Abstract
There is convincing evidence that particles produced by the wear of joint prostheses are causal in the peri-prosthetic loss of bone, or osteolysis, which, if it progresses, leads to the phenomenon of aseptic loosening. It is important to fully understand the biology of this bone loss because it threatens prosthesis survival, and loosened implants can result in peri-prosthetic fracture, which is disastrous for the patient and presents a difficult surgical scenario. The focus of this review is the bioactivity of polyethylene (PE) particles, since there is evidence that these are major players in the development and progression of osteolysis around prostheses which use PE as the bearing surface. The review describes the biological consequences of interaction of PE particles with macrophages, osteoclasts and cells of the osteoblast lineage, including osteocytes. It explores the possible cellular mechanisms of action of PE and seeks to use the findings to date to propose potential non-surgical treatments for osteolysis. In particular, a non-surgical approach is likely to be applicable to implants containing newer, highly cross-linked PEs (HXLPEs), for which osteolysis seems to occur with much reduced PE wear compared with conventional PEs. The caveat here is that we know little as yet about the bioactivity of HXLPE particles and addressing this constitutes our next challenge.
Keywords: Polyethylene, Aseptic loosening, Osteolysis, Wear particle, Peri-prosthetic
INTRODUCTION
Total hip replacement (THR), although a highly successful procedure, can fail prematurely for a number of reasons. The most common cause of failure of THR in the mid- to long-term is aseptic loosening associated with peri-implant bone loss (termed osteolysis)[1,2]. Revision THR is considerably more difficult than primary THR and carries a higher rate of morbidity and mortality. The loss of bone stock jeopardises surgery to revise the prosthesis and reconstruct the joint. Furthermore, when osteolysis has weakened the bone to the extent that peri-prosthetic fracture occurs, the result is not only devastating to the affected individual but is also extremely challenging surgically, with poor long-term success rates in rebuilding the fractured bone, especially in the pelvis. Unless implants can be made to last longer in patients, the numbers of revision surgeries will continue to increase because of population aging, increasing life expectancies and a growing expectation of joint replacement surgery by young, active individuals with arthritic joints.
POLYETHYLENE WEAR PARTICLES AS A CAUSE OF PERI-PROSTHETIC OSTEOLYSIS
The concept that particulates released from prosthetic components by wear of the articular surfaces are important causative agents in peri-prosthetic osteolysis is well accepted[3], although other factors are also likely to be important, including access of particles to bone sites and hydrostatic pressure[4,5]. Most commonly, hip prostheses have metal or ceramic on polyethylene (PE) articulations. It is now well established that PE, metal[6], alumina[7] and polymethylmethacrylate (PMMA) particles[8] all have bioactivity and could therefore be involved in the events leading to osteolysis. Evidence suggests that PE wear particles may be most important in peri-prosthetic bone loss around articulations with PE linings[9-11]. Our own results show that wear of PE bearing surfaces correlates strongly with the extent of osteolysis, and that patients with a high volumetric wear rate exhibited the greatest progression of lesion volume[12]. This review will therefore focus on PE as a key agent of bone loss in osteolysis and on the bioactivity of particulates of this material. In addition, the review will deal exclusively with THR. Wear-related osteolysis also occurs adjacent to total knee replacements in the medium to long-term (reviewed by Gupta et al[13]) but the features of this are sufficiently distinct as to require separate consideration.
EFFECT OF SIZE, MORPHOLOGY AND CHEMICAL FORM OF PE PARTICLES
Most of the investigative studies into PE particles to date are of conventional ultra high molecular weight PE (UHMWPE), with some more recent studies of PE that has been cross-linked by various methodologies. It is clear that the size of PE particles is a factor in cell responses in vivo[14] and in vitro. A comparison by Matthews et al[15] showed that human monocyte-like cells were most responsive to particles in the size range 0.21-7.2 μm, depending on the readout, with larger particles of 88 μm evoking little response. The morphology of particles also appears to contribute to cellular responses, with UHMWPE debris with a roughened surface and a fibular shape provoking a greater response in terms of inflammatory cytokine production in a murine inflammation model than particles with a smooth surface and a globular shape[16]. The clinical evidence cited above showing strong relationships between PE wear and osteolysis is for implants that contain conventional UHMWPE. In order to reduce wear and as a consequence also reduce osteolysis, highly cross-linked PEs (HXLPE) have been developed (reviewed in[17]), which are now used almost universally in hip replacements. Although measurable wear still occurs, HXLPE components do show greatly reduced rates of wear, both in hip simulators and in clinical use[18,19]. However, while the use of HXLPE has reduced the early incidence of osteolysis compared with conventional PE[20], it has not eliminated the problem, with osteolysis still being observed 5-7 years after THR with prostheses containing HXLPE bearings[21-24]. It will therefore be important to understand the bioactivity of HXLPE particles in comparison with conventional PE particles, since there is a paucity of data so far. Endo et al[25] found that higher percentages of small wear particles, namely those in the 0.1-1μm range, were produced during laboratory wear of cross-linked PE than conventional PE. These authors also found that smaller numbers of HXLPE particles than for conventional PE were required to stimulate cytokine production from macrophages, possibly because of the higher percentage of smaller particles of HXLPE and the increased ability of cells to phagocytose smaller particles[15]. HXLPE particles may interact with cells differently, since Illgen et al[26] showed that crosslinked PE particles had altered bioactivity, which was unrelated to particle size. Particles of PE were crosslinked by electron-beam irradiation in nitrogen at 10 and 40 MRad to produce particles of the same size but with different extents of crosslinking. The crosslinked particles produced more inflammation and osteolysis (35%) in a murine calvarial osteolysis model than did control non-crosslinked particles (9%). Finally, endotoxin adherent to wear particles is also involved in the biological responses to these particles[27]. Taken together, these data strongly suggest that the bioactivity of PE particles depends on the size and material properties of the PE.
