SUMMARY
Chronic stress could trigger maladaptive changes associated with stress-related mental disorders, however, the underlying mechanisms remain elusive. In this study, we found that exposing juvenile male rats to repeated stress significantly impaired the temporal order recognition memory, a cognitive process controlled by prefrontal cortex (PFC). Concomitantly, significantly reduced AMPAR- and NMDAR-mediated synaptic transmission and glutamate receptor expression were found in PFC pyramidal neurons from repeatedly stressed animals. All these effects relied on activation of glucocorticoid receptors and the subsequent enhancement of ubiquitin/proteasome-mediated degradation of GluR1 and NR1 subunits, which was controlled by the E3 ubiquitin ligase Nedd4-1 and Fbx2, respectively. Inhibition of proteasomes or knockdown of Nedd4-1 and Fbx2 in PFC prevented the loss of glutamatergic responses and recognition memory in stressed animals. Our results suggest that repeated stress dampens PFC glutamatergic transmission by facilitating glutamate receptor turnover, which causes the detrimental effect on PFC-dependent cognitive processes.
Keywords: stress, corticosterone, glucocorticoid receptor, NMDA receptor, AMPA receptor, ubiquitination, degradation, recognition memory
INTRODUCTION
Adrenal corticosterone, the major stress hormone, through the activation of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR), can induce long-lasting influences on cognitive and emotional processes (McEwen, 2007). Mounting evidence suggest that inappropriate stress responses act as a trigger for many mental illnesses (de Kloet et al., 2005). For example, depression is associated with hypercortisolaemia (excessive cortisol, Holsboer, 2000; Van Praag, 2004), while post-traumatic stress disorder (PTSD) has been linked to hypocortisolaemia (insufficient cortisol) resulting from an enhanced negative feedback by cortisol (Yehuda, 2002). Thus, corticosteroid hormones are thought to serve as a key controller for adaptation and maintenance of homeostasis in situations of acute stress, as well as maladaptive changes in response to chronic and repeated stress that lead to cognitive and emotional disturbances symptomatic of stress-related neuropsychiatric disorders (Newport and Nemeroff, 2000; Caspi et al., 2003; de Kloet et al., 2005; Joëls, 2006; McEwen, 2007).
One of the primary targets of stress hormones is prefrontal cortex (McEwen, 2007), a region controlling high level “executive” functions including working memory, inhibition of distraction, novelty seeking, and decision making (Miller, 1999; Stuss and Knight, 2002). Chronic stress or glucocorticoid treatment has been found to cause structural remodeling and behavioral alterations in PFC from adult animals, such as dendritic shortening, spine loss, and neuronal atrophy (Cook and Wellman, 2004; Radley et al., 2004; 2006), as well as impairment in cognitive flexibility and perceptual attention (Cerqueira et al., 2005; 2007; Liston et al., 2006). However, little is known about the physiological consequences and molecular targets of long-term stress in PFC, especially during the adolescent period when the brain is more sensitive to stressors (Lupien et al., 2009).
It has been proposed that glutamate receptor-mediated synaptic transmission that controls PFC neuronal activity is crucial for working memory (Goldman-Rakic, 1995; Lisman et al., 1998). Our recent studies have found that acute stress induces a sustained potentiation of glutamate receptor membrane trafficking and glutamatergic transmission in rat PFC (Yuen et al., 2009; 2011), providing a molecular and cellular mechanism for the beneficial effects of acute stress on working memory. Since dysfunction of glutamatergic transmission is considered the core feature and fundamental pathology of mental disorders (Tsai and Coyle 2002; Moghaddam, 2003; Frankle et al., 2003), in this study, we sought to determine whether repeated (subchronic) stress might negatively influence PFC-mediated cognitive processes by disturbing glutamatergic signaling in juvenile animals.
RESULTS
Exposing to repeated stress impairs object recognition memory
To test the impact of stress on cognitive functions, we measured the recognition memory task, a fundamental explicit memory process requiring judgments of the prior occurrence of stimuli based on the relative familiarity of individual objects, the association of objects and places, or the recency information (Ennaceur and Delacour, 1988; Dix and Aggleton, 1999; Mitchell and Laiacona, 1998). Lesion studies have shown that medial prefrontal cortex plays an obligatory role in the temporal order recognition (TOR) memory (Barker et al., 2007), so this behavioral task was used. Young (4-week-old) male rats, which had been exposed to 7-day repeated behavioral stressors, were examined at 24 hrs after stressor cessation.
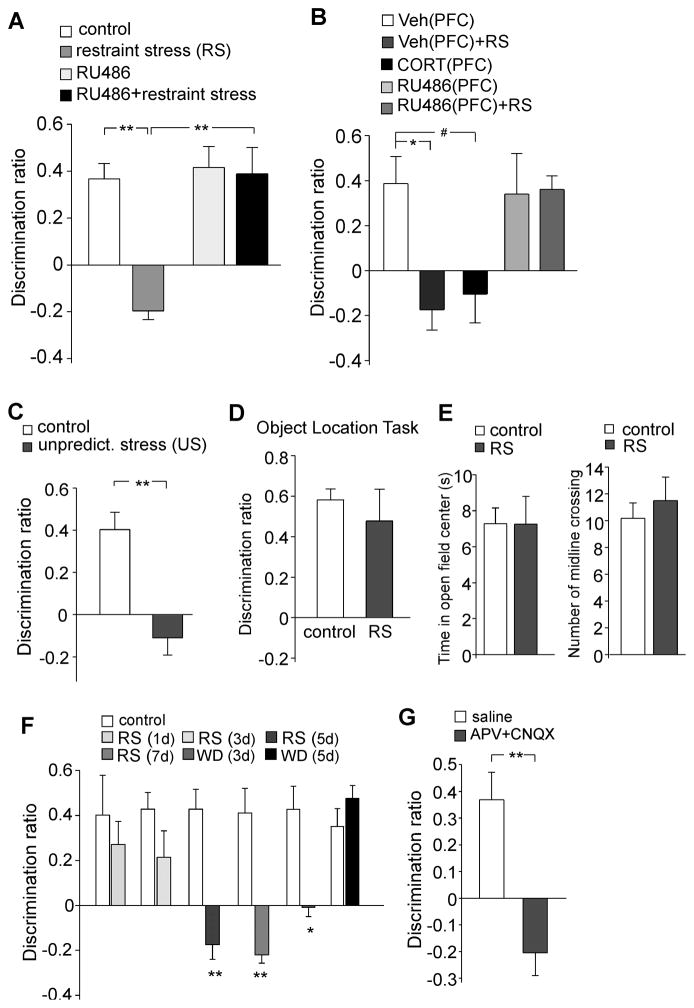
The control groups spent much more time exploring the novel (less recent) object in the test trial (familiar recent object: 9.9±2.4 s, novel object: 19.9±2.4 s, n=7, p<0.01), while the stressed rats (restraint, 2 hr/day, 7d) lost the preference to the novel object (familiar recent object: 15.2±2.4 s; novel object: 11.0±2.8 s, n=5, p>0.05). The discrimination ratio (DR), an index of the object recognition memory, showed a significant main effect (Figure 1A, F3,24=9.8, p<0.001, ANOVA). Post-hoc analysis indicated a profound impairment of TOR memory by repeated stress (DR in control: 36.7±6.6%, n=7; DR in stressed: −19.6±3.8%, n=5, p<0.001), which was blocked by systemic injection of the GR antagonist RU486 (DR in RU486: 41.6±9.0%, n=6; DR in RU486+stress: 38.8±11.2%, n=7, p>0.05).
Figure 1. Rats exposed to repeated stress or infused with glutamate receptor antagonists to PFC exhibit worse performance on the temporal order recognition (TOR) memory task.
(A) Bar graphs showing the discrimination ratio (DR) of TOR tasks in control groups vs. animals exposed to 7-day restraint stress without or with RU486 injection (10mg/kg, i.p. daily at 30 min before stress). **: p<0.001, ANOVA. (B) Bar graphs showing the DR of TOR tasks in control groups vs. stressed animals (restraint, 7d) with PFC infusion of vehicle or RU486 (1.4 nmol/g, daily at 40 min before stress). Another group of animals was given repeated injections of CORT to the PFC (0.87 nmol/g, 7d). *: p<0.01; #: p<0.05, ANOVA. (C) Bar graphs showing the DR of TOR tasks in control groups vs. animals exposed to 7-day unpredictable stress. **: p<0.001, t test. (D) Bar graphs showing the DR of object location tasks in control groups vs. animals exposed to 7-day restraint stress. (E) Bar graphs showing the time spent at the center in open-field tests and the number of midline crossing in control vs. stressed (restraint, 5d) rats. (F) Bar graphs showing the DR of TOR tasks in control groups, stressed animals (restraint for 1, 3, 5, 7d), and animals withdrawn (WD, for 3 or 5d) from 7-day restraint stress. **: p<0.001; *: p<0.01, t test. (G) Bar graphs showing the DR of TOR tasks in animals with PFC infusion of saline vs. glutamate receptor antagonists (APV: 1 mM, CNQX: 0.2 mM, 1 μl each side). The infusion was performed via an implanted cannula at 20 min before behavioral experiments. **: p<0.001, t test.
To test whether GR in the PFC mediates the detrimental effect of repeated stress on cognition, we performed stereotaxic injections of RU486, vehicle control or corticosterone to PFC prelimbic regions bilaterally via an implanted guide cannula (Yuen et al., 2011). A significant main effect was found (Figure 1B, F4,30=5.1, p<0.005, ANOVA), and post-hoc analysis indicated that repeated restraint stress impaired TOR memory in rats injected with vehicle (DR in veh: 38.7±12.0%, n=7; DR in veh+stress: −17.5±9.1%, n=6, p<0.01), an effect mimicked by repeated CORT injections (0.87 nmol/g, 7d, −10.5±12.7%, n=6, p<0.05), while such impairment was prevented by RU486 delivered to PFC (1.4 nmol/g, 7d, DR in RU486: 34.2±17.8%, n=6; DR in RU486+stress: 36.1±6.1%, n=6, p>0.05). It suggests that repeated stress influences cognitive processes via GR activation in the PFC.
