P value | β | SE | P value | β | SE | P value | |
| Intercept | −0.705 | 0.208 | 0.001 | −3.42 | 0.44 | <0.001 | −0.806 | 0.246 | 0.001 | −3.51 | 0.51 | <0.001 | 0.120 | 0.250 | 0.63 | −1.33 | 0.48 | 0.006 |
| Age, mo | 0.009 | 0.017 | 0.61 | −0.005 | 0.017 | 0.79 | 0.010 | 0.021 | 0.63 | 0.004 | 0.024 | 0.87 | −0.041 | 0.024 | 0.08 | −0.051 | 0.024 | 0.038 |
| Group | ||||||||||||||||||
| Historical control | 0.333 | 0.248 | 0.18 | 0.131 | 0.235 | 0.58 | 0.890 | 0.299 | 0.003 | 0.716 | 0.322 | 0.028 | −0.181 | 0.305 | 0.55 | −0.264 | 0.332 | 0.43 |
| Intervention2 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||
| Group x age | −0.047 | 0.021 | 0.027 | −0.050 | 0.021 | 0.02 | −0.092 | 0.026 | <0.001 | −0.096 | 0.030 | 0.001 | 0.004 | 0.029 | 0.90 | −0.002 | 0.030 | 0.95 |
| Birth weight, kg | 1.13 | 0.14 | <0.001 | 1.07 | 0.15 | <0.001 | 0.637 | 0.138 | <0.001 | |||||||||
| Mother‘s level of education | ||||||||||||||||||
| <Secondary school | −0.503 | 0.139 | <0.001 | −0.373 | 0.153 | 0.016 | −0.381 | 0.141 | 0.007 | |||||||||
| ≥Secondary school2 | — | — | — | — | — | — | ||||||||||||
Adjusted for birth weight and level of maternal education. LAZ, length-for-age Z-score; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
Reference category.
FIGURE 2.
LAZ by age predicted using unadjusted multilevel models for change in children in the intervention and historical control groups. *Group × age, P < 0.001. LAZ, length-for-age Z-score.
Caregiver knowledge.
Overall, the percentage of caregivers who identified positive infant feeding and hygiene practices increased from wk 0 to 24, confirming the impact of the education intervention on knowledge (Table 5).
TABLE 5.
Reported changes in infant feeding-related knowledge from wk 0 to 24 of caregivers of children in the intervention group1
| wk 0 |
wk 24 |
||||
| n | % | n | % | P-value | |
| How can you encourage a child who does not want to eat | |||||
| Feed with patience | 73 | 17.8 | 72 | 51.4 | <0.001 |
| Talk to child | 73 | 13.7 | 72 | 55.6 | <0.001 |
| How can you treat diarrhea? | |||||
| Give ORS/home fluids | 73 | 100.0 | 72 | 98.6 | 0.50 |
| Give child same or more food during diarrhea | 73 | 17.8 | 72 | 36.1 | 0.02 |
| Give child more liquid during diarrhea | 73 | 6.8 | 72 | 45.8 | <0.001 |
| Give child an extra plate of food per day after diarrhea3 | 72 | 76.4 | 72 | 95.8 | 0.0012 |
| How can you protect your child from germs? | |||||
| Wash hands | 70 | 15.7 | 72 | 47.2 | <0.001 |
| Put pants on child | 70 | 7.1 | 72 | 23.6 | 0.007 |
| Wash fruits and vegetables | 70 | .4 | 72 | 16.7 | 0.0012 |
| Treat water | 70 | 4.3 | 72 | 8.3 | 0.49 |
Values are percentages, varies by time point and group as indicated. ORS, oral rehydration solution.
Analysis by Fischer's exact test due to cell with count <5.
Mothers were questioned directly about feeding after diarrhea (e.g., Should you feed an extra plate per day?) Responses to other questions were not prompted by interviewer.
Discussion
HIV-exposed, uninfected, replacement-fed GHESKIO infants who participated in the infant feeding support intervention had lower rates of underweight and stunting at the end of the intervention period compared to HIV-exposed uninfected, replacement-fed children of the same age who were seen at GHESKIO during the year prior to the intervention. Although there was no difference in Z-score between groups at 12 mo, anthropometric indices declined from 6 to 12 mo in the historical control but not in the intervention group. The pattern of reduced prevalence of stunting or wasting between groups without a difference in growth Z-scores was previously shown and suggests that children who were smaller at enrollment had greater potential to benefit from the intervention (10). The greater SD in anthropometry measures among the historical control suggest that the measuring technique might not have been as controlled during this period compared to the intervention. This may have reduced our ability to detect significant differences between groups.
To assess whether the growth impact observed in our study was attributable to intervention participation, we answered 3 questions: 1) Were the intervention and historical control groups comparable at age of enrollment? 2) Was the intervention actually delivered and utilized by participants? and 3) Were the direction and magnitude of changes in intermediate outcomes consistent with program impact pathways? (18, 19)
Comparability at baseline.