PE PARTICLES IN PERIPROSTHETIC TISSUES
Bone that is removed by the osteolytic process is replaced by a granulomatous connective tissue consisting of numerous cell types, including monocytes, macrophages, lymphocytes, endothelial cells and fibrohistiocytic infiltration in association with particulate debris[28-32]. Jacobs et al[30] have also made the point that these cells are often in close association with osteoblasts and osteoclasts so that elucidating the biology of periprosthetic bone loss requires taking into account the coordinated actions of all these cell types. Takagi et al[29] administered fluorescent label to patients prior to revision surgery for loose hip prostheses and found by histological analysis that sites of osteolysis were characterized by a unique high turnover bone remodeling. They reported evidence of osteoclastic resorption accompanied by increased mineral apposition rate and bone formation rate, producing apparently immature bone matrices with poor bone quality, containing abundant osteocytes. In the granulomatous tissues adjacent to PE containing prostheses, PE particulates are found in large numbers, usually engulfed by cells with a macrophage appearance[33,34]. Kobayashi et al[35] analysed peri-implant tissue from patients having revision knee replacement surgery and concluded that PE particles were most concentrated at sites of osteolysis. We and others have probed the molecular milieu of the tissue at sites of osteolysis and the factors identified combine to present a distinct catabolic profile. Matrix metalloproteinases (MMP), including MMPs-1, 2, 3, 9 and MT1-MMP, are abundant in this tissue and show an imbalance with their respective endogenous inhibitors[36,37]. The interface tissues around failed prostheses have also been found to be acidic and to contain high levels of the collagenase cathepsin K[38]. Production of these matrix degrading enzymes is likely secondary to the large number of cytokines, many proinflammatory, which have been identified in these tissues. These include interleukin (IL)-1α and β, IL-2, IL-6, IL-11, macrophage colony stimulating factor (M-CSF), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, transforming growth factor β, tumor necrosis factor (TNF)-α, granulocyte macrophage (GM)-CSF, platelet derived growth factor and interferon γ[31,39-43]. In addition to these factors, we have shown that an accumulation of PE particles is also frequently associated with a marked increase in receptor activator of nuclear factor-κB (RANK) and RANK ligand (RANKL) expression[34,40]. It is now established that the activated RANKL/RANK ligand-receptor complex promotes physiological osteoclast differentiation and activity[44]. The expression and activity of RANKL is known to be induced by a number of proinflammatory cytokines factors and there is evidence[40,45] that TNF-α can greatly enhance RANKL activity. The implication of this is that RANKL expression, induced in cells in the inflammatory tissue in response to prosthetic wear particles, could promote the influx of osteoclast precursors and drive osteoclastic differentiation and activity, thus promoting osteolysis. In support of this, RANKL inhibitors appear to be a promising treatment modality for particle-induced osteolysis (see below). Confirmation that the pathological changes seen in tissues adjacent to osteolysis, and osteolysis itself, are caused by PE particles, has been obtained by the demonstration in animal models that infusion of PE particles recapitulates many of the phenomena described above. For example, intramedullary infusion of PE particles in murine models resulted in reduced bone volume[46] and formation of proliferative fibrous tissue with increased expression of IL-1, IL-6 and TNF-α[11].
PE PARTICLES AND MACROPHAGES
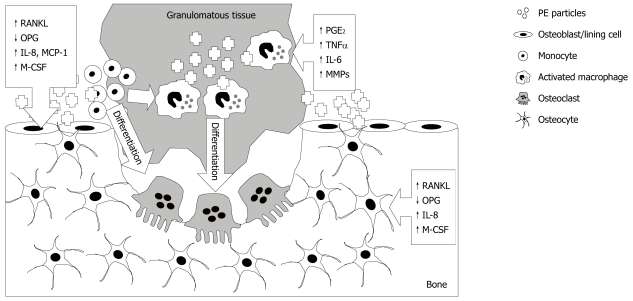
As reviewed previously[47], the prevailing view of the cellular mechanism of particle-induced osteolysis is that macrophages in the periprosthetic tissues phagocytose wear particles and become activated, releasing an array of cytokines, leading to increased osteoclastic resorption of the adjacent bone and the production of the granulomatous tissue that fills the resorbed space (Figure 1). Experiments performed to better understand the interaction between PE particles and monocyte/macrophages are challenging because of the extreme hydrophobicity of PE particles, reflected by the relatively small number of reports. However, Ren et al[48] performed elegant experiments to investigate whether responses to PE particles were by resident or infiltrating macrophages. In a mouse model of UHMWPE-induced osteolysis and using a bioluminescent approach to monitor systemic migrating macrophages in vivo, it was found that infusion of particles stimulated systemic migration of remotely injected macrophages, leading to net bone loss at the site of PE particles. Evidence that PE particles directly affect macrophages comes from a study by Horowitz et al[49], in which J774 macrophage-like cells exposed to PE particles produced increased TNF-α, prostaglandin (PG)E2 and IL-6. In addition, it was shown that conditioned media from the macrophages exposed to PE particles increased the release of 45Ca from pre-labelled mouse calvariae, which could be prevented by co-addition of pamidronate bisphosphonate. An important role for macrophages is also inferred from a study by Ren et al[50], in which depletion of macrophages using clodronate significantly reduced inflammation in a mouse air pouch model of UHMWPE-induced tissue inflammation. Together, these experiments provide proof of principle that macrophages exposed to PE particles release mediators that can stimulate bone resorption. Finally, we[51,52] and others[53] have shown that macrophages isolated from peri-prosthetic tissues that have phagocytosed wear particles, are themselves capable of differentiating into bone resorbing osteoclasts.
Figure 1.