Next, we examined whether other stressors could produce a similar effect. As shown in Figure 1C, rats exposed to repeated unpredictable stress (7-day) also lost the preference to the novel object in TOR memory tasks (DR in control: 40.3±8.2%, n=9; DR in stressed: −11.0±8.3%, n=9, p<0.001). To test the specificity of this stress-induced memory deficit, we also subjected animals to the object location task, a paradigm for the PFC-independent memory (Barker et al., 2007). As shown in Figure 1D, both control groups and stressed animals (restraint, 7d) showed similar discrimination between the object that had changed position than the object that had remained in a constant position (DR in control: 58.1±5.4%, n=6; DR in stressed: 47.7±15.7%, n=6, p>0.05).
In contrast to the impaired temporal order recognition memory, rats exposed to repeated restraint stress showed no changes in anxiety-related behavior or locomotive activity (Figure 1E), as indicated by the amount of time spent in the open-field center (control: 7.3±0.9 sec; stressed: 7.3±1.5 sec, n=8 pairs, p>0.05) and the number of midline crossing in a cage (control: 10.2±1.2, stressed: 11.5±1.8, n=6 pairs, p>0.05).
To find out the onset of the detrimental effects of stress on cognition, we exposed young male rats to various days (1, 3, 5 and 7) of restraint stress. As shown in Figure 1F, TOR memory was largely unchanged by 1- or 3-day stress, but was significantly impaired in animals exposed to 5- or 7-day stress (p<0.001, n=6 pairs per group). After 3-day withdrawal from the repeated stress, TOR memory still showed deficiency (p<0.01, n=6 pairs), but recovered after 5-day withdrawal (n=6 pairs).
To test whether glutamatergic transmission in PFC is critical for the object recognition memory, we gave animals a stereotaxic injection of the NMDAR antagonist APV and AMPAR antagonist CNQX to PFC prelimbic regions bilaterally. As shown in Figure 1G, APV+CNQX-injected animals lost the normal preference to the novel (less recent) object (DR in saline: 36.8±10.3%, n=7; DR in APV+CNQX: −20.4±8.7%, n=11, p<0.001), similar to the animals exposed to repeated stress. The total exploration time in the two sample phases and the subsequent test trial was unchanged by any of these treatments (Figure S1). Taken together, it suggests that repeated stress has a detrimental effect on recognition memory, which may be due to the loss of glutamatergic transmission in PFC.
Animals exposed to repeated stress show the depression of glutamatergic transmission in PFC
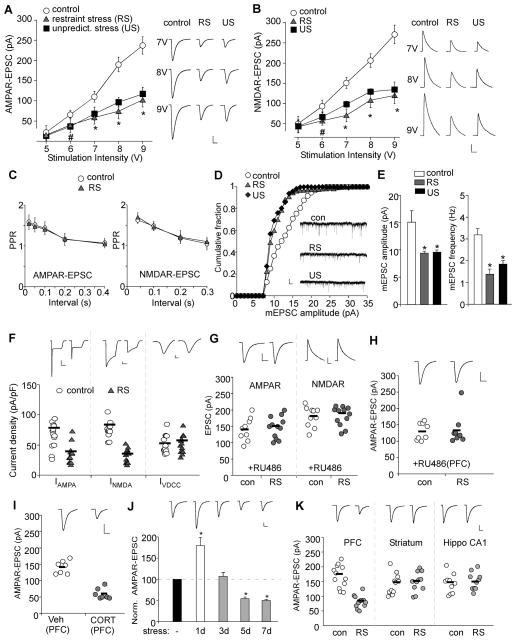
To find out the impact of repeated stress on glutamatergic transmission, we examined the input/output curves of AMPAR- and NMDAR-mediated synaptic currents (EPSC) in PFC pyramidal neurons from stressed young male rats (4-week-old). As shown in Figure 2A and 2B, AMPAR-EPSC and NMDAR-EPSC induced by a series of stimulus intensities were markedly reduced in neurons from animals exposed to repeated (7-day) restraint stress or unpredictable stress (AMPA: 40–60% decrease, p<0.01, ANOVA, n=16–29 per group; NMDA: 38–57% decrease, p<0.01, ANOVA, n=19–28 per group).
Figure 2. Repeated stress impairs glutamatergic transmission in PFC pyramidal neurons via a post-synaptic mechanism.
(A, B) Summarized input-output curves of AMPAR-EPSC (A) or NMDAR-EPSC (B) in response to a series of stimulation intensity in control vs. animals exposed to 7-day repeated restraint stress (RS) or unpredictable stress (US). *: p<0.01, #: p<0.05, ANOVA. Inset: representative EPSC traces. Scale bars: 50pA, 20ms (A) or 100ms (B). (C) Plot of PPR of AMPAR-EPSC and NMDAR-EPSC evoked by double pulses with various intervals in control or stressed rats. (D, E) Cumulative distribution and bar graphs showing the effect of repeated stress on mEPSC amplitude and frequency. *: p<0.01, ANOVA. Inset (D): representative mEPSC traces. Scale bars: 10pA, 1s. (F) Dot plots summarizing the AMPAR, NMDAR and VDCC current density in PFC neurons acutely dissociated from control vs. stressed animals. Inset: representative current traces. Scale bars: 100pA, 1s (AMPA, NMDA) or 2ms (VDCC). (G) Dot plots showing the amplitude of AMPAR-EPSC and NMDAR-EPSC in PFC pyramidal neurons taken from control or stressed animals (restraint, 7-day) with systemic injections of RU486 (10mg/kg). Inset: representative EPSC traces. Scale bars: 50pA, 20ms (AMPA) or 100ms (NMDA). (H) Dot plots showing the amplitude of AMPAR-EPSC in control or stressed animals (restraint, 7-day) with local injections of RU486 (1.4 nmol/g, 7d) to the PFC. (I) Dot plots showing the amplitude of AMPAR-EPSC in animals with local injections of CORT (0.87 nmol/g, 7d) or vehicle control to the PFC. Inset (H, I): representative AMPAR-EPSC traces. Scale bars: 50pA, 20ms. (J) Bar graphs demonstrating the bi-phasic effect of stress on AMPAR-EPSC in rats exposed to various durations of restraint stress.*: p<0.01, ANOVA. Inset: representative AMPAR-EPSC traces. Scale bars: 25pA, 20ms. (K) Dot plots showing the AMPAR-EPSC amplitude in PFC pyramidal neurons, striatal medium spiny neurons and hippocampal CA1 pyramidal neurons from control or stressed rats (restraint, 7-day).
To test whether the reduced synaptic transmission by repeated stress may result from a presynaptic mechanism, we measured the paired pulse ratio (PPR) of AMPAR- and NMDAR-EPSC, a readout sensitive to presynaptic glutamate release. As shown in Figure 2C, PPR was not different in control vs. stressed animals, suggesting a lack of gross change in presynaptic function.
To further confirm the involvement of postsynaptic glutamate receptors, we measured miniature EPSC (mEPSC), a synaptic response resulting from quantal release of single glutamate vesicles, in PFC slices. As shown in Figure 2D and 2E, repeatedly stressed animals had markedly reduced mEPSC amplitude (control: 15.1±2.1pA, n=8; restraint stress: 9.4±0.3pA, n=7, unpredictable stress: 9.6±0.4pA, n=9, F2,26=8.8, p<0.01, ANOVA) and frequency (control: 3.2±0.3Hz, n=8; restraint stress: 1.4±0.2Hz, n=7, unpredictable stress: 1.9±0.2Hz, n=9, F2,23=15.5, p<0.01, ANOVA). Moreover, we measured whole-cell ionic current elicited by AMPA (100 μM) or NMDA (100 μM) application in acutely dissociated PFC neurons (a pure postsynaptic preparation). As shown in Figure 2F, animals exposed to repeated restraint stress had significantly smaller AMPA current density (pA/pF) (control: 81.9±6.8, n=14; stressed: 42.9±5.1, n=14, p<0.01) and NMDAR current density (control: 93.3±4.6; stressed: 40.4±4.0, n=13; p<0.01). In contrast, the voltage-dependent calcium channel (VDCC) current density was not altered (control: 59.4±4.9; stressed: 63.1±4.9, n=14; p>0.05).
Systemic injections of the GR antagonist RU486 blocked the decreasing effect of repeated restraint stress on AMPAR-EPSC (Figure 2G, control: 141.3±11.7pA, n=9; stressed: 147.4±9.5pA, n=12, p>0.05) and NMDAR-EPSC (Figure 2G, control: 180.2±9.8pA, n=10; stressed: 181.3±8.5pA, n=12, p>0.05). Local injections of RU486 to the PFC (1.4 nmol/g, 7d) also prevented the reduction of AMPAR-EPSC by repeated stress (Figure 2H, control: 135.4±16.9pA, n=8; stressed: 130.4±9.4pA, n=8, p>0.05). Repeated injections of CORT to the PFC (0.87 nmol/g, 7d) produced a significant reduction of AMPAR-EPSC (Figure 2I, control: 141.4±7.5pA, n=7; CORT: 59.4±6.2pA, n=7, p<0.01), similar to the effect of behavioral stressors. It suggests that repeated stress down-regulates glutamatergic transmission via GR activation in the PFC.
