Abstract
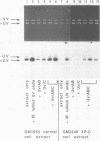
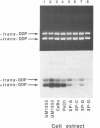
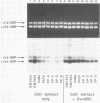
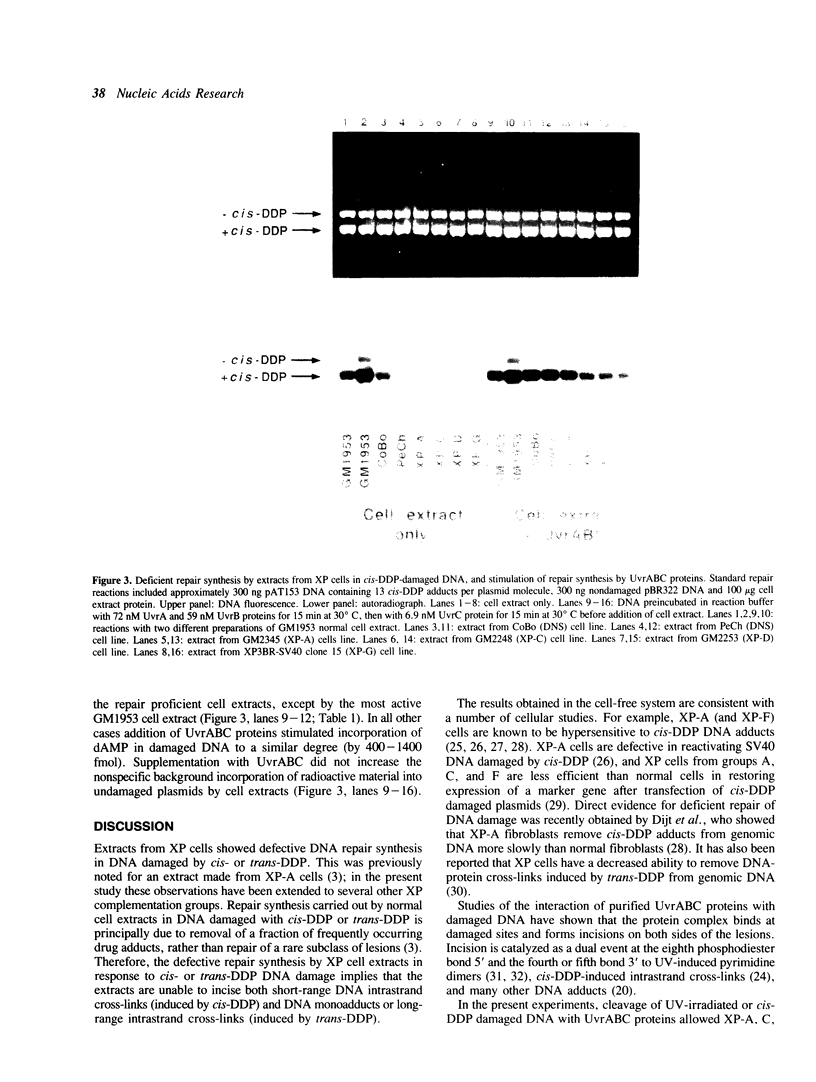
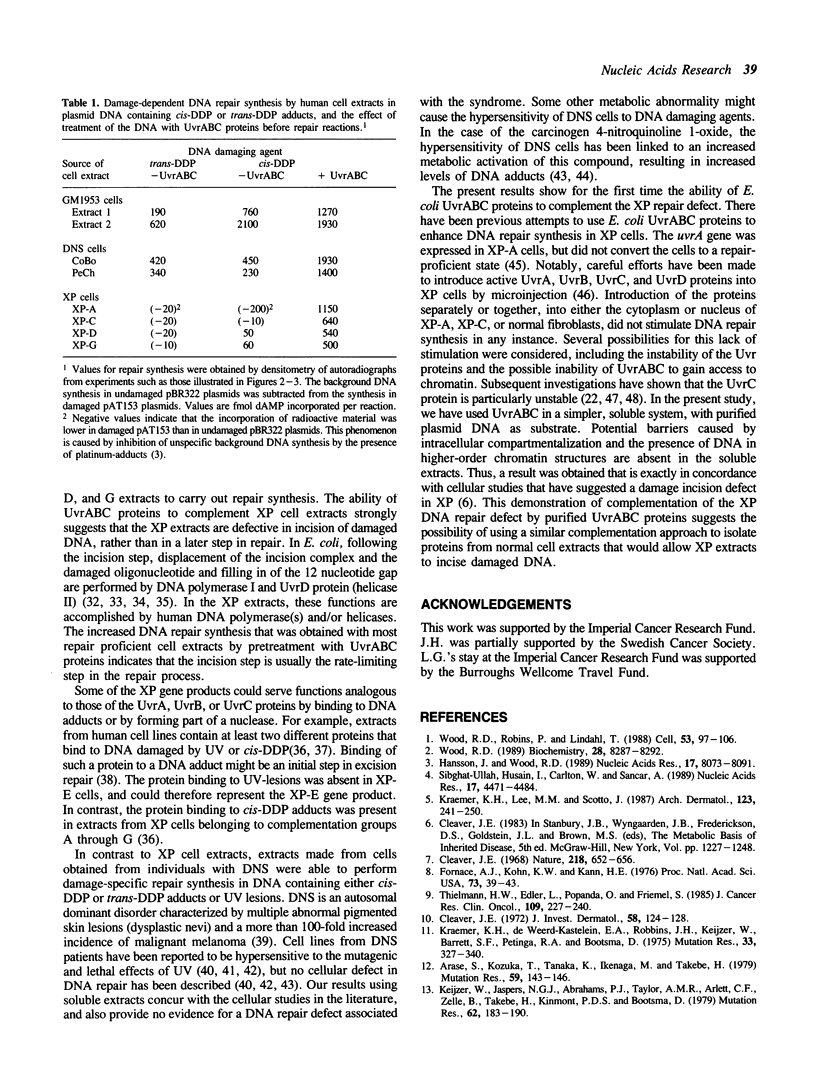
A newly developed cell-free system was used to study DNA repair synthesis carried out by extracts from human cell lines in vitro. Extracts from a normal human lymphoid cell line and from cell lines established from individuals with hereditary dysplastic nevus syndrome perform damage-dependent repair synthesis in plasmid DNA treated with cis- or trans-diamminedichloro-platinum(II) or irradiated with ultraviolet light. Cell extracts of xeroderma pigmentosum origin (complementation groups A, C, D, and G) are deficient in DNA repair synthesis. When damaged plasmid DNA was pretreated with purified Escherichia coli UvrABC proteins, xeroderma pigmentosum cell extracts were able to carry out DNA repair synthesis. The ability of E. coli UvrABC proteins to complement xeroderma pigmentosum cell extracts indicates that the extracts are deficient in incision, but can carry out later steps of repair. Thus the in vitro system provides results that are in agreement with the incision defect found from studies of xeroderma pigmentosum cells.
Full text
PDF

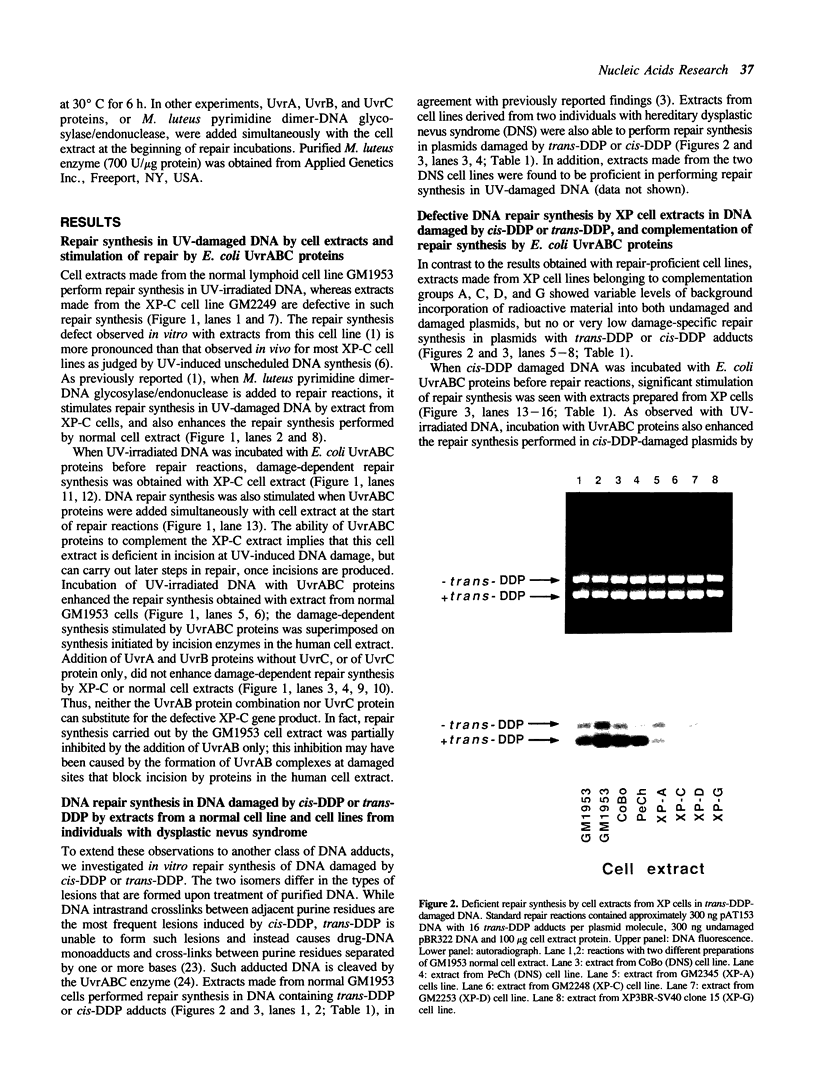

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arase S., Kozuka T., Tanaka K., Ikenaga M., Takebe H. A sixth complementation group in xeroderma pigmentosum. Mutat Res. 1979 Jan;59(1):143–146. doi: 10.1016/0027-5107(79)90202-1. [DOI] [PubMed] [Google Scholar]
- Arrand J. E., Squires S., Bone N. M., Johnson R. T. Restoration of u.v.-induced excision repair in Xeroderma D cells transfected with the denV gene of bacteriophage T4. EMBO J. 1987 Oct;6(10):3125–3131. doi: 10.1002/j.1460-2075.1987.tb02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Popoff S., Sancar A., Rupp W. D. Reactions of the UVRABC excision nuclease with DNA damaged by diamminedichloroplatinum(II). Nucleic Acids Res. 1985 Oct 25;13(20):7395–7412. doi: 10.1093/nar/13.20.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Grossman L. Incision of damaged versus nondamaged DNA by the Escherichia coli UvrABC proteins. Nucleic Acids Res. 1988 Aug 25;16(16):7855–7865. doi: 10.1093/nar/16.16.