Multi-cellular effects of polyethylene wear particles, following their release from the bearing surface, resulting in osteolysis. Polyethylene (PE) engulfment by monocytes and macrophages, and possibly other cells including fibroblasts, as described in the text, causes their activation and release of pro-inflammatory cytokines, prostaglandins and matrix degrading enzymes. These and other factors promote differentiation of macrophages into bone resorbing osteoclasts. PE effects on osteoblastic lineage cells (osteoblasts, lining cells and potentially osteocytes) following contact with and/or engulfment of particles include promotion of a catabolic phenotype, up-regulation of potent chemotactic agents for osteoclast precursors and osteoclastogenic mediators. The responses in each cell type are indicated by boxed arrows. RANKL:receptor activator of nuclear factor-κB ligand; OPG: osteoprotegerin; M-CSF: macrophage colony stimulating factor; MMPs: Matrix metalloproteinases.
PE PARTICLES AND OSTEOCLASTS
Although it is possible that wear particle-activated macrophages present in granulomatous tissue participate directly in bone degradation in periprosthetic osteolysis, bone resorption is more likely a function of osteoclasts recruited to sites of osteolysis and activated by the osteoclastogenic molecules that are expressed in abundance at such sites. These molecules, listed above, comprise chemoattractants that result in the influx of osteoclast precursors, thought to be cells of the monocyte lineage, and cytokines that stimulate osteoclast differentiation. It is generally acknowledged that the major pathway of osteoclastogenesis is the production of RANKL by osteoblastic stromal cells[54,55] and ligation of RANKL to its cognate receptor, RANK, on the surface of osteoclast precursors, stimulating these cells to differentiate into mature, active osteoclasts capable of resorbing bone. The natural antagonist of RANKL is osteoprotegerin (OPG), whose role is to negatively regulate the activity of RANKL, and therefore bone resorption. Other cell types, including fibroblasts[56,57], osteocytes[58] and activated T cells[59], also produce RANKL and are capable of stimulating osteoclastogenesis. We have shown that the RANKL pathway is subverted in a range of pathologies characterised by bone loss, including peri-implant osteolysis[34,60-62]. Studies on healthy and pathologic synovial tissues show that a number of cell types may be important in the ectopic production of RANKL in the tissues adjacent to localized bone loss. Importantly, there is evidence for a reduction of the OPG:RANKL ratio in PE-induced osteolysis, seen for example in experiments in which mouse calvariae were implanted with PE particles, associated with reduction of bone volume[63] and in peri-prosthetic human tissues[31].
Various cytokines collaborate with RANKL to promote osteoclast formation, in particular M-CSF, which is an essential co-activator of osteoclastogenesis from immature precursors[54,64]. However, we recently showed that RANKL and M-CSF alone were not sufficient for osteoclast differentiation of osteoclasts from a murine macrophage cell line RAW 264.7 and that other cytokines and steroidal hormones were essential in this process. Indeed, in a defined culture medium in the absence of serum, osteoclast formation from primary cells, such as human peripheral blood mononuclear cells, did not occur at all, despite the presence of high concentrations of RANKL, M-CSF and other co-factors[65], underscoring the likely significant contributions of the complex milieu present in peri-prosthetic granulomatous tissue.
TNF-α is also present in the tissues adjacent to peri-prosthetic osteolysis and has been reported to synergise with RANKL in promoting osteoclast formation[45]. It has also been claimed that, in the presence of M-CSF, TNF-α is sufficient for inducing human osteoclast differentiation from arthroplasty macrophages[53]. By immunohistochemical analysis of tissues associated with osteolysis obtained at revision surgery, RANK, RANKL and TNF-α were all found to be strongly expressed by large multinucleated cells containing PE wear debris[40]. Control synovial tissue stained weakly for these cytokines. A strong statistical correlation was found between the parameters of: volume of bone lost (determined by quantitative computed tomography; r = 0.65, P = 0.01); PE wear debris (number of particles; r = 0.67, P < 0.01), RANK (r = 0.67, P < 0.01), RANKL (r = 0.81, P < 0.01) and TNF-α (r = 0.65, P = 0.01) expression. In the same study, it was shown that RANKL and TNF-α synergise to increase the volume of bone resorbed. These data suggested that the interaction of TNF-α and RANKL may promote osteoclast activity associated with PE particles and perhaps that therapies targeting TNF activity may be useful to treat peri-implant osteolysis.
Chemokines are involved in the recruitment of many types of cells but it is those that are involved in the recruitment of monocytes/macrophages and lymphocytes that are likely to be most important in peri-prosthetic osteolysis. Chemokines present in periprosthetic tissues include MCP-1, MIP-1α, RANTES and IL-8, whose release is stimulated by wear particles and which are chemotactic for monocytic osteoclast precursors[66]. Finally, RANKL expression itself is stimulated by many of the pro-inflammatory cytokines that are found in the peri-implant tissues. The above effects of PE particles are summarised in Figure 1.