Our previous studies show that acute stress (e.g. single 2 hr restraint) enhances PFC glutamatergic transmission and working memory (Yuen et al., 2009; 2011). To understand the complex actions of stress hormones, we exposed animals to various days of restraint stress. As shown in Figure 2J, a bi-directional effect on AMPAR-EPSC was detected in stressed animals (F4,63=11.4, p<0.01, ANOVA, n=12–14 per group). Post hoc analysis indicated that AMPAR synaptic transmission was significantly increased by 1-day (2 hr) stress (79.6±19.8% increase, p<0.01), largely unchanged by 3-day stress (10.1±9.4% increase, p>0.05), and significantly decreased by 5-day stress (45.2±3.7% decrease, p<0.01) or 7-day stress (51.3±3.1% decrease, p<0.01). These results suggest that stress exerts a bi-phasic effect on PFC glutamatergic transmission depending on the duration of stressor. The onset of the impairing effect of repeated stress on glutamatergic transmission parallels that on recognition memory (Figure 1F), further suggesting the causal link between them.
To test the regional specificity of the effect of repeated stress, we also examined glutamatergic transmission in striatum and hippocampus from young male rats (Figure 2K). In contrast to the significant effect in PFC (control: 168.3±11.2pA, n=12; stressed: 81.8±5.9pA, n=12, p<0.01), repeated stress did not significantly alter AMPAR-EPSC in striatal medium spiny neurons (control: 142.9±10.6pA, n=11; stressed: 149.9±10.1pA, n=11, p>0.05) or CA1 pyramidal neurons (control: 142.4±10.3pA, n=10; stressed: 150.2±9.4pA, n=10, p>0.05).
Repeated stress decreases the total and surface levels of AMPAR and NMDAR subunits in PFC
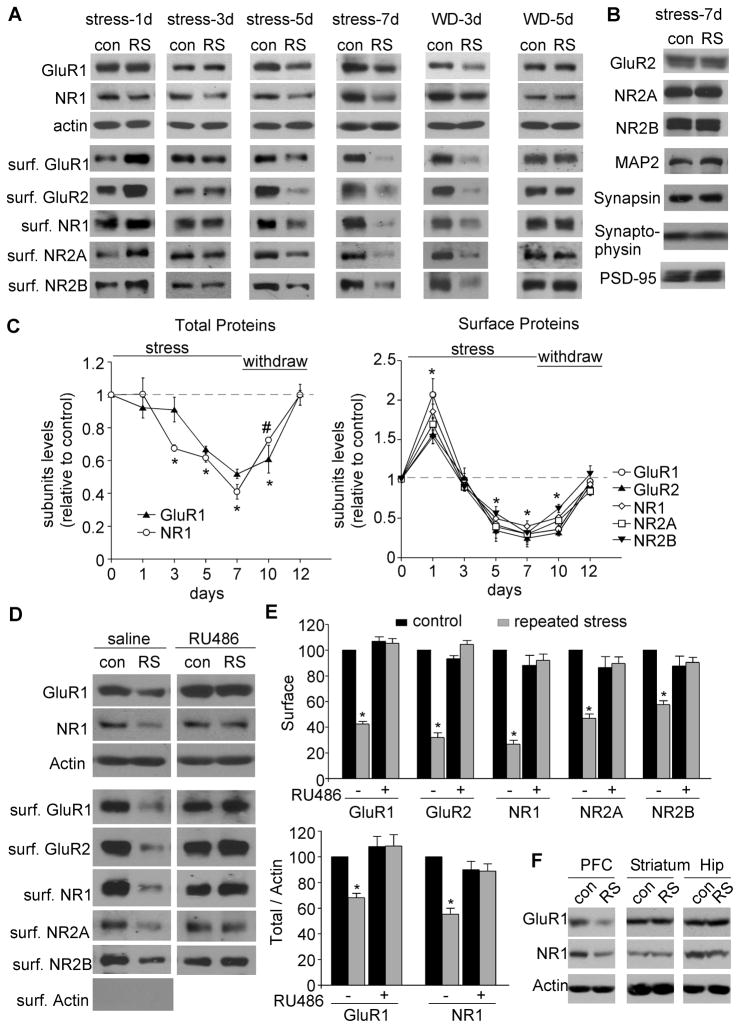
The suppression of glutamatergic transmission by repeated stress could result from the reduced number of glutamate receptors. To test this, we performed Western blotting and surface biotinylation experiments to detect the total and surface level of AMPAR and NMDAR subunits in PFC slices from stressed young male rats (4-week-old). As shown in Figure 3A, animals exposed to acute restraint stress (single time, 2 hr) showed a significant increase in surface AMPAR and NMDAR subunits (35–86% increase; n=4 pairs, p<0.01), while the total proteins remained unchanged, consistent with our previous findings (Yuen et al., 2009; 2011). Animals exposed to 3-day restraint stress showed no difference (n=4 pairs). Animals exposed to 5 or 7-day restraint stress showed a significant decrease in the amount of GluR1 and NR1 subunits (Figure 3C, GluR1: 45–51% decrease, NR1: 55–63% decrease, n=21 pairs, p<0.01). Moreover, repeated stress did not affect the total level of other glutamate receptor subunits (Figure 3B), such as GluR2, NR2A and NR2B (n=16 pairs), nor the expression of MAP2 (a dendritic marker), synapsin, synaptophysin (presynaptic markers) or PSD-95 (a postsynaptic marker, n=10 pairs), suggesting that no general dendritic or synaptic loss has occurred under such conditions. The amount of AMPAR and NMDAR subunits in the surface pool was all significantly decreased by repeated stress (Figure 3C, surface GluR1/2: 62–70% decrease, surface NR1/2A/2B: 55–70% decrease, n=6 pairs, p<0.01), indicating the loss of glutamate receptors at the plasma membrane.
Figure 3. Repeated stress decreases the total and surface levels of AMPAR and NMDAR subunits in PFC through GR activation.
(A, C) Immunoblots (A) and quantification analysis (C) of the total and surface AMPAR and NMDAR subunits in PFC from control (con) vs. rats exposed to 1–7 day of restraint stress (RS). Some animals were withdrawn (WD) for different durations (3 or 5 day) after being exposed to 7-day restraint stress. #: p<0.05; *: p<0.01, t test. (B) Immunoblots of the total proteins in PFC from control vs. repeatedly stressed (7-day restraint) rats. (D, E) Immunoblots (D) and quantification analysis (E) of the total and surface AMPAR and NMDAR subunits in PFC from control vs. repeatedly stressed animals without or with RU486 injection (10mg/kg). *: p<0.01, t test. (F) Immunoblots of total GluR1 and NR1 in PFC, striatum and hippocampus from control vs. repeatedly stressed (7-day restraint) rats.
To find out how long the effect of repeated stress can last, we exposed young animals to 7-day restraint stress, and examined at 3–5 days after stressor cessation. As shown in Figure 3A and 3C, after 3-day withdrawal of stress, the expression of total and surface AMPARs and NMDARs was still at a partially reduced level (total GluR1: ~39% decrease, total NR1: ~27% decrease, surface GluR1/2: 60–62% decrease, surface NR1/2A/2B: 40–55% decrease, n=3 pairs, p<0.01), but returned to the control level after 5-day withdrawal (n=3 pairs).
Injecting the GR antagonist RU486 abolished the decreasing effects of repeated restraint stress on total GluR1, total NR1, surface GluR1/2 and surface NR1/2A/2B (Figure 3D and 3E, n=3 pairs). It suggests that repeated stress down-regulates glutamate receptor expression via GR activation.
In contrast to the significant reduction of total GluR1 and NR1 expression in PFC by repeated restraint stress (Figure 3F, GluR1: ~52% of control; NR1: ~51% of control, p<0.01), no significant changes were found in other brain areas including striatum and hippocampus (Figure 3F, striatum: GluR1: ~108% of control; NR1: ~110% of control; hippocampus: GluR1: ~103% of control; NR1: 93% of control, n=3–5 pairs, p>0.05), confirming the region specificity of stress effects.
Similar to restraint stress, young male rats exposed to repeated unpredictable stress (7-day) also had significantly reduced levels of total GluR1 and NR1, as well as surface AMPAR and NMDAR subunits in PFC (Figure S2).
Since stress hormones elicit distinct effects throughout the lifespan (Lupien et al., 2009), we also examined older animals. As shown in Figure S3, adult (7-week-old) male rats, which had been exposed to 7-day repeated restraint or unpredictable stress, had normal levels of total and surface AMPAR and NMDAR subunits in the PFC. It suggests that the loss of PFC glutamate receptors induced by one-week repeated stress is a phenomenon specifically occurring in the adolescent period.
