Abstract
Spondyloarthropathies (SpA) are a group of inflammatory arthritis which consist of ankylosing spondylitis (AS), reactive arthritis, arthritis/spondylitis associated with psoriasis (PsA), and arthritis/spondylitis associated with inflammatory bowel diseases. It is now more important than ever to diagnose and treat SpA early. New therapeutic agents including blockers of tumor necrosis factor have yielded tremendous responses not only in advanced disease but also in the early stages of the disease. Sacroiliitis on conventional radiography is the result of structural changes which may appear late in the disease process. However, magnetic resonance imaging (MRI) can visualize active inflammation at sacroiliac joints and spine in recent onset disease. The modified New York criteria, the European Spondyloarthropathy Study Group criteria and the Amor criteria do not include advanced imaging techniques like MRI which is very sensitive to the early Inflammatory changes. Assessment of SpondyloArthritis international Society has defined MRI methods for the assessment of sacroiliac joints and spine, criteria for inflammatory back pain and developed new criteria for classification of axial and peripheral spondyloarthritis. These new criteria are intended to be used for patients with SpA at the very early stage of their disease. Also, classification of psoriatic arthritis study group developed criteria for the classification of PsA. The widespread use of these criteria in clinical trials will provide evidence for a better definition of early disease and recognize many patients who may further develop classical AS or PsA. These efforts will guide therapeutic trials of potent drugs like biological agents in the early stage of these diseases.
Keywords: Classification criteria, Spondyloarthritis, Psoriatic arthritis, Ankylosing spondylitis
INTRODUCTION
Spondyloarthropathies (SpA) are a group of inflammatory arthritis that consist of ankylosing spondylitis (AS), reactive arthritis, arthritis/spondylitis associated with psoriasis (PsA) and arthritis/spondylitis associated with inflammatory bowel diseases (IBD). The association with human leukocyte antigen (HLA)-B27, peripheral joint involvement predominantly of the lower extremities, sacroiliitis, spondylitis, enthesitis, dactylitis, uveitis, enteric mucosal lesions and skin lesions are the shared manifestations of the diseases[1,2]. Categorization of an individual patient into a subset of SpA can be difficult due to the lack of well-defined criteria for the diagnosis[3]. The newly developed Assessment of SpondyloArthritis International Society (ASAS) classification criteria proposes to classify the SpA according to leading clinical manifestations; predominantly axial or predominantly peripheral, with or without associated psoriasis, IBD or preceding infection[4,5].
The new developments in the clinical and scientific aspects of SpA were pursued by the need for new strategies for definition of early diagnosis and outcome criteria for clinical studies. There is a long delay, approximately 5-6 years, between the first occurrence of the SpA symptoms and the diagnosis of the disease especially for female, juvenile onset or HLA-B27 negative patients[6,7]. The major reason for this delay may be the low awareness of AS among the physicians as well as a lack of well defined criteria for identifying patients with inflammatory back pain (IBP) from chronic low back pain of mechanical origin. Relatively late appearance of sacroiliitis on plain radiographs, due to insidious nature of AS, is another reason for delay. Recent developments demonstrated that inflammation of sacroiliac joints could be well visualized by magnetic resonance imaging (MRI) long before than radiographic changes take place[8].
WHAT ARE CLASSIFICATION CRITERIA?
Classification criteria serve to define disease groups for clinical and epidemiological studies[9]. These sets of classification criteria combine different types of information like symptoms, signs, laboratory findings, imaging, genetic factors and etiological agents.
Classification criteria should not contain too many false positives and should have high specificity. Because of the inverse relationship, it has low sensitivity. In clinical studies, classification criteria provide homogeneous patient groups which thus enable comparisons. On the other hand, diagnostic criteria should have high sensitivity in order to make a correct diagnosis; this means that it may contain false positives and may have low specificity. Most of the rheumatic diseases do not have unique or specific diagnostic tests and classification criteria have been developed to identify homogeneous patient populations for clinical trials. It should be noted that most of the criteria sets in rheumatology have been developed as classification criteria for clinical research but unfortunately are widely used as diagnostic tools in daily practice. This is, for example, the case with the formerly the American Rheumatism Association criteria (for the classification of rheumatoid arthritis) and the European Spondylarthropathy Study Group (ESSG) preliminary criteria for the classification of spondyloarthropathies[10].