PE PARTICLES AND CELLS OF THE OSTEOBLAST LINEAGE
PE particles can interact with cells of the osteoblast lineage indirectly via products released by PE activated macrophages[67]. There is also evidence of direct effects of PE particles on osteoblasts. Exposure of osteoblast-like cells to PE was shown to induce changes in the rate of cell proliferation[68-70], to decrease alkaline phosphatase activity[69], and to increase the production of osteoclastogenic mediators, such as PGE2[69], IL-6[71,72], GM-CSF, RANKL[73] and nitric oxide[74]. The nature of the response may be dependent on the way in which the PE particles are presented to the cells and to the maturation state of the osteoblasts[74]. However, the overall effect of PE particles on osteoblasts is one of promoting a phenotype that is anti-anabolic and pro-osteoclastic. For example, UHMWPE particles increased the release of RANKL from human osteoblasts, while inhibiting their expression of OPG. Consistent with this, conditioned media from the PE-treated osteoblasts strongly promoted osteoclastogenesis from human peripheral blood mononuclear cells[73]. Given the extreme hydrophobicity of the particles, we developed a 3-dimensional type I collagen gel culture system, allowing long-term culture of osteoblastic cells in the continued juxtaposition of PE particles in a bone connective tissue-like matrix. This system allowed human bone-derived osteoblasts obtained at joint replacement surgery to undergo differentiation into a mature osteocyte-like phenotype over a 21-28 day culture period[58,75]. Following release of the cells from the gel, it was evident that PE particles could form close contacts with these cells and in some cases appeared to be engulfed[58]. Importantly, cells exposed to PE particles showed an increase in mRNA expression of the late osteoblast/osteocyte markers E11, dentin matrix protein 1 and the gene encoding sclerostin (SOST) and increased expression of several of the genes discussed above to be associated with osteoclast formation and activity (RANKL, M-CSF and IL-8), with a concomitant decrease in the expression of OPG[58]. Furthermore, it appeared that under the influence of PE, the key transcription factor RUNX2 in these cells switched from controlling matrix production genes (type I collagen) to inducing the expression of genes associated with osteoclast recruitment, differentiation and activation. This study provided further evidence that PE particles switch mature osteoblastic cells from an anabolic to a catabolic phenotype, a concept further supported by the finding that PE particles induced expression of RANKL mRNA in the mouse osteocyte cell line, MLO-Y4. Overall, our results suggest that PE particles act directly on cells of the osteoblast lineage to induce a change in the phenotype of mature osteoblasts and osteocytes[58], consistent with the net loss of bone near orthopedic implants.
INVOLVEMENT OF THE INNATE IMMUNE RESPONSE IN THE RESPONSE TO PE PARTICLES
Little is known about the way in which PE particles interact with cells or how this interaction is transduced into intracellular signals. It appears that particles need to be phagocytosed to elicit a signal in macrophages and osteoclasts, and possibly also osteoblasts and osteocytes, as discussed above. It is possible that cells first interact with PE particles via proteins adsorbed to the particle surface, rather than with the PE material itself, either with endotoxin[27] or other proteins[76]. There is evidence that activation of Toll-like receptors (TLRs) may contribute to the biological activity of the wear particles. Maitra et al[77], in a study which explored the cellular mechanisms of interaction of UHMWPE with peripheral blood monocytes, found that polymeric alkane UHMWPE breakdown products with side chain oxidation directly bound and activated the TLR1/2 signalling pathway. They showed that phagocytosis of particulates in the larger micron- and nanometer sized range induced enlargement, fusion and disruption of endosomal compartments, resulting in lysosomal damage and enzymatic leakage with release of cathepsins S and B, as well as caspase 1 activation and processing of pro-IL-1 and pro-IL-18. The authors reported that the two processes of TLR activation and phagocytosis synergistically initiated a strong inflammatory response associated with cell death and extracellular matrix degradation. Hao et al[78] reported that heat-shock protein (Hsp) expression was elevated when monocytes were exposed to UHMWPE particles. They showed that TLR4 was involved in the recognition of particles and subsequent induction of intracellular signalling cascades and that anti-sense oligonucleotide down-regulation of TLR4 expression suppressed cytokine production in both exogenous Hsp60- and particle-stimulated cultures. Adherent endotoxin was found to be important in the activation of monocytes with titanium particles[27]. Mice that were ‘endotoxin resistant’ due to a mutation in TLR4 were less responsive to endotoxin coated titanium particles than cells from wild-type mice were. Taken together, the extant studies point to the innate immune response as being responsible for at least some of the cellular responses to PE and other wear particles, with a possible role for both adherent endotoxin (and other proteins) interacting with TLRs.
NON-SURGICAL TREATMENT OF OSTEOLYSIS
With conventional PE, large volumetric osteolysis is almost always accompanied by considerable wear of the PE liner, and in this case liner replacement at revision surgery is indicated. In the case of implants with HXLPE liners, acetabular osteolysis occurs with relatively little obvious wear due to the wear resistant nature of this material. Thus, while there may currently be limited opportunities to treat osteolysis from conventional PE non-surgically, such an approach may be feasible with HXLPE, the caveat being that we know very little to date about the bioactivity of HXLPE particles.
Bisphosphonates are a class of drug with the dual features of potent anti-resorptive efficacy and high uptake in the skeleton. While most commonly prescribed for the treatment of osteoporosis, Paget’s disease and for certain types of cancer, wider applicability including the treatment of peri-prosthetic osteolysis has also been proposed[79]. Indeed, several bisphosphonates show promise as therapeutic agents for particle-induced bone loss. Alendronate has proved efficacious in both rat[80] and dog[81] models of PE-induced periprosthetic osteolysis. Similarly, the bisphosphonate TRK-530 suppressed bone loss in a rat model of continuous infusion of PE particles into the bone and reduced the expression of inflammatory mediators[82]. Pamidronate was able to prevent resorption of explanted calvariae exposed to conditioned medium from PE-treated macrophages[49]. In a clinical case study, O’Hara et al[83] reported that oral alendronate halted the progression of osteolysis over the course of 1 year prior to revision surgery to replace the PE liner. By virtue of what is currently understood regarding the general mechanism of action of bisphosphonates, these data suggest that osteoclasts represent a valuable potential target to prevent or treat osteolysis. Thus, other treatment strategies that target osteoclasts may also be applicable. For example, it is possible that anti-RANKL strategies could be effective in blocking osteolysis. In support of this, OPG has been shown to suppress both UHMWPE[84] and titanium[85] particle induced osteolysis in the mouse calvarial model. Interestingly, RANK:Fc, an alternative approach to blocking RANKL action, was also effective in the calvarial model, where it also allowed restoration of bone lost by osteolysis[86]. Another therapeutic approach to osteolysis may be to treat the underlying inflammation, which creates a catabolic environment, including the production of RANKL and MMPs. Approaches to this that have been experimentally successful include local treatment with the inflammatory suppressor, IL-10, which prevented a fibrotic reaction and allowed bone growth in the presence of PE particles[87]. Also, anti-TNF-α gene therapy was able to inhibit a resorptive response to titanium particles in the mouse calvarial model[88], although administration of the TNF antagonist etanercept to patients with peri-prosthetic osteolysis produced equivocal results, perhaps because the study was underpowered[89]. Such a targeted approach in human patients may be ineffectual, or at least not generally applicable, because of the multi-factorial nature of the inflammatory response to particles, as discussed above. MMP inhibitors have also been suggested for use in the context of osteolysis[36], given the abundance of MMPs in periprosthetic tissues. However, anti-MMP approaches have proven disappointing to date[90], perhaps awaiting more specific agents or better methods for local application. Perhaps, as suggested by Schwarz[91], prophylactic treatment immediately post operatively to optimise the osseo-integration of the implant and therefore reduce access to the bone of wear particles, may inhibit the initiation and progression of particle-induced osteolysis.