In vitro long-term corticosterone treatment reduces synaptic AMPARs through GR activation
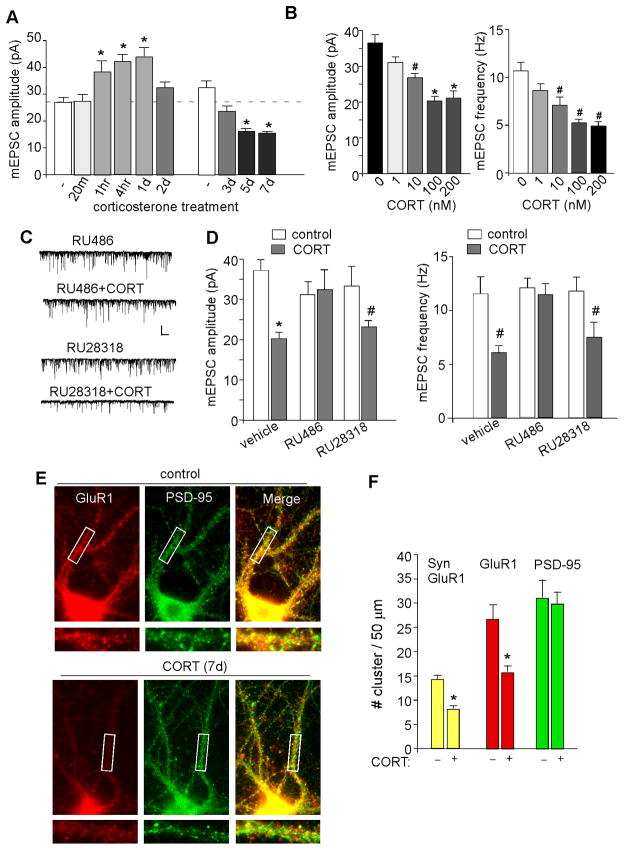
We next examined whether the effect of repeated stress in vivo may be mimicked by corticosterone (CORT) application in vitro. To do so, we treated PFC cultures with different durations and doses of CORT, and examined mEPSC. As shown in Figure 4A, mEPSC amplitude was bi-directionally changed in response to short- or long-term CORT (100 nM) treatment (F9,99=21.0, p<0.001, ANOVA, n=5–14 per group). Post hoc analysis indicated that acute CORT treatment significantly increased mEPSC amplitude (DIV21 control: 25.0±1.3pA, 1-hr CORT: 38.5±3.9pA, 4-hr CORT: 42.4±2.5pA, 1-day CORT: 44.2±3.3pA, p<0.01), similar to what we found before (Yuen et al., 2011; Liu et al., 2010), while a significant decrease was found with prolonged CORT treatment (DIV26 control: 32.6±2.7pA, 5-day CORT: 16.3±0.9pA, 7-day CORT: 15.4±0.5pA, p<0.01). Dose response studies (Figure 4B) indicated that different doses of CORT treatment (7-day) had different effects on mEPSC (amplitude: F4,42=15.3, p<0.01, frequency: F4,36=13.0, p<0.05, ANOVA, n=7–10 per group), with a small reducing effect at 10 nM and a saturated reducing effect at 100–200 nM. The effect of CORT (100 nM, 7-day) on mEPSC was lost in neurons incubated with RU486 (10 μM, Figure 4C and 4D, RU486: 31±3.1pA, 12.1±0.8Hz, n=7; RU486+CORT: 32.4±4.9pA, 11.3±0.98Hz, n=9, p>0.05), but not the MR antagonist RU28318 (10 μM, RU28318: 33.3±4.7pA, 11.8±1.3Hz, n=7; RU28318+CORT: 22.9±1.4pA, 7.4±1.4Hz, n=9, p<0.05), suggesting that GR mediates the effect of chronic CORT treatment.
Figure 4. In vitro chronic CORT treatment reduces AMPAR synaptic currents and synaptic GluR1 clusters via GR activation.
(A, B) Bar graphs showing the effect of different durations (A) and concentrations (B) of CORT on mEPSC. *: p<0.01, #: p<0.05, ANOVA. (C, D) Representative mEPSC traces (C) and statistic summary (D) showing the effect of CORT (100 nM, 7-day) on mEPSC amplitude and frequency in the presence of GR or MR antagonists in cultured PFC neurons (DIV28–30). Scale bars: 50pA, 1s. *: p<0.01, #: p<0.05, t test. (E) Immunostaining of total GluR1 and PSD-95 in PFC cultures treated with or without CORT (100 nM, 7-day). (F) Bar graphs showing the cluster density of synaptic GluR1 (co-localized, yellow puncta), total GluR1 (red puncta) and PSD-95 (green puncta) in response to CORT treatment. *: p<0.01, t test.
To test whether the CORT-induced reduction of mEPSC was due to the decreased number of AMPARs at synapses, we performed immunocytochemical experiments to measure the cluster density (# clusters/50μm dendrite) of total GluR1 and synaptic GluR1 (co-localized with the synaptic marker PSD-95) in PFC cultures. As shown in Figure 4E and 4F, CORT treatment (100 nM, 7-day) significantly reduced total GluR1 cluster density (control: 26.6±3.1, n=14; CORT: 15.6±1.3, n=12, p<0.01) and synaptic GluR1 cluster density (control: 14.0±1.0, n=11; CORT: 7.8±0.7, n=12, p<0.01). Taken together, these results suggest that, similar to in vivo repeated stress, prolonged in vitro CORT treatment also reduces AMPAR expression and function through GR activation.
Ubiquitin/proteasome-dependent degradation of glutamate receptors underlies the effect of repeated stress
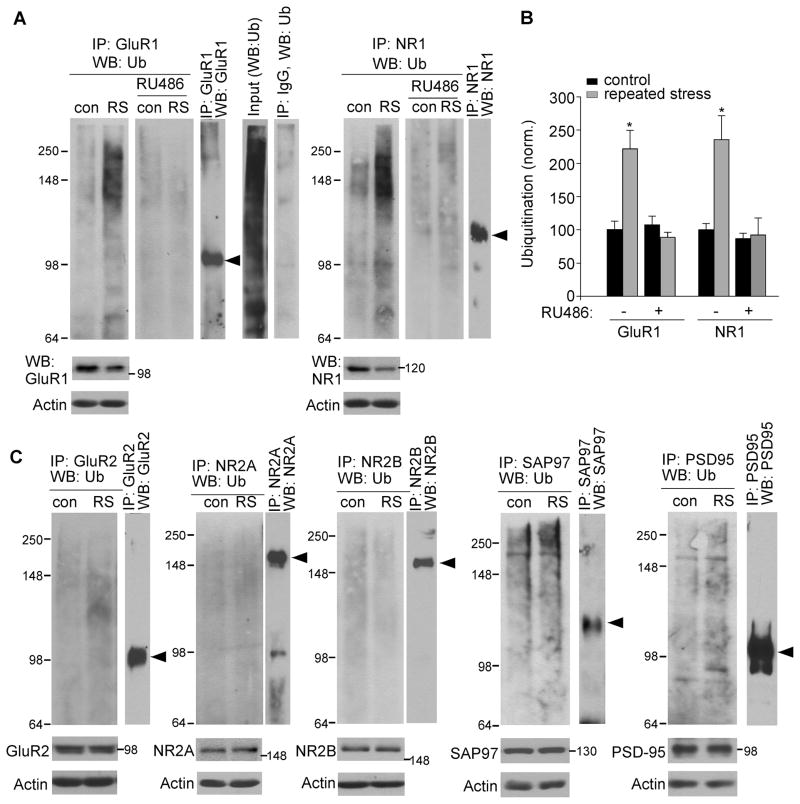
Since the total level of NR1 and GluR1 was reduced in repeatedly stressed animals, we examined whether it could be due to the decreased synthesis or increased degradation of glutamate receptors. As shown in Figure S4, repeated stress did not significantly alter the mRNA level of AMPAR and NMDAR subunits, suggesting that protein synthesis is intact. Thus, the reducing effect of repeated stress on NR1 and GluR1 expression may be due to the increased ubiquitin/proteasome-dependent protein degradation. Consistent with this, the level of ubiquitinated GluR1 and NR1 was significantly increased in animals exposed to repeated restraint stress (Figure 5A and 5B, Ub-GluR1: 121.6±28.3% increase, Ub-NR1: 135.9±35.6% increase, n=6 pairs, p<0.01), which was abolished by RU486 injection (n=3). The level of ubiquitinated GluR2, NR2A, or NR2B subunits remained unchanged (n=4 pairs, Figure 5C). Repeated stress also failed to alter the ubiquitination of SAP97 (a GluR1 binding protein) and PSD-95 (an NR1 binding protein, n=3 pairs, Figure 5C). These results provide direct evidence showing that prolonged GR activation selectively increases ubiquitin conjugation of GluR1 and NR1 subunits in PFC and thus enhances the susceptibility of these proteins to proteasome-mediated degradation.
Figure 5. Repeated stress increases the ubiquitination level of GluR1 and NR1 subunits.
(A, B) Representative blots (A) and quantification (B) showing the ubiquitination of GluR1 and NR1 subunits in control vs. stressed (7-day restraint) animals without or with RU486 injection (10 mg/kg). *: p<0.01, t test. Lysates of PFC slices were immunoprecipitated with an antibody against GluR1 or NR1, and then blotted with a ubiquitin antibody. Also shown are the input control, the immunoprecipitation control, and the immunoblots of total proteins in control vs. stressed animals. Note, in stressed rats, the immunoprecipitated GluR1 or NR1 showed ubiquitin staining at a molecular mass heavier than the unmodified protein itself. The ladder of ubiquitinated GluR1 or NR1 is typical of proteins that are polyubiquitinated to signal their degradation. (C) Ubiquitination of GluR2, NR2A, NR2B, SAP97 and PSD-95 in control vs. stressed (7-day restraint) animals.