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Berg P. DNA cross-linked by cisplatin: a new probe for the DNA repair defect in xeroderma pigmentosum. Mol Biol Med. 1987 Oct;4(5):277–290. [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972 Mar;58(3):124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- Dickstein R., Huh N. D., Sandlie I., Grossman L. The expression of the Escherichia coli uvrA gene in human cells. Mutat Res. 1988 Jan;193(1):75–86. doi: 10.1016/0167-8817(88)90009-0. [DOI] [PubMed] [Google Scholar]
- Dijt F. J., Fichtinger-Schepman A. M., Berends F., Reedijk J. Formation and repair of cisplatin-induced adducts to DNA in cultured normal and repair-deficient human fibroblasts. Cancer Res. 1988 Nov 1;48(21):6058–6062. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Kohn K. W., Kann H. E., Jr DNA single-strand breaks during repair of UV damage in human fibroblasts and abnormalities of repair in xeroderma pigmentosum. Proc Natl Acad Sci U S A. 1976 Jan;73(1):39–43. doi: 10.1073/pnas.73.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Seres D. S. Repair of trans-Pt(II) diamminedichloride DNA-protein crosslinks in normal and excision-deficient human cells. Mutat Res. 1982 Jun;94(2):277–284. doi: 10.1016/0027-5107(82)90291-3. [DOI] [PubMed] [Google Scholar]
- Fraval H. N., Rawlings C. J., Roberts J. J. Increased sensitivity of UV-repair-deficient human cells to DNA bound platinum products which unlike thymine dimers are not recognized by an endonuclease extracted from Micrococcus luteus. Mutat Res. 1978 Jul;51(1):121–132. doi: 10.1016/0027-5107(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Park L., Grossman L. Enzymatic repair of pyrimidine dimer-containing DNA. A 5' dimer DNA glycosylase: 3'-apyrimidinic endonuclease mechanism from Micrococcus luteus. J Biol Chem. 1982 Nov 25;257(22):13465–13474. [PubMed] [Google Scholar]
- Greene M. H., Clark W. H., Jr, Tucker M. A., Elder D. E., Kraemer K. H., Guerry D., 4th, Witmer W. K., Thompson J., Matozzo I., Fraser M. C. Acquired precursors of cutaneous malignant melanoma. The familial dysplastic nevus syndrome. N Engl J Med. 1985 Jan 10;312(2):91–97. doi: 10.1056/NEJM198501103120205. [DOI] [PubMed] [Google Scholar]