One of the potential problems of a non-surgical approach to the treatment of osteolysis associated with HXLPE liners may be the problem of replacing the lost bone. It is noteworthy that while some agents have been shown to promote bone formation in the context of particle induced mouse calvarial osteolysis[92,93], it remains to be seen if these or other approaches will be effective in more refractory skeleton of the aging human adult. Of the new and emerging anabolic agents in bone, sclerostin antagonists to date appear promising, with evidence of widespread new bone growth throughout the skeleton in both healthy bone and in a number of conditions of bone loss[94].
CONCLUSION
This review shows that we have come a long way in understanding the bioactivity of PE particles and the mechanisms by which an accumulation of these particles elicits a characteristic tissue response leading to bone loss around PE-containing implants. This knowledge is important because it has the potential to enable non-surgical approaches to managing PE-induced osteolysis, particularly in implants containing the newer HXLPEs, for which osteolysis seems, from observations so far, to occur without substantial PE wear. The caveat here is that we currently know little about the bioactivity of HXLPE particles, and our next task needs to be to address this knowledge gap. It will also be important to address the issue of bone regrowth in a non-surgically treated individual.
ACKNOWLEDGMENTS
GJA was supported by a National Health and Medical Research Council of Australia R Douglas Wright Fellowship. The authors thank Dr. Masakazu Kogawa, University of Adelaide, for his critical comments.
Footnotes
Peer reviewers: Antonio Herrera, Professor, Orthopaedic Surgery and Traumatology Miguel Servet University Hospital, Avenue Isabel la Catolican1, Zaragoza 50009, Spain; Nuria Vilaboa, Dr., Hospital Universitario La-Paz-IdiPAZ Edificio de I+D Paseo de La Castellana 261, 28046 Madrid, Spain; Konstantin I Momot, PhD, Queensland University of Technology, GPO Box 2434, Brisbane, Qld 4001, Australia
Supported by National Health and Medical Research Council of Australia
S- Editor Yang XC L- Editor Roemmele A E- Editor Zheng XM
References
- 1.Harris WH, Schiller AL, Scholler JM, Freiberg RA, Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg Am. 1976;58:612–618. [PubMed] [Google Scholar]
- 2.Wang ML, Sharkey PF, Tuan RS. Particle bioreactivity and wear-mediated osteolysis. J Arthroplasty. 2004;19:1028–1038. doi: 10.1016/j.arth.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Total hip replacement. NIH Consens Statement. 1994;12:1–31. [PubMed] [Google Scholar]
- 4.Skoglund B, Holmertz J, Aspenberg P. Systemic and local ibandronate enhance screw fixation. J Orthop Res. 2004;22:1108–1113. doi: 10.1016/j.orthres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy A, Jeyam M, Ferrier G, Evans CE, Andrew JG. Synergistic effect of particles and cyclic pressure on cytokine production in human monocyte/macrophages: proposed role in periprosthetic osteolysis. Bone. 2002;30:171–177. doi: 10.1016/s8756-3282(01)00658-5. [DOI] [PubMed] [Google Scholar]
- 6.Haynes DR, Rogers SD, Hay S, Pearcy MJ, Howie DW. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J Bone Joint Surg Am. 1993;75:825–834. doi: 10.2106/00004623-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84:464–474. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- 8.Jones LC, Frondoza C, Hungerford DS. Effect of PMMA particles and movement on an implant interface in a canine model. J Bone Joint Surg Br. 2001;83:448–458. doi: 10.1302/0301-620x.83b3.10734. [DOI] [PubMed] [Google Scholar]
- 9.Revell PA, Weightman B, Freeman MA, Roberts BV. The production and biology of polyethylene wear debris. Arch Orthop Trauma Surg. 1978;91:167–181. doi: 10.1007/BF00379748. [DOI] [PubMed] [Google Scholar]
- 10.Looney RJ, Boyd A, Totterman S, Seo GS, Tamez-Pena J, Campbell D, Novotny L, Olcott C, Martell J, Hayes FA, et al. Volumetric computerized tomography as a measurement of periprosthetic acetabular osteolysis and its correlation with wear. Arthritis Res. 2002;4:59–63. doi: 10.1186/ar384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KJ, Kobayashi Y, Itoh T. Osteolysis model with continuous infusion of polyethylene particles. Clin Orthop Relat Res. 1998:46–52. [PubMed] [Google Scholar]
- 12.Howie DW, Neale SD, Stamenkov R, McGee MA, Taylor DJ, Findlay DM. Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J Bone Joint Surg Am. 2007;89:1818–1825. doi: 10.2106/JBJS.E.01305. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SK, Chu A, Ranawat AS, Slamin J, Ranawat CS. Osteolysis after total knee arthroplasty. J Arthroplasty. 2007;22:787–799. doi: 10.1016/j.arth.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Ingram RT, Bonde SK, Riggs BL, Fitzpatrick LA. Effects of transforming growth factor beta (TGF beta) and 1,25 dihydroxyvitamin D3 on the function, cytochemistry and morphology of normal human osteoblast-like cells. Differentiation. 1994;55:153–163. doi: 10.1046/j.1432-0436.1994.5520153.x. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JB, Besong AA, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000;52:296–307. doi: 10.1002/1097-4636(200011)52:2<296::aid-jbm8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Sieving A, Wu B, Mayton L, Nasser S, Wooley PH. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J Biomed Mater Res A. 2003;64:457–464. doi: 10.1002/jbm.a.10368. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Barrena E, Puertolas JA, Munuera L, Konttinen YT. Update on UHMWPE research: from the bench to the bedside. Acta Orthop. 2008;79:832–840. doi: 10.1080/17453670810016939. [DOI] [PubMed] [Google Scholar]