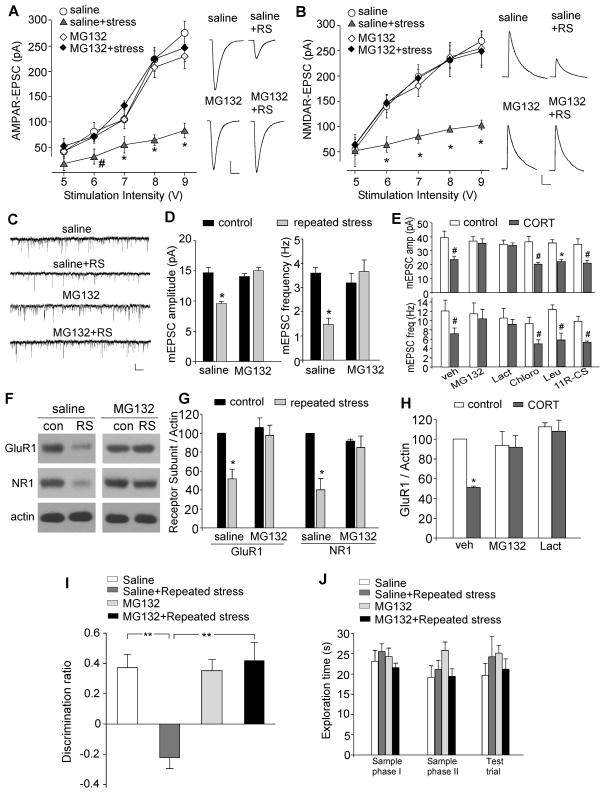
To further test the role of glutamate receptor degradation in chronic stress-induced reduction of synaptic transmission, we injected the proteasome inhibitor MG132 to PFC via an implanted cannula (0.5μg each side; 21pmol/g b.w., daily at 1 hr before stress). As shown in Figure 6A and 6B, the effects of repeated restraint stress on glutamatergic transmission were significantly different in saline- vs. MG132-injected animals (AMPA: p<0.01, ANOVA, n=9–12 per group; NMDA: p<0.01, ANOVA, n=11–14 per group). Post hoc analysis showed that repeated stress caused a substantial down-regulation of eEPSC amplitude in saline-injected animals (AMPA: 50–59% decrease; NMDA: 44–52% decrease, p<0.01), but had little effect in MG132-injected animals (AMPA: 3–7% decrease; NMDA: 2–5% decrease, p>0.05). Injection of MG132, but not saline, also blocked the reducing effect of repeated stress on mEPSC amplitude and frequency in PFC slices (Figure 6C and 6D, MG132: 14.0±0.5pA, 3.2±0.4Hz, n=8; MG132+stress: 15.0±0.5pA, 3.6±0.5Hz, n=10, p>0.05).
Figure 6. Infusion of a proteasome inhibitor into PFC prevents the loss of glutamate receptors and recognition memory by repeated stress.
(A, B) Summarized input-output curves of AMPAR-EPSC (A) or NMDAR-EPSC (B) in control vs. repeatedly stressed (7-day restraint) animals with local injection of the proteasome inhibitor MG132 or saline control. *: p<0.01, #: p<0.05, ANOVA. Inset: representative EPSC traces. Scale bars: 50pA, 20ms (A); 50pA, 100ms (B). (C, D) Representative mEPSC traces and bar graph summary of mEPSC amplitude and frequency in control vs. repeatedly stressed animals with PFC infusion of MG132 or saline. *: p<0.01, t test. Scale bars (C): 25pA, 1s. (E) Bar graphs showing the effect of CORT (100 nM, 7-day) on mEPSC amplitude and frequency in cultured PFC neurons pre-treated with the specific inhibitors of proteasome, lysosome or calpain. *: p<0.01, #: p<0.05, t test. (F, G) Immunoblots and quantification analysis of GluR1 and NR1 expression in control vs. repeatedly stressed animals with PFC infusion of MG132 or saline. *: p<0.01, t test. (H) Quantification analysis of GluR1 expression in control vs. CORT (100 nM, 7-day)-treated PFC cultures pre-incubated without or with proteasome inhibitors. *: p<0.01, t test. (I, J) Bar graphs showing the discrimination ratio (I) and total exploration time (J) of TOR tasks in control groups vs. repeatedly stressed animals (7-day restraint) with stereotaxic injections of saline or MG132 into PFC via an implanted cannula. **: p<0.001, ANOVA.
In vitro studies further confirmed that the proteasome-mediated degradation of glutamate receptors may underlie the reduction of mEPSC by long-term CORT treatment. As shown in Figure 6E, CORT (100 nM, 7d) significantly decreased mEPSC in vehicle-treated neurons (control: 37.1±2.9pA, 12.1±1.8Hz, n=9; CORT: 23.3±2.9pA, 7.1±1.2Hz, n=7, p<0.05), but failed to do so in MG132 (1 μM)-treated neurons (MG132: 36.8±3.2pA, 11.5±2.3Hz, n=11; MG132+CORT: 35.4±2.8pA, 10.4±1.9Hz, n=7, p>0.05). Another proteasome inhibitor lactacystin (1 μM) gave similar blockade (lact: 34.5±3.0pA, 10.5±2.0Hz, n=8; lact+CORT: 33.9±1.8pA, 9.2±1.1Hz, n=8, p>0.05). However, the reducing effect of CORT was insensitive to the general lysosomal enzyme inhibitor chloroquine (200 μM, Chlq: 36.2±3.9pA, 9.4±1.4Hz, n=6; Chlq+CORT: 22.4±1.2pA, 5.0±0.8Hz, n=6, p<0.05), the lysosomal protease inhibitor leupeptin (200 μM, leu: 35.9±2.4pA, 12.2±0.9Hz, n=8; leu+CORT: 22.3±1.3pA, 5.6±1.4Hz, n=8, p<0.05), or the membrane-permeable calpain protease inhibitory peptide 11R-CS (2 μM, Wu et al., 2005; 11R-CS: 34.9±3.9pA, 9.8±1.2Hz, n=7; 11R-CS+CORT: 21.0±1.9pA, 5.2±0.3Hz, n=5, p<0.05).
Biochemical measurement of glutamate receptor subunits in PFC slices (Figure 6F and 6G) indicated that MG132-injected rats exhibited the normal level of GluR1 and NR1 after being exposed to 7-day restraint stress (GluR1: 6.6±10.7% decrease; NR1: 10.5±12.8% decrease, n=4 pairs, p>0.05), which was in sharp contrast to the reduced expression of GluR1 and NR1 in saline-injected rats after repeated stress (GluR1: 48.3±10.1% decrease; NR1: 59.7±11.9% decrease, n=4 pairs, p<0.01). In addition, the CORT (100 nM, 7d)-induced decrease of GluR1 expression (49.0±1.4% decrease, n=6, p<0.01) was abolished by proteasome inhibitors (Figure 6H, MG132: 8.2±11.7% decrease; lactacystin: 7.9±11.2% decrease, n=4, p>0.05). Taken together, these results suggest that repeated behavioral stress or long-term CORT treatment induces the ubiquitin/proteasome-dependent degradation of GluR1 and NR1, leading to the depression of glutamatergic transmission in PFC.
To find out whether the proteasome-dependent degradation of glutamate receptors induced by repeated stress may underlie its detrimental effect on cognitive processes, we examined the temporal order recognition memory in animals with stereotaxic injections of MG132 into PFC prelimbic regions bilaterally. A significant main effect was observed (Figure 6I, F3,28=7.9, p<0.001, ANOVA), and post-hoc analysis indicated that repeated stress caused a significant deficit in the recognition of novel (less recent) object in saline-injected animals (DR in control: 37.1±8.9%, n=7; DR in stressed: −22.3±7.4%, n=7, p<0.001), while the deficit was blocked in MG132-injected animals (DR in control: 36.4±6.7%, n=6; DR in stressed: 42.2±12.3%, n=9, p>0.05). The total exploration time was unchanged in the sample phases and test trial (Figure 6J). These behavioral data, in combination with electrophysiological and biochemical data, suggest that the cognitive impairment by repeated stress may be due to the proteasome-dependent degradation of glutamate receptors in PFC.
The specific regulation of AMPAR and NMDAR subunits in PFC by repeated stress involves different E3 ubiquitin ligases
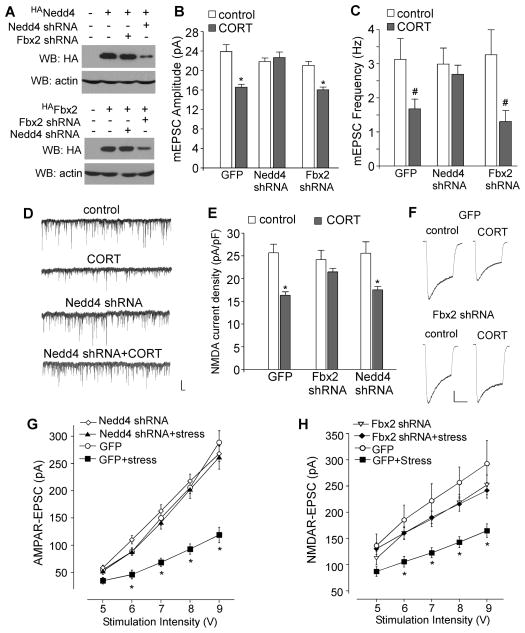
Given the role of proteasome-dependent degradation of glutamate receptors in the detrimental effects of repeated stress, we would like to know which E3 ubiquitin ligases are potentially involved in the stress-induced ubiquitination of GluR1 and NR1 subunits in PFC. The possible candidates are Nedd4-1 (neural-precursor cell-expressed developmentally downregulated gene 4-1), an E3 ligase necessary for GluR1 ubiquitination in response to the agonist AMPA (Schwarz et al., 2010; Lin et al., 2011), and Fbx2, an E3 ligase in the ER that ubiquitinates NR1 subunits (Kato et al., 2005). Thus, we performed RNA interference-mediated knockdown of Nedd4-1 or Fbx2 in vitro or in vivo, and examined the impact of long-term CORT treatment or repeated stress on glutamatergic transmission in PFC neurons. As illustrated in Figure 7A, Nedd4-1 or Fbx2 shRNA caused a specific and effective suppression of the expression of these E3 ligases.
Figure 7. The E3 ubiquitin ligases Nedd4-1 and Fbx2 are involved in the downregulation of AMPAR- and NMDAR-mediated synaptic reponses by long-term CORT treatment or repeated stress.