- Hansson J., Wood R. D. Repair synthesis by human cell extracts in DNA damaged by cis- and trans-diamminedichloroplatinum(II). Nucleic Acids Res. 1989 Oct 25;17(20):8073–8091. doi: 10.1093/nar/17.20.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J. N., Greene M. H., Corner R. C., Maher V. M., McCormick J. J. Fibroblasts from patients with hereditary cutaneous malignant melanoma are abnormally sensitive to the mutagenic effect of simulated sunlight and 4-nitroquinoline 1-oxide. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1179–1183. doi: 10.1073/pnas.81.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer W., Jaspers N. G., Abrahams P. J., Taylor A. M., Arlett C. F., Zelle B., Takebe H., Kinmont P. D., Bootsma D. A seventh complementation group in excision-deficient xeroderma pigmentosum. Mutat Res. 1979 Aug;62(1):183–190. doi: 10.1016/0027-5107(79)90231-8. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., De Weerd-Kastelein E. A., Robbins J. H., Keijzer W., Barrett S. F., Petinga R. A., Bootsma D. Five complementation groups in xeroderma pigmentosum. Mutat Res. 1975 Dec;33(2-3):327–340. doi: 10.1016/0027-5107(75)90208-0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kumura K., Sekiguchi M., Steinum A. L., Seeberg E. Stimulation of the UvrABC enzyme-catalyzed repair reactions by the UvrD protein (DNA helicase II). Nucleic Acids Res. 1985 Mar 11;13(5):1483–1492. doi: 10.1093/nar/13.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Belle M., Linn S. In vivo excision of pyrimidine dimers is mediated by a DNA N-glycosylase in Micrococcus luteus but not in human fibroblasts. Photochem Photobiol. 1982 Sep;36(3):319–324. doi: 10.1111/j.1751-1097.1982.tb04381.x. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Samuels M., Sharp P. A. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- Patterson M., Chu G. Evidence that xeroderma pigmentosum cells from complementation group E are deficient in a homolog of yeast photolyase. Mol Cell Biol. 1989 Nov;9(11):5105–5112. doi: 10.1128/mcb.9.11.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera M. I., Um K. I., Greene M. H., Waters H. L., Bredberg A., Kraemer K. H. Hereditary dysplastic nevus syndrome: lymphoid cell ultraviolet hypermutability in association with increased melanoma susceptibility. Cancer Res. 1986 Feb;46(2):1005–1009. [PubMed] [Google Scholar]