- 18.Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A double-blind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty. 2008;23:337–343. doi: 10.1016/j.arth.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 19.Thomas GER, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Gill HS, Murray DW, Glyn-Jones S. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93:716–22. doi: 10.2106/JBJS.J.00287. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262–2277. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engh CA, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21:17–25. doi: 10.1016/j.arth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Leung SB, Egawa H, Stepniewski A, Beykirch S, Engh CA, Engh CA. Incidence and volume of pelvic osteolysis at early follow-up with highly cross-linked and noncross-linked polyethylene. J Arthroplasty. 2007;22:134–139. doi: 10.1016/j.arth.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Mall NA, Nunley RM, Zhu JJ, Maloney WJ, Barrack RL, Clohisy JC. The incidence of acetabular osteolysis in young patients with conventional versus highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:372–381. doi: 10.1007/s11999-010-1518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder DT, Kelly NH, Wright TM, Parks ML. Retrieved highly crosslinked UHMWPE acetabular liners have similar wear damage as conventional UHMWPE. Clin Orthop Relat Res. 2011;469:387–394. doi: 10.1007/s11999-010-1552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng H. 2002;216:111–122. doi: 10.1243/0954411021536333. [DOI] [PubMed] [Google Scholar]
- 26.Illgen RL, Forsythe TM, Pike JW, Laurent MP, Blanchard CR. Highly crosslinked vs conventional polyethylene particles--an in vitro comparison of biologic activities. J Arthroplasty. 2008;23:721–731. doi: 10.1016/j.arth.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 27.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 28.Takagi M. Bone-implant interface biology: foreign body reaction and periprosthetic osteolysis in artificial hip joints. J Clin Exp Hematopathol. 2001;41:81–87. [Google Scholar]
- 29.Takagi M, Santavirta S, Ida H, Ishii M, Takei I, Niissalo S, Ogino T, Konttinen YT. High-turnover periprosthetic bone remodeling and immature bone formation around loose cemented total hip joints. J Bone Miner Res. 2001;16:79–88. doi: 10.1359/jbmr.2001.16.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Haynes DR, Crotti TN, Zreiqat H. Regulation of osteoclast activity in peri-implant tissues. Biomaterials. 2004;25:4877–4885. doi: 10.1016/j.biomaterials.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Goodman SB, Lind M, Song Y, Smith RL. In vitro, in vivo, and tissue retrieval studies on particulate debris. Clin Orthop Relat Res. 1998:25–34. [PubMed] [Google Scholar]
- 33.McGee MA, Howie DW, Neale SD, Haynes DR, Pearcy MJ. The role of polyethylene wear in joint replacement failure. Proc Inst Mech Eng H. 1997;211:65–72. doi: 10.1243/0954411971534692. [DOI] [PubMed] [Google Scholar]
- 34.Crotti TN, Smith MD, Findlay DM, Zreiqat H, Ahern MJ, Weedon H, Hatzinikolous G, Capone M, Holding C, Haynes DR. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials. 2004;25:565–573. doi: 10.1016/s0142-9612(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Freeman MA, Bonfield W, Kadoya Y, Yamac T, Al-Saffar N, Scott G, Revell PA. Number of polyethylene particles and osteolysis in total joint replacements. A quantitative study using a tissue-digestion method. J Bone Joint Surg Br. 1997;79:844–848. doi: 10.1302/0301-620x.79b5.7602. [DOI] [PubMed] [Google Scholar]
- 36.Takagi M, Santavirta S, Ida H, Ishii M, Akimoto K, Saotome K, Konttinen YT. The membrane-type-matrix metalloproteinase/matrix metalloproteinase-2/tissue inhibitor of metalloproteinase-2 system in periprosthetic connective-tissue remodeling in loose total-hip prostheses. Lab Invest. 1998;78:735–742. [PubMed] [Google Scholar]
- 37.Takagi M, Santavirta S, Ida H, Ishii M, Mandelin J, Konttinen YT. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in loose artificial hip joints. Clin Orthop Relat Res. 1998:35–45. [PubMed] [Google Scholar]
- 38.Konttinen YT, Takagi M, Mandelin J, Lassus J, Salo J, Ainola M, Li TF, Virtanen I, Liljestrom M, Sakai H, et al. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. J Bone Miner Res. 2001;16:1780–1786. doi: 10.1359/jbmr.2001.16.10.1780. [DOI] [PubMed] [Google Scholar]
- 39.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, Sibley R. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80:531–539. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 40.Holding CA, Findlay DM, Stamenkov R, Neale SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ, Howie DW, et al. The correlation of RANK, RANKL and TNFalpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials. 2006;27:5212–5219. doi: 10.1016/j.biomaterials.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 41.al Saffar N, Revell PA. Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. Br J Rheumatol. 1994;33:309–316. doi: 10.1093/rheumatology/33.4.309. [DOI] [PubMed] [Google Scholar]
- 42.Takei I, Takagi M, Ida H, Ogino T, Santavirta S, Konttinen YT. High macrophage-colony stimulating factor levels in synovial fluid of loose artificial hip joints. J Rheumatol. 2000;27:894–899. [PubMed] [Google Scholar]