(A) Representative Western blots in HEK293 cells transfected with HA-tagged rat Nedd4-1 or Fbx2 in the absence or presence of Nedd4-1 shRNA or Fbx2 shRNA. (B, C) Summary data (mean ± SEM) showing the mEPSC amplitude and frequency in control vs. CORT (100 nM, 7d)-treated PFC neurons transfected with Nedd4-1 shRNA, Fbx2 shRNA or GFP control. *: p<0.01, #: p<0.05, t test. (D) Representative mEPSC traces in control vs. CORT-treated PFC neurons with different transfections. Scale bar: 20 pA, 1 sec. (E) Summary data (mean ± SEM) showing the NMDAR current density in control vs. CORT (100 nM, 7d)-treated PFC neurons transfected with Fbx2 shRNA, Nedd4-1 shRNA or GFP control. *: p<0.01, t test. (F) Representative NMDAR currents in control vs. CORT-treated PFC neurons with different transfections. Scale bar: 200 pA, 1 sec. (G, H) Summarized input-output curves of AMPAR-EPSC (G) or NMDAR-EPSC (H) in control vs. repeatedly stressed (7-day restraint) rats with the PFC injection of Nedd4-1 shRNA lentivirus (G), Fbx2 shRNA lentivirus (H), or GFP lentivirus control. *: p<0.01, ANOVA.
In PFC cultures transfected with Nedd4-1 shRNA, CORT treatment (100 nM, 7d) lost the capability to reduce mEPSC (Figure 7B–D, control: 21.8±0.7pA, 3.0±0.5Hz, n=20; CORT: 22.6±1.2pA, 2.7±0.3Hz, n=15, p>0.05), while the reducing effect of CORT on mEPSC was unaltered in Fbx2 shRNA neurons (control: 21.1±0.8pA, 3.3±0.7Hz, n=10; CORT: 16.1±0.6pA, 1.3±0.3Hz, n=12, p<0.05) or GFP-transfected neurons (control: 23.9±1.4pA, 3.1±0.6Hz, n=9; CORT: 16.6±0.6pA, 1.7±0.3Hz, n=14, p<0.05). On the other hand, in PFC cultures transfected with Fbx2 shRNA, long-term CORT failed to decrease NMDAR current density (pA/pF) (Figure 7E and 7F, control: 24.2±2.0, n=13; CORT: 21.5±0.8, n=13, p>0.05), while the suppressing effect of CORT on NMDAR current was intact in Nedd4 shRNA-transfected neurons (control: 25.6±2.5, n=9; CORT: 17.5±0.8, n=9, p<0.01) or GFP-transfected neurons (control: 25.7±1.9, n=13; CORT: 16.4±0.8, n=8, p<0.01).
Next, we delivered Nedd4-1 or Fbx2 shRNA lentivirus to rat frontal cortex via a stereotaxic injection (Liu et al., 2011), and tested the involvement of these E3 ligases in the action of repeated stress. As shown in Figure 7G and 7H, the effects of repeated restraint stress on AMPAR-EPSC or NMDAR-EPSC were significantly different in animals with different viral infections (AMPA: p<0.01, ANOVA, n=13–15 per group; NMDA: p<0.01, ANOVA, n=13–19 per group). Post hoc analysis showed that repeated stress caused a substantial down-regulation of the eEPSC amplitude in GFP lentivirus-injected animals (AMPA: 48–58% decrease; NMDA: 38–52% decrease, p<0.01), but had little effect on AMPAR-EPSC in Nedd4 shRNA lentivirus-injected animals (7–10% decrease, p>0.05) or on NMDAR-EPSC in Fbx2 shRNA lentivirus-injected animals (5–7% decrease, p>0.05). These electrophysiological results suggest that Nedd4-1 and Fbx2 mediate the long-term CORT or repeated stress-induced downregulation of AMPAR and NMDAR responses in PFC, respectively.
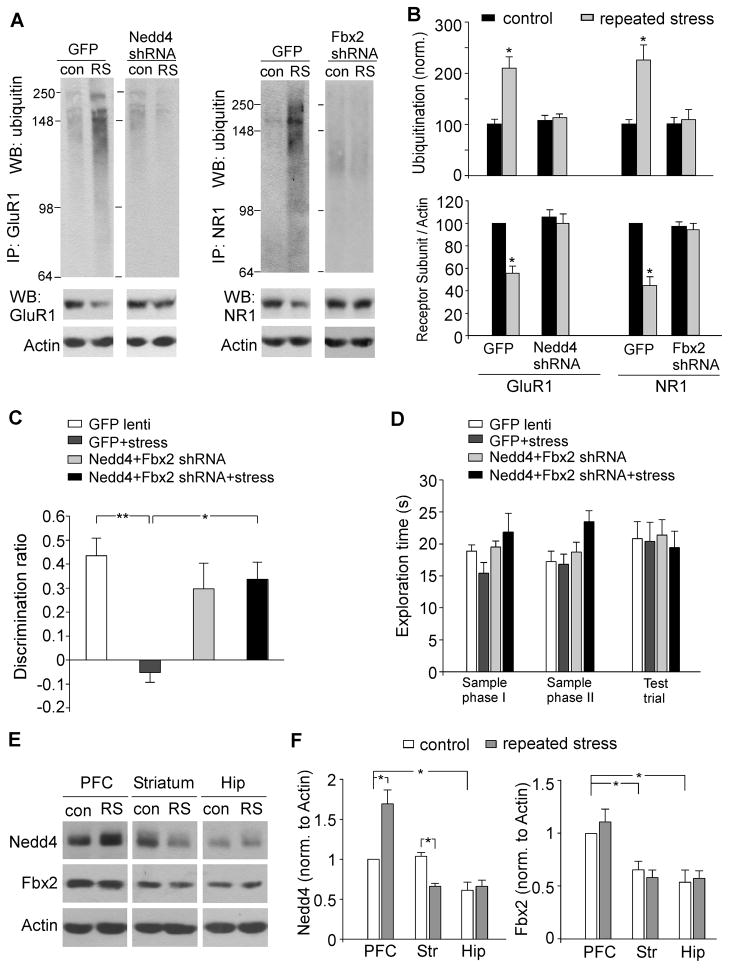
We further examined the involvement of Nedd4-1 and Fbx2 in the stress-induced glutamate receptor ubiquitination by in vivo delivery of the shRNA lentivirus against these E3 ligases to PFC. As shown in Figure 8A and 8B, Nedd4-1 shRNA or Fbx2 shRNA lentivirus-injected rats failed to show the increased level of ubiquitinated GluR1 or NR1 after being exposed to 7-day restraint stress (Ub-GluR1: 5.0±4.5% increase; Ub-NR1: 6.4±9.3% increase, n=4 pairs for each, p>0.05), which was significantly different from the effects seen in GFP lentivirus-injected rats after repeated stress (Ub-GluR1: 115.0±24.6% increase; NR1: 136.4±31.3% increase, n=6 pairs, p<0.01). Moreover, in contrast to the significantly lower level of GluR1 and NR1 expression in GFP lentivirus-injected rats following stress (GluR1: 46.8±8.3% decrease; NR1: 57.2±8.8% decrease, n=6 pairs, p<0.01), Nedd4-1 shRNA or Fbx2 shRNA lentivirus-injected rats exhibited the normal level of GluR1 or NR1 after repeated stress (GluR1: 7.3±8.7% decrease; NR1: 5.5±8.8% decrease, n=4 pairs for each, p>0.05). These biochemical results suggest that Nedd4-1 and Fbx2 mediate the repeated stress-induced ubiquitination and degradation of GluR1 and NR1 subunits in PFC, respectively.
Figure 8. Nedd4-1 and Fbx2 are involved in the stress-induced ubiquitination/degradation of GluR1 and NR1 subunits and impairment of recognition memory, and they show differential expression in various brain regions of rats with or without stress exposure.
(A, B) Representative blots (A) and quantification (B) showing the ubiquitination and expression of GluR1 and NR1 subunits in control vs. stressed (7-day restraint) animals with PFC injection of GFP lentivirus, Nedd4-1 shRNA lentivirus or Fbx2 shRNA lentivirus *: p<0.01, t test. (C, D) Bar graphs showing the discrimination ratio (C) and total exploration time (D) of TOR tasks in control groups vs. repeatedly stressed animals (7-day restraint) with PFC injection of GFP lentivirus or Nedd4-1 shRNA+Fbx2 shRNA lentiviruses. **: p<0.001, *: p<0.01, ANOVA. (E, F) Representative Western blots and quantification showing the expression of Nedd4-1 and Fbx2 in PFC, striatum and hippocampus of control vs. repeatedly stressed (RS) rats. Actin was used as the loading control. *: p<0.01, ANOVA.
To find out the role of Nedd4-1 and Fbx2 in the stress-induced detrimental effect on cognitive processes, we examined the temporal order recognition memory in animals with in vivo knockdown of both E3 ligases in PFC. As shown in Figure 8C, repeated stress caused a significant deficit in the recognition of novel (less recent) object in GFP lentivirus-injected animals (DR in control: 43.6±7.3%, n=7; DR in stressed: −5.2±4.1%, n=8, p<0.001), while the deficit was blocked in animals injected with both Nedd4-1 and Fbx2 shRNA lentiviruses to PFC (DR in control: 29.7±10.7%, n=7; DR in stressed: 33.7±7.1%, n=8, p>0.05). The total exploration time was unchanged in the sample phases and test trial (Figure 8D). These behavioral data suggest that the cognitive impairment by repeated stress may be due to the Nedd4-1 and Fbx2-dependent loss of glutamate receptors in PFC.
To understand the potential mechanism underlying the region specificity of the effects of repeated stress on glutamate receptor expression and function, we examined the level of Nedd4-1 and Fbx2 in PFC, striatum and hippocampus from control vs. stressed young male rats. As shown in Figure 8E, the level of Nedd4-1 was significantly higher in PFC or striatum than in hippocampus from control animals (p<0.01, n=8). After repeated stress, Nedd4-1 was significantly elevated in PFC (~70% increase, p<0.01, n=6 pairs), but was significantly reduced in striatum (~35% decrease, p<0.01, n=7 pairs) and unchanged in hippocampus (p>0.05, n=8 pairs). Moreover, the level of Fbx2 was significantly higher in PFC than in striatum or hippocampus from control or stressed animals (Figure 8F, p<0.01, n=7 pairs). These results provide a potential reason for the higher sensitivity of PFC to repeated stress than other brain regions like striatum and hippocampus.