- Plooy A. C., van Dijk M., Berends F., Lohman P. H. Formation and repair of DNA interstrand cross-links in relation to cytotoxicity and unscheduled DNA synthesis induced in control and mutant human cells treated with cis-diamminedichloroplatinum(II). Cancer Res. 1985 Sep;45(9):4178–4184. [PubMed] [Google Scholar]
- Poll E. H., Abrahams P. J., Arwert F., Eriksson A. W. Host-cell reactivation of cis-diamminedichloroplatinum(II)-treated SV40 DNA in normal human, Fanconi anaemia and xeroderma pigmentosum fibroblasts. Mutat Res. 1984 Nov-Dec;132(5-6):181–187. doi: 10.1016/0167-8817(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sato K., Howell J. N., Greene M. H., Maher V. M., McCormick J. J. Relationship between sensitivity of cells from patients with hereditary cutaneous malignant melanoma to killing and mutations by 4-nitroquinoline 1-oxide and adduct formation. Cancer Res. 1988 Sep 15;48(18):5145–5150. [PubMed] [Google Scholar]
- Sibghatullah, Husain I., Carlton W., Sancar A. Human nucleotide excision repair in vitro: repair of pyrimidine dimers, psoralen and cisplatin adducts by HeLa cell-free extract. Nucleic Acids Res. 1989 Jun 26;17(12):4471–4484. doi: 10.1093/nar/17.12.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Greene M. H., Adams D., Paterson M. C. Abnormal responses to the carcinogen 4-nitroquinoline 1-oxide of cultured fibroblasts from patients with dysplastic nevus syndrome and hereditary cutaneous malignant melanoma. Carcinogenesis. 1983;4(7):911–916. doi: 10.1093/carcin/4.7.911. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Greene M. H., Devlin D. A., McKeen E. A., Paterson M. C. Abnormal sensitivity to UV-radiation in cultured skin fibroblasts from patients with hereditary cutaneous malignant melanoma and dysplastic nevus syndrome. Int J Cancer. 1982 Jul 15;30(1):39–45. doi: 10.1002/ijc.2910300108. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hayakawa H., Sekiguchi M., Okada Y. Specific action of T4 endonuclease V on damaged DNA in xeroderma pigmentosum cells in vivo. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2958–2962. doi: 10.1073/pnas.74.7.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielmann H. W., Edler L., Popanda O., Friemel S. Xeroderma pigmentosum patients from the Federal Republic of Germany: decrease in post-UV colony-forming ability in 30 xeroderma pigmentosum fibroblast strains is quantitatively correlated with a decrease in DNA-incising capacity. J Cancer Res Clin Oncol. 