- 43.Nakashima Y, Sun DH, Trindade MC, Chun LE, Song Y, Goodman SB, Schurman DJ, Maloney WJ, Smith RL. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg Br. 1999;81:155–162. doi: 10.1302/0301-620x.81b1.8884. [DOI] [PubMed] [Google Scholar]
- 44.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma T, Huang Z, Ren PG, McCally R, Lindsey D, Smith RL, Goodman SB. An in vivo murine model of continuous intramedullary infusion of polyethylene particles. Biomaterials. 2008;29:3738–3742. doi: 10.1016/j.biomaterials.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 48.Ren PG, Huang Z, Ma T, Biswal S, Smith RL, Goodman SB. Surveillance of systemic trafficking of macrophages induced by UHMWPE particles in nude mice by noninvasive imaging. J Biomed Mater Res A. 2010;94:706–711. doi: 10.1002/jbm.a.32744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz SM, Gonzales JB. Effects of polyethylene on macrophages. J Orthop Res. 1997;15:50–56. doi: 10.1002/jor.1100150108. [DOI] [PubMed] [Google Scholar]
- 50.Ren W, Markel DC, Schwendener R, Ding Y, Wu B, Wooley PH. Macrophage depletion diminishes implant-wear-induced inflammatory osteolysis in a mouse model. J Biomed Mater Res A. 2008;85:1043–1051. doi: 10.1002/jbm.a.31665. [DOI] [PubMed] [Google Scholar]
- 51.Haynes DR, Crotti TN, Potter AE, Loric M, Atkins GJ, Howie DW, Findlay DM. The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis. J Bone Joint Surg Br. 2001;83:902–911. doi: 10.1302/0301-620x.83b6.10905. [DOI] [PubMed] [Google Scholar]
- 52.Neale SD, Fujikawa Y, Sabokbar A, Gundle R, Murray DW, Graves SE, Howie DW, Athanasou NA. Human bone-derived cells support formation of human osteoclasts from arthroplasty-derived cells in vitro. J Bone Joint Surg Br. 2000;82:892–900. doi: 10.1302/0301-620x.82b6.10175. [DOI] [PubMed] [Google Scholar]
- 53.Sabokbar A, Kudo O, Athanasou NA. Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J Orthop Res. 2003;21:73–80. doi: 10.1016/S0736-0266(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 56.Quinn JM, Horwood NJ, Elliott J, Gillespie MT, Martin TJ. Fibroblastic stromal cells express receptor activator of NF-kappa B ligand and support osteoclast differentiation. J Bone Miner Res. 2000;15:1459–1466. doi: 10.1359/jbmr.2000.15.8.1459. [DOI] [PubMed] [Google Scholar]
- 57.Mandelin J, Li TF, Hukkanen M, Liljeström M, Salo J, Santavirta S, Konttinen YT. Interface tissue fibroblasts from loose total hip replacement prosthesis produce receptor activator of nuclear factor-kappaB ligand, osteoprotegerin, and cathepsin K. J Rheumatol. 2005;32:713–720. [PubMed] [Google Scholar]
- 58.Atkins GJ, Welldon KJ, Holding CA, Haynes DR, Howie DW, Findlay DM. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials. 2009;30:3672–3681. doi: 10.1016/j.biomaterials.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 59.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 60.Crotti T, Smith MD, Hirsch R, Soukoulis S, Weedon H, Capone M, Ahern MJ, Haynes D. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res. 2003;38:380–387. doi: 10.1034/j.1600-0765.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 61.Crotti TN, Smith MD, Weedon H, Ahern MJ, Findlay DM, Kraan M, Tak PP, Haynes DR. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61:1047–1054. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haynes DR, Barg E, Crotti TN, Holding C, Weedon H, Atkins GJ, Zannetino A, Ahern MJ, Coleman M, Roberts-Thomson PJ, et al. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology (Oxford) 2003;42:123–134. doi: 10.1093/rheumatology/keg047. [DOI] [PubMed] [Google Scholar]
- 63.Masui T, Sakano S, Hasegawa Y, Warashina H, Ishiguro N. Expression of inflammatory cytokines, RANKL and OPG induced by titanium, cobalt-chromium and polyethylene particles. Biomaterials. 2005;26:1695–1702. doi: 10.1016/j.biomaterials.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 64.Findlay DM, Haynes DR. Mechanisms of bone loss in rheumatoid arthritis. Mod Rheumatol. 2005;15:232–240. doi: 10.1007/s10165-005-0412-z. [DOI] [PubMed] [Google Scholar]
- 65.Vincent C, Kogawa M, Findlay DM, Atkins GJ. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J Bone Miner Metab. 2009;27:114–119. doi: 10.1007/s00774-008-0018-6. [DOI] [PubMed] [Google Scholar]
- 66.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–5050. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gallo J, Raska M, Mrázek F, Petrek M. Bone remodeling, particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57:339–349. doi: 10.33549/physiolres.931140. [DOI] [PubMed] [Google Scholar]
- 68.Martínez ME, Medina S, del Campo MT, García JA, Rodrigo A, Munuera L. Effect of polyethylene particles on human osteoblastic cell growth. Biomaterials. 1998;19:183–187. doi: 10.1016/s0142-9612(97)00250-0. [DOI] [PubMed] [Google Scholar]
- 69.Dean DD, Schwartz Z, Liu Y, Blanchard CR, Agrawal CM, Mabrey JD, Sylvia VL, Lohmann CH, Boyan BD. The effect of ultra-high molecular weight polyethylene wear debris on MG63 osteosarcoma cells in vitro. J Bone Joint Surg Am. 1999;81:452–461. doi: 10.2106/00004623-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J Biomed Mater Res A. 2009;89:242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 71.Vermes C, Chandrasekaran R, Jacobs JJ, Galante JO, Roebuck KA, Glant TT. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J Bone Joint Surg Am. 2001;83-A:201–211. doi: 10.2106/00004623-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigo AM, Martinez ME, Saldaña L, Vallés G, Martinez P, González-Carrasco JL, Cordero J, Munuera L. Effects of polyethylene and alpha-alumina particles on IL-6 expression and secretion in primary cultures of human osteoblastic cells. Biomaterials. 2002;23:901–908. doi: 10.1016/s0142-9612(01)00200-9. [DOI] [PubMed] [Google Scholar]