DISCUSSION
In the present study, we have identified glutamate receptors as an important molecular substrate of repeated stress. Given the significance of glutamatergic signaling in PFC-mediated cognitive processes (Goldman-Rakic, 1995; Lisman et al., 1998), it is not surprising that repeated stress impairs the object recognition memory, which is reminiscent of the memory deficits following bilateral infusion of glutamate receptor antagonists directly into PFC. The loss of PFC glutamatergic responses could also underlie the stress-induced other behavioral impairments found earlier (Liston et al., 2006; Cerqueira et al., 2005; 2007).
Mounting evidence has suggested that stress induces divergent changes in different brain regions (de Kloet et al., 2005; McEwen, 2007). Chronic stress causes atrophy of dendrites in the CA3 region, suppresses neurogenesis of dentate gyrus granule neurons, and impairs hippocampal-dependent cognitive functions (McEwen, 1999; Joëls et al., 2007). High levels of corticosterone or chronic stress also impair long-term potentiation (LTP) and facilitate long-term depression (LTD) induced by electrical stimulation in hippocampus (Kim and Diamond, 2002; Alfarez et al., 2003). On the other hand, chronic stress has been shown to enhance amygdala-dependent fear conditioning (Conrad et al., 1999) and anxiety-like behavior (Mitra et al., 2005), which may be correlated to the stress-induced dendritic growth and spinogenesis in this region (Vyas et al., 2002; Mitra et al., 2005). In this study, we have demonstrated that glutamatergic transmission in PFC pyramidal neurons is significantly suppressed in young male rats exposed to repeated stress, without the apparent loss of synapses. In contrast, no such effect is observed in striatal medium spiny neurons or CA1 pyramidal neurons, consistent with the lack of effect of chronic stress on synaptic currents in hippocampal dentate gyrus neurons (Karst and Joëls, 2003). It suggests that PFC is a more sensitive area in response to repeated stress, especially during the adolescent period when this region is still undergoing significant development (Lupien et al., 2009). The GR-induced suppression of glutamatergic transmission in PFC might serve as a form of LTD that precedes structural plasticity.
In addition to the region specificity, the outcome of stress is also determined by the duration and severity of the stressor (de Kloet et al., 2005; Joëls, 2008). While acute stressful experience has been found to enhance associative learning (Shors et la., 1992; Joëls et al., 2006) in a glucocorticoid-dependent manner (Beylin and Shors, 2003), severe or chronic stress has been shown to impair working memory and prefrontal function (Liston et al., 2006; Cerqueira et al., 2007; Arnsten, 2009). We have found that acute stressors induce a long-lasting potentiation of glutamatergic transmission in PFC and facilitate working memory (Yuen et al., 2009; 2011), which is in contrast to the strong suppression of PFC glutamatergic transmission and impairment of object recognition memory by repeated stress. Thus, glutamate receptors seem to be the neural substrate that underlies the biphasic effects of stress and glucocorticoids on synaptic plasticity and memory (Diamond et al., 1992; Groc et al., 2008; Krugers et al., 2010).
Different downstream mechanisms have been identified in the dual effects of stress on PFC glutamatergic signaling. Acute stress enhances the surface delivery of NMDARs and AMPARs via a mechanism depending on the induction of serum- and glucocorticoid-inducible kinase (SGK) and the activation of Rab4 (Yuen et al., 2009; 2011; Liu et al., 2010). In contrast, repeated stress reduces the expression of GluR1 and NR1 subunits, as well as functional AMPAR and NMDAR channels at cell surface.
Our data suggest that the loss of glutamate receptors after repeated stress may involve the increased ubiquitin/proteasome-mediated degradation of GluR1 and NR1 subunits. Posttranslational modification through the ubiquitin pathway at the postsynaptic membrane has emerged as a key mechanism for remodeling synaptic networks and altering synaptic transmission (Mabb and Ehlers, 2010). Following chronic changes in synaptic activity of hippocampal cultures, many PSD scaffold proteins, such as Shank, GKAP and AKAP, are up- or down-regulated through the ubiquitin-proteasome system (UPS, Ehlers, 2003). Abnormalities in the brain UPS have been implied in a variety of neurodegenerative and mental disorders (Ciechanover and Brundin, 2003; Middleton et al., 2002), however little is known about the circumstances under which AMPAR and NMDAR ubiquitination occurs under normal and disease conditions. In the present study, we demonstrate that the ubiquitination of GluR1 and NR1 subunits, but not their anchoring proteins, is specifically increased in PFC slices upon GR activation following repeated stress. The effect of repeated stress or prolonged CORT treatment on glutamatergic responses and GluR1/NR1 expression is blocked by the specific inhibitors of proteasomes, but not lysosomes. It suggests that GR-induced ubiquitination of GluR1 and NR1 subunits tags them for degradation by proteasomes in the cytoplasm, therefore fewer heteromeric AMPARs and NMDARs channels are assembled and delivered to the synaptic membrane. Interestingly, infusion of a proteasome inhibitor into PFC prevents the loss of recognition memory in stressed animals, providing a potential approach to block the detrimental effects of repeated stress.
To further understand the mechanisms underlying the specific ubiquitination of GluR1 and NR1 in PFC by repeated stress, we have explored the potentially participating E3 ubiquitin ligase, which determines selectivity for ubiquitination by bridging target proteins to E2 ubiquitin-conjugating enzyme and ubiquitin. NR1 subunits are found to be ubiquitinated by the E3 ligase Fbx2 in the ER (Kato et al., 2005), a process affecting the assembly and surface expression of NMDARs. Studies in C. elegans also indicate that GLR-1 is ubiquitinated in vivo, which regulates the GLR-1 abundance at synapses (Burbea et al., 2002; Juo & Kaplan, 2004; Park et al., 2009). Moreover, the E3 ligase Nedd4-1 has been recently shown to mediate the agonist-induced GluR1 ubiquitination in neuronal cultures, which affects AMPAR endocytosis and lysosomal trafficking (Schwarz et al., 2010; Lin et al., 2011). Using RNA interference-mediated knockdown in vitro and in vivo, we demonstrate that the suppression of AMPAR and NMDAR responses induced by long-term CORT treatment or repeated stress requires Nedd4-1 and Fbx2, respectively. Moreover, Nedd4-1 is required for the increased GluR1 ubiquitination and Fbx2 is required for the increased NR1 ubiquitination in repeatedly stressed animals. Both E3 ligases are also required for the stress-induced impairment of cognitive processes. The higher expression level of these E3 ubiquitin ligases in PFC than other brain regions, along with the upregulation of Nedd4-1 in PFC from stressed animals, potentially underlies the selective increase of GluR1 and NR1 ubiquitination and degradation in PFC neurons after repeated stress. Future studies will further examine the biochemical signaling cascades underlying the GR-induced changes in the activity and/or expression of Nedd4-1 and Fbx2.
Taken together, this study indicates that in response to repeated stress, the key AMPAR and NMDAR subunits, GluR1 and NR1, are degraded by the ubiquitin-proteasome pathway in PFC neurons, causing the loss of glutamate receptor expression and function, which leads to the deficit of PFC-mediated cognitive processes. Since PFC dysfunction has been implicated in various stress-related mental disorders (Andreasen et al., 1997; Brody et al., 2001; Davidson et al., 2000; Shin et al., 2001), delineating molecular mechanisms by which stress affects PFC functions should be critical for understanding the role of stress in influencing the disease process (Moghaddam and Jackson, 2004; Cerqueira et al., 2007).
EXPERIMENTAL PROCEDURES
Repeated stress paradigm
All experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo. Juvenile (3–4 weeks old) SD male rats were used in this study. For repeated restraint stress, rats were placed in air-assessable cylinders for 2 hr daily (10:00 am to 12:00 pm) for 5–7 days. The container size was similar to the animal size, which made the animal almost immobile in the container. For repeated unpredictable stress (7-day), rats were subjected each day to two stressors that were randomly chosen from six different stressors, forced swim (RT, 30 min), elevated platform (30 min), cage movement (30 min), lights on overnight, immobilization (RT, 1 hr), food and water deprivation overnight. Experiments were performed 24 hrs after the last stressor exposure.
Animal surgery
For drug delivery to PFC, rats (~3wk) were implanted with double guide cannulas (Plastics One Inc.) using a stereotaxic apparatus (David Kopf Instruments) as we described before (Yuen et al., 2011). The PFC coordinates were: 2.5 mm anterior to bregma; 0.75 mm lateral; 2.5 mm dorsal to ventral. The injection cannula extended 1.5 mm beyond the guide. After the implantation surgery, animals were allowed to recover for 2–3 days. Drugs were injected via the cannula bilaterally into PFC using a Hamilton syringe (22-gauge needle).
Behavioral testing
The temporal order recognition (TOR) task was conducted as previously described (Barker et al., 2007). All objects were affixed to a round platform (diameter: 61.4 cm). Each rat was habituated twice on the platform for 5 min on the day of behavioral experiments. This TOR task comprised two sample phases and one test trial. In each sample phase, the animals were allowed to explore two identical objects for a total of 3 min. Different objects were used for sample phases I and II, with a 1-hr delay between the sample phases. The test trial (3-min duration) was given 3-hr after sample phase II. During the test trial, an object from sample phase I and an object from sample phase II were used. The positions of the objects in the test and sample phases were counterbalanced between the animals. All behavioral experiments were performed at late afternoon and early evening in dim light. If temporal order memory is intact, the animals will spend more time exploring the object from sample I (i.e., the “novel” object presented less recently), compared with the object from sample II (i.e., the “familiar” object presented more recently). We calculated a discrimination ratio, the proportion of time spent exploring the novel (less recent) object (i.e., the difference in time spent exploring the “novel” and “familiar” objects divided by the total time spent exploring both objects) during the test trial. This measure takes into account individual differences in the total amount of exploration time.