There were no significant differences in anthropometric outcomes in the intervention and historical control groups at birth (birth weight, prevalence of low birth weight) or at age 6 mo (wk 0). At age 6 mo, the intervention and historical control groups were similar in all other baseline characteristics except completion of perinatal ARV prophylaxis regimen, a difference that might have favored the historical control group. However, a previous study in the GHESKIO population showed no association between completion of ARV prophylaxis and growth in late infancy (9).
Delivery and utilization.
Overall delivery and utilization of the intervention was high. It was not possible to directly measure supplement consumption. However, supplement disappearance measured using the weighed approach was consistent with caregiver reports, suggesting that overall supplement consumption was high. We did not explicitly question caregivers at each visit about sharing of the supplement within the household. Mothers reported limited sharing during qualitative interviews conducted at the end of intervention.
Caregivers were present for about three-quarters of the group teaching times. If a caregiver missed a particular club session, they were likely exposed to the session’s key messages in subsequent group sessions and individual counseling. Increases in vitamin A coverage and receipt of household food rations demonstrated that the intervention increased access to other services. It was not possible to assess what contribution these inputs made to growth effect, but it was assumed to be minimal due to reports from clinic staff that recipients often sold the rations. No data on receipt of household rations were available for the historical control even though the same ration program was in place at GHESKIO during that period.
Intermediates along causal pathway.
The intervention was designed to affect growth outcomes through two causal pathways based on known determinants of child growth: increased nutrient intake through supplement use and infant feeding education, and decreased diarrhea-related nutrient losses through education about hygiene and diarrhea care (20). Although the evaluation was not designed to statistically distinguish the relative contribution of the supplement compared with the behavior change education components, the data support that both causal pathways contributed to the impact on growth outcomes. There were significant improvements in knowledge about responsive feeding, diarrhea care, and hygiene from wk 0 to 24. Despite the potential pitfalls of noncompliance or sharing of the supplement within the household, it appears that the supplement was well received and appropriately used.
Relative effect size.
There is an emerging body of literature around supplementary or complementary feeding interventions targeting children 6–24 mo that use energy-dense, lipid-based nutrient supplements. Dewey and Adu-Afarwuah (10) reviewed two efficacy trials and six program evaluations of infant feeding interventions that provided infants age 6–24 mo food (not all lipid based) along with another strategy, usually education, for at least 4 mo. Reductions in the prevalence of growth faltering ranged from +1.3 to −9.4 percentage points in underweight and +2.9 to −6.8 percentage points in stunting. In our trial, the differences between intervention and historical control in prevalence of underweight (−14.0 percentage points) and stunting (−11.6 percentage points) were much larger. In rural Malawi, among 125 children fed 200 kcal (6–9 mo) to 300 kcal nutrient (after 9 mo) of lipid-based supplement per day (without nutrition education targeted at the mother), weight gain was 1110 ± 440 g and length gain was 6.6 ± 1.4 cm from 6 to 12 mo of age (21). In our trial, weight gain in the intervention group was 1552 ± 588 g and length gain was 7.5 ± 2.1 cm over the same age range. The relatively large effect sizes observed in our study compared to these other trials may be related to breastfeeding status. The non-breast-fed GHESKIO infants may have had greater potential to benefit from a food supplement compared to the breastfeeding infants in these studies.
Study limitations.
The lack of randomization to treatment or a concurrent control group increased the likelihood of confounding in the observed association between intervention participation and improved growth. However, the use of similar historical controls and assessment of program delivery and intermediate outcomes allowed us to infer that the observed effect was caused by the intervention.
Aspects of the intervention design were highly contextualized to the high rates of replacement feeding at GHESKIO during the study period. Because the supplement was intended to compensate for lack of breast milk intake, the 65-g dose was higher than other contexts that have used 20–55 g of lipid-based nutrient supplement in the 6- to 12-mo age group (21, 22).
The findings of our study demonstrate that implementation of a clinic-based, infant feeding support strategy that combines a supplement and infant feeding education is associated with reduced risk of growth faltering in HIV-exposed, uninfected children from 6 to 12 mo of age. Further research is needed to refine the supplement dosing and education strategy to reflect the shift in HIV and infant feeding policies in Haiti and much of Africa toward continued breastfeeding through at least the first year of life (6). Implementation of the infant feeding support intervention is ongoing at the GHESKIO Centers and is being expanded to include all HIV-exposed children 0–23 mo of age. To reduce the time burden on staff and participants, visits are scheduled every 4 wk from 0 to 12 mo of age and bimonthly thereafter. This adapted model will be evaluated with the aim of scaling-up throughout the country.