- 73.Granchi D, Ciapetti G, Amato I, Pagani S, Cenni E, Savarino L, Avnet S, Peris JL, Pellacani A, Baldini N, et al. The influence of alumina and ultra-high molecular weight polyethylene particles on osteoblast-osteoclast cooperation. Biomaterials. 2004;25:4037–4045. doi: 10.1016/j.biomaterials.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 74.Lohmann CH, Dean DD, Bonewald LF, Schwartz Z, Boyan BD. Nitric oxide and prostaglandin E2 production in response to ultra-high molecular weight polyethylene particles depends on osteoblast maturation state. J Bone Joint Surg Am. 2002;84-A:411–419. doi: 10.2106/00004623-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 75.Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2009;297:C1358–C1367. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- 76.Elfick AP, Green SM, McCaskie AW, Birch MA. Opsonization of polyethylene wear particles regulates macrophage and osteoblast responses in vitro. J Biomed Mater Res B Appl Biomater. 2004;71:244–251. doi: 10.1002/jbm.b.30095. [DOI] [PubMed] [Google Scholar]
- 77.Maitra R, Clement CC, Scharf B, Crisi GM, Chitta S, Paget D, Purdue PE, Cobelli N, Santambrogio L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol Immunol. 2009;47:175–184. doi: 10.1016/j.molimm.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao HN, Zheng B, Nasser S, Ren W, Latteier M, Wooley P, Morawa L. The roles of monocytic heat shock protein 60 and Toll-like receptors in the regional inflammation response to wear debris particles. J Biomed Mater Res A. 2010;92:1373–1381. doi: 10.1002/jbm.a.32474. [DOI] [PubMed] [Google Scholar]
- 79.Landesberg R, Eisig S, Fennoy I, Siris E. Alternative indications for bisphosphonate therapy. J Oral Maxillofac Surg. 2009;67:27–34. doi: 10.1016/j.joms.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A:236–249. doi: 10.2106/00004623-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997:33–43. [PubMed] [Google Scholar]
- 82.Iwase M, Kim KJ, Kobayashi Y, Itoh M, Itoh T. A novel bisphosphonate inhibits inflammatory bone resorption in a rat osteolysis model with continuous infusion of polyethylene particles. J Orthop Res. 2002;20:499–505. doi: 10.1016/S0736-0266(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 83.O’Hara LJ, Nivbrant B, Röhrl S. Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip arthroplasty: a case report. J Orthop Surg (Hong Kong) 2004;12:114–121. doi: 10.1177/230949900401200121. [DOI] [PubMed] [Google Scholar]
- 84.von Knoch F, Heckelei A, Wedemeyer C, Saxler G, Hilken G, Brankamp J, Sterner T, Landgraeber S, Henschke F, Löer F, et al. Suppression of polyethylene particle-induced osteolysis by exogenous osteoprotegerin. J Biomed Mater Res A. 2005;75:288–294. doi: 10.1002/jbm.a.30441. [DOI] [PubMed] [Google Scholar]
- 85.Goater JJ, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–173. doi: 10.1016/S0736-0266(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 86.Childs LM, Paschalis EP, Xing L, Dougall WC, Anderson D, Boskey AL, Puzas JE, Rosier RN, O’Keefe RJ, Boyce BF, et al. In vivo RANK signaling blockade using the receptor activator of NF-kappaB: Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res. 2002;17:192–199. doi: 10.1359/jbmr.2002.17.2.192. [DOI] [PubMed] [Google Scholar]
- 87.Goodman S, Trindade M, Ma T, Lee M, Wang N, Ikenou T, Matsuura I, Miyanishi K, Fox N, Regula D, et al. Modulation of bone ingrowth and tissue differentiation by local infusion of interleukin-10 in the presence of ultra-high molecular weight polyethylene (UHMWPE) wear particles. J Biomed Mater Res A. 2003;65:43–50. doi: 10.1002/jbm.a.10279. [DOI] [PubMed] [Google Scholar]
- 88.Childs LM, Goater JJ, O’Keefe RJ, Schwarz EM. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. J Bone Joint Surg Am. 2001;83-A:1789–1797. doi: 10.2106/00004623-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Schwarz EM, Campbell D, Totterman S, Boyd A, O’Keefe RJ, Looney RJ. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. J Orthop Res. 2003;21:1049–1055. doi: 10.1016/S0736-0266(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 90.Dormán G, Cseh S, Hajdú I, Barna L, Kónya D, Kupai K, Kovács L, Ferdinandy P. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs. 2010;70:949–964. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 91.Schwarz EM. What potential biologic treatments are available for osteolysis? J Am Acad Orthop Surg. 2008;16 Suppl 1:S72–S75. doi: 10.5435/00124635-200800001-00015. [DOI] [PubMed] [Google Scholar]
- 92.Zhou X, Zhang P, Zhang C, Zhu Z. Promotion of bone formation by naringin in a titanium particle-induced diabetic murine calvarial osteolysis model. J Orthop Res. 2010;28:451–456. doi: 10.1002/jor.21002. [DOI] [PubMed] [Google Scholar]
- 93.von Knoch F, Wedemeyer C, Heckelei A, Saxler G, Hilken G, Brankamp J, Sterner T, Landgraeber S, Henschke F, Löer F, et al. Promotion of bone formation by simvastatin in polyethylene particle-induced osteolysis. Biomaterials. 2005;26:5783–5789. doi: 10.1016/j.biomaterials.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–1904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]