Details regarding the object location task, open-field and locomotion tests are included in Supplementary Experimental Procedure.
Electrophysiological Recordings
PFC-containing slices were positioned in a perfusion chamber attached to the fixed stage of an upright microscope (Olympus) and submerged in continuously flowing oxygenated ACSF (in mM: 130 NaCl, 26 NaHCO3, 3 KCl, 5 MgCl2, 1.25 NaH2PO4, 1 CaCl2, 10 Glucose, pH 7.4, 300 mOsm). Bicuculline (10 μM) and CNQX (25 μM) were added in NMDAR-EPSC recordings. Bicuculline and D-APV (25 μM) were added in AMPAR-EPSC recordings. Patch electrodes contained internal solution (in mM): 130 Cs-methanesulfonate, 10 CsCl, 4 NaCl, 10 HEPES, 1 MgCl2, 5 EGTA, 2.2 QX-314, 12 phosphocreatine, 5 MgATP, 0.2 Na3GTP, 0.1 leupeptin, pH 7.2–7.3, 265–270 mOsm. Layer V mPFC pyramidal neurons were visualized with a 40X water-immersion lens and recorded with the Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA). Evoked EPSC were generated with a pulse from a stimulation isolation unit controlled by a S48 pulse generator (Grass Technologies, West Warwick, RI). A bipolar stimulating electrode (FHC, Bowdoinham, ME) was placed ~100 μm from the neuron under recording. Membrane potential was maintained at −70mV for AMPAR-EPSC recordings. For NMDAR-EPSC, the cell (clamped at −70 mV) was depolarized to +60mV for 3 s before stimulation to fully relieve the voltage-dependent Mg2+ block. ACSF was modified to contain 1 mM MgCl2 to record miniature EPSC in PFC slices.
To obtain the input-output responses, EPSC was elicited by a series of stimulation intensities with the same duration of pulses (0.6 ms for NMDAR-EPSC; 0.06 ms for AMPAR-EPSC). In other experiments, synaptic currents evoked by the same stimulation intensity were recorded in individual neurons across groups with different manipulations. To control recording variability between cells, a few criteria were used as we previously described (Yuen et al., 2009; 2011). Recordings from control vs. stressed animals were interleaved throughout the course of all experiments. Data analyses were performed with Clampfit (Molecular Devices) and Kaleidagraph (Albeck Software).
Details regarding whole-cell recordings in isolated neurons and miniature EPSC recordings in cultured PFC neurons are included in Supplementary Experimental Procedure.
Biochemical measurement of surface and total proteins
The surface AMPA and NMDA receptors were detected as previously described (Yuen et al., 2009). In brief, PFC slices were incubated with ACSF containing 1 mg/ml sulfo-N-hydroxysuccinimide- LC-Biotin (Pierce Chemical Co., Rockford, IL) for 20 min on ice. The slices were then rinsed three times in Tris-buffered saline to quench the biotin reaction, followed by homogenization in modified radioimmunoprecipitation assay buffer. The homogenates were centrifuged at 14,000 × g for 15 min at 4°C, incubated with 50% Neutravidin Agarose (Pierce Chemical Co.) for 2 hr at 4°C, and bound proteins were resuspended in SDS sample buffer and boiled. Quantitative Western blots were performed on both total and biotinylated (surface) proteins (See Supplementary Experimental Procedure for details).
Immunoprecipitation
PFC slices were collected and homogenized in lysis buffer (in mM: 50 NaCl, 30 sodium pyrophosphate, 50 NaF, 10 Tris, 5 EDTA, 0.1 Na3VO4, 1 PMSF, with 1% Triton X-100 and protease inhibitor tablet). Lysates were ultracentrifuged (200,000 × g) at 4°C for 1 hr. Supernatant fractions were incubated with primary antibodies (see Supplementary Experimental Procedure for antibody details) for overnight at 4°C, followed by incubation with 50 μl of protein A/G plus agarose (Santa Cruz Biotechnology) for 1 hr at 4°C. Immunoprecipitates were washed three times with lysis buffer, then boiled in 2×SDS loading buffer for 5 min, and separated on 7.5% SDS-polyacrylamide gels. Western blotting experiments were performed with anti-ubiquitin (1:1000, Santa Cruz Biotechnology, sc-8017).
ShRNA Lentiviral Knockdown
The full-length open reading frame of Nedd4-1 or Fbx2 was amplified from rat brain cDNA by PCR, and an HA tag was added to the N-terminal in frame. The PCR product was cloned to T/A vector, and then subcloned to pcDNA3.1 expression vector. The construct was verified by DNA sequencing. The shRNA oligonucleotide targeting rat Nedd4 sequence (GGAGAATTAT GGGTGTGAAGA, Open Biosystem) or rat Fbx2 sequence (CCACTGGCAACAGTTCTACTT, Open Biosystem) was inserted to the lentiviral vector pLKO.3G (Addgene), which contains an eGFP marker. To test the knockdown effect, the plasmid HANedd4-1 or HAFbx2 was transfected to HEK293 cells with Nedd4 shRNA or Fbx2 shRNA plasmid. Two days after transfection, the cells were harvested and subjected to Western blotting with Anti-HA (1:1000, Roche). Actin was used as a loading control.
For the production of lentiviral particles, a mixture containing the pLKO.3G shRNA plasmid (against Nedd4-1 or Fbx2), psPAX2 packaging plasmid and pMD2.G envelope plasmid (Addgene) was transfected to HEK-293FT cells using Lipofectmine 2000. The transfection reagent was removed 12–15 hours later, and cells were incubated in fresh DMEM (containing 10% FBS + penicillin/streptomycin) for 24 hrs. The media harvested from the cells, which contained lentiviral particles, was concentrated by centrifugation (2,000 × g, 20 min) with Amicon Ultra Centrifugal Filter (Ultracel-100K, Millipore). The concentrated virus was stored at −80°C. In vivo delivery of the viral suspension (2 μl) was achieved by stereotaxic injection into the PFC prelimbic regions bilaterally with a Hamilton syringe (needle gauge 31) as we previously described (Liu et al., 2011). Electrophysiological, biochemical or behavioral experiments were performed at ~10 days after the viral injection.
Immunocytochemical Staining
Synaptic glutamate receptors in PFC cultures were detected as we previously described (Yuen et al., 2011, see Supplementary Experimental Procedure for details).
Quantitative real-time RT-PCR
A similar protocol was used as described before (Gu et al., 2007, see Supplementary Experimental Procedure for details).
Statistics
All data are expressed as the mean ± SEM. Experiments with two groups were analyzed statistically using unpaired Student’s t-tests. Experiments with more than two groups were subjected to one-way ANOVA, followed by post hoc Tukey tests.
Supplementary Material
Acknowledgments
We would like to thank Xiaoqing Chen for her excellent technical support. This work was supported by NIH grants (MH85774, MH84233) to Z.Y. None of the authors have a financial interest related to this work.
Footnotes
Supplemental Information includes four figures and supplementary experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal Order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pêgo JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation-a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Dix S, Aggleton J. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–216. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Yan Z. RGS4 modulates serotonin signaling in prefrontal cortex and links to serotonin dysfunction in a rat model of schizophrenia. Mol Pharmacol. 2007;71:1030–1039. doi: 10.1124/mol.106.032490. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joëls M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Karst H, Joëls M. Effect of chronic stress on synaptic currents in rat hippocampal dentate gyrus neurons. J Neurophysiol. 2003;89:625–633. doi: 10.1152/jn.00691.2002. [DOI] [PubMed] [Google Scholar]
- Kato A, Rouach N, Nicoll RA, Bredt DS. Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc Natl Acad Sci USA. 2005;102:5600–5605. doi: 10.1073/pnas.0501769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nature Reviews Neuroscience. 2010;11:675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, Man HY. Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem. 2011;119:27–39. doi: 10.1111/j.1471-4159.2011.07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Dou F, Feng J, Yan Z. RACK1 is involved in beta-amyloid impairment of muscarinic regulation of GABAergic transmission. Neurobiol Aging. 2011;32:1818–1826. doi: 10.1016/j.neurobiolaging.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Yan Z. The stress hormone corticosterone increases synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via serum- and glucocorticoid-inducible kinase (SGK) regulation of the GDI-Rab4 complex. J Biol Chem. 2010;285:6101–6108. doi: 10.1074/jbc.M109.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Ehlers MD. Ubiquitination in Postsynaptic Function and Plasticity. Annu Rev Cell Dev Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22:2718–2729. doi: 10.1523/JNEUROSCI.22-07-02718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: complex neural properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Laiacona J. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behav Brain Res. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotox Res. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Nemeroff CB. Neurobiology of posttraumatic stress disorder. Curr Opin Neurobiol. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- Park EC, Glodowski DR, Rongo C. The ubiquitin ligase RPM-1 and the p38 MAPK PMK-3 regulate AMPA receptor trafficking. PLoS One. 2009;4:e4284. doi: 10.1371/journal.pone.0004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30:16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of frontal lobe function. Oxford University Press; 2002. [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharm Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Yuen EY, Lu YF, Matsushita M, Matsui H, Yan Z, Tomizawa K. Regulation of N-methyl-D-aspartate receptors by calpain in cortical neurons. J Biol Chem. 2005;280:21588–93. doi: 10.1074/jbc.M501603200. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.