1985;109(3):227–240. doi: 10.1007/BF00390362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney J. H., Donahue B. A., Kellett P. J., Bruhn S. L., Essigmann J. M., Lippard S. J. Isolation of cDNAs encoding a human protein that binds selectively to DNA modified by the anticancer drug cis-diamminedichloroplatinum(II) Proc Natl Acad Sci U S A. 1989 Nov;86(21):8328–8332. doi: 10.1073/pnas.86.21.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Analysis of sequential steps of nucleotide excision repair in Escherichia coli using synthetic substrates containing single psoralen adducts. J Biol Chem. 1988 Nov 15;263(32):16553–16560. [PubMed] [Google Scholar]
- Weinfeld M., Gentner N. E., Johnson L. D., Paterson M. C. Photoreversal-dependent release of thymidine and thymidine monophosphate from pyrimidine dimer-containing DNA excision fragments isolated from ultraviolet-damaged human fibroblasts. Biochemistry. 1986 May 6;25(9):2656–2664. doi: 10.1021/bi00357a055. [DOI] [PubMed] [Google Scholar]
- Wood R. D. Repair of pyrimidine dimer ultraviolet light photoproducts by human cell extracts. Biochemistry. 1989 Oct 17;28(21):8287–8292. doi: 10.1021/bi00447a005. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Yoakum G. H., Grossman L. The purification of the Escherichia coli UvrABC incision system. Nucleic Acids Res. 1986 Nov 11;14(21):8535–8556. doi: 10.1093/nar/14.21.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwetsloot J. C., Barbeiro A. P., Vermeulen W., Arthur H. M., Hoeijmakers J. H., Backendorf C. Microinjection of Escherichia coli UvrA, B, C and D proteins into fibroblasts of xeroderma pigmentosum complementation groups A and C does not result in restoration of UV-induced unscheduled DNA synthesis. Mutat Res. 1986 Jul;166(1):89–98. doi: 10.1016/0167-8817(86)90044-1. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., Vermeulen W., Keijzer W., Hoeijmakers J. H., Bootsma D. Microinjection of Micrococcus luteus UV-endonuclease restores UV-induced unscheduled DNA synthesis in cells of 9 xeroderma pigmentosum complementation groups. Mutat Res. 1985 Jun-Jul;150(1-2):99–105. doi: 10.1016/0027-5107(85)90106-x. [DOI] [PubMed] [Google Scholar]