Supplementary Material
Acknowledgments
The authors thank the GHESKIO study team (Marie-Jean Mona Maitre, Ghislaine Saint Louis, Adeline Bernard, Suzette Fleury), Kathleen Rasmussen, Jean-Pierre Habicht, and Patsy Brannon for support to study design and analysis and Francoise Vermeylen and Jason Barry of the Cornell Statistical Consulting Unit for guidance on statistical analysis. R.H., R.S., D.F., and J.P. designed the research; R.H. conducted the research, analyzed data, wrote the paper, and had primary responsibility for final content; and R.S. provided detailed comments on the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported in part by the President’s Emergency Plan for AIDS Relief and by NIH National Institute of Diabetes and Digestive and Kidney Diseases Nutrition Training Grant T32DK007158.
This trial was registered at clinicaltrials.gov as NCT01434238.
Abbreviations used: ARV, antiretroviral; GHESKIO, Groupe Haïtien d’Etude du Sarcome de Kaposi et des Infections Opportunistes; LAZ, length-for-age Z-score; PMTCT, prevention of mother-to-child transmission; WAZ, weight-for-age Z-score; WLZ, weight-for-length Z-score.
Literature Cited
- 1.Alderman H, Hoddinott J, Kinsey B. Long term consequences of early childhood malnutrition. In: FCND Discussion Paper No. 10. Washington, DC: International Food Policy Research Institute; 2003.
- 2.Bhandari N, Bahl R, Taneja S, Strand T, Molbak K, Ulvik RJ, Sommerfelt H, Bhan MK. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics. 2002;109:e86. [DOI] [PubMed] [Google Scholar]
- 3.Shrimpton R, Victora CG, de Onis M, Lima RC, Blossner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics. 2001;107:E75. [DOI] [PubMed] [Google Scholar]
- 4.Allen L. Malnutrition and human function: a comparison of conclusions from the INCAP and nutrition CRSP studies. J Nutr. 1995;125:1119S–26S [DOI] [PubMed] [Google Scholar]
- 5.Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet. 1999;354:203–9 [DOI] [PubMed] [Google Scholar]
- 6.WHO. Guidelines on HIV and infant feeding 2010: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: WHO; 2010. [PubMed]
- 7.President's Emergency Plan for AIDS Relief. PEFPAR guidance on integrating prevention of mother to child transmission of HIV, maternal, neonatal, and child health and pediatric HIV services. Washington, DC: U.S. Department of State; 2011.
- 8.Noel F, Mehta S, Zhu Y, Rouzier PDM, Marcelin A, Shi JR, Nolte C, Severe L, Deschamps MM, Fitzgerald DW, et al. Improving outcomes in infants of HIV-infected women in a developing country setting. PLoS ONE. 2008;3:e3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidkamp R. Evaluation of an infant feeding support strategy among HIV-exposed infants in urban Haiti. Ithaca: Cornell University; 2011.
- 10.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4 Suppl 1:24–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E. Haider Ba, Kirkwood B, Morris SS, Sachdev H. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–40 [DOI] [PubMed] [Google Scholar]
- 12.Caulfield L, Huffman S, Piwoz E. Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Washington, DC: Linkages Project; Academy for Educational Development; 1999. [Google Scholar]
- 13.Dewey KG, Brown KH. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr Bull. 2003;24:5–28 [DOI] [PubMed] [Google Scholar]
- 14.Dewey KG, Cohen RJ, Rollins NC. WHO technical background paper: feeding of non-breastfed children from 6 to 24 months of age in developing countries. Food Nutr Bull. 2004;25:377–402 [DOI] [PubMed] [Google Scholar]
- 15.WHO. Guiding principles for feeding non-breastfed children 6–24 months of age. Geneva: WHO; 2005.
- 16.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. In. Geneva: WHO; 2006.
- 17.Kuusipalo H, Maleta K, Briend A, Manary M, Ashorn P. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. J Pediatr Gastroenterol Nutr. 2006;43:525–32 [DOI] [PubMed] [Google Scholar]
- 18.Habicht JP, Victora C, Vaughan JP. Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. Int J Epidemiol. 1999;28:10. [DOI] [PubMed] [Google Scholar]
- 19.Victora CG, Habicht JP, Bryce J. Evidence-based public health: moving beyond randomized trials. Am J Public Health. 2004;94:400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engle P, Menon P, Haddad L. Care and nutrition: concepts and measurement. In: Washington, DC: International Food Policy Research Institute; 1997.
- 21.Lin CA, Manary MJ, Maleta K, Briend A, Ashorn P. An energy-dense complementary food is associated with a modest increase in weight gain when compared with a fortified porridge in Malawian children aged 6–18 months. J Nutr. 2008;138:593–8 [DOI] [PubMed] [Google Scholar]
- 22.Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr. 2007;86:412–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.