Inflammatory back pain
Inflammatory back pain is the leading symptom of the SpA and mirrors inflammation of sacroiliac joints, spine and spinal entheses. However its value for the diagnosis, classification and screening in primary care settings is not well recognized. Clinical history has been proposed as a screening test to identify patients with SpA among those who have chronic back pain[11]. In general, criteria for IBP were derived from studies comparing patients with AS and patients with back pain of other etiologies and from studies based on expert opinion. Although IBP is considered as the foremost clinical symptom for axial SpA, its sensitivity and specificity with respect to diagnosis of axial SpA does not exceed 80%[12].
Calin et al[13] examined 42 patients with AS and 24 patients with other origin of back pain for 5 features of back pain: (1) insidious onset; (2) age at onset < 40 years; (3) duration of back pain ≥ 3 mo; (4) associated with morning stiffness; and (5) improvement with exercise. IBP was considered in the presence of 4 of 5 features, and these were the first criteria for IBP (Table 1). However, Calin’s criteria had some limitations. Duration of morning stiffness was later reported by Gran; a duration more than 30 min is associated with AS, and has 64% sensitivity and 58% specificity[14]. In the original study, Calin’s criteria have 95% specificity and 76% sensitivity but the subsequent studies showed low sensitivity and specificity[14,15]. Adding a single criterion “getting out of the bed at night” improved the sensitivity of these criteria[14].
Table 1.
Inflammatory back pain criteria sets and mnemonic for assessment of spondyloarthritis international society criteria[11-13,17]
| Calin’s criteria for IBP | Berlin criteria for IBP | ASAS IBP criteria mnemonic for criteria “iPAIN” |
| Age at onset < 40 yr | Morning stiffness of > 30 min duration | Insidious onset |
| Duration of back pain > 3 mo | Improvement in back pain with exercise but not with rest | Pain at night (with improvement upon getting up) |
| Insidious onset | ||
| Morning stiffness | Nocturnal awakening (second half of the night only) | Age at onset < 40 yr |
| Improvement with exercise | Alternating buttock pain | Improvement with exercise |
| No improvement with rest | ||
| Requires the presence of four of five criteria | The sensitivity is 70% specificity 81% if two of the four criteria are fulfilled | The sensitivity is 77.0% and specificity 91.7% if at least four out of five criteria are fulfilled |
IBP: Inflammatory back pain; ASAS: Assessment of spondyloarthritis international society; iPAIN: Inflammatory PAIN.
Modified New York Criteria (mNY) for AS integrated features of the Calin’s criteria made the definition of back pain in patients with AS: low back pain and stiffness more than 3 mo, improving with exercise but is not relieved by rest[16]. Various combinations of IBP features were evaluated in 101 patients with AS and 112 patients with mechanical low back pain by Rudwaleit et al[11]. Clinical features of back pain were: (1) morning stiffness > 30 min; (2) age of onset; (3) no improvement by rest; (4) awakening because of the pain in the second half of the night only; (5) alternating buttock pain; and (6) duration of back pain. None of the single parameters differentiated AS from MLBP. Based on a good balance between sensitivity, specificity and feasibility the Berlin criteria were proposed with 70% sensitivity and 81% specificity (Table 1).
In 2009, thirteen internationally well-known rheumatologists, considered as experts in AS/SpA and members of ASAS, participated in the development of new classification criteria for IBP. They presented new ASAS IBP criteria without major differences from formerly established IBP criteria (Table 1). ASAS IBP criteria have 77.0% sensitivity and 91.7% specificity when at least four out of five parameters are present. Calin criteria had a higher sensitivity but a lower specificity. Berlin criteria had a lower sensitivity and a higher specificity with respect to newly developed criteria[12]. Mnemonic for ASAS IBP criteria (iPAIN: Inflammatory PAIN) has been recently published[17] (Table 1).
Imaging
Imaging of the sacroiliac joints and the spine has an important role in the diagnosis, classification and monitoring for patients with SpA. Sacroiliitis on conventional radiography became an important diagnosis in AS and was given an outstanding role in the development of classification criteria in 1961 and mNY criteria in 1984 (Table 2) Usually bilateral grade ≥ 2 or unilateral grade ≥ 3 sacroiliitis are considered critical for the diagnosis of AS[16]. However, radiographic sacroiliitis reflects structural changes which may appear late in the disease process at least in a subset of patients[18]. Thus, it has low specificity especially for patients at the early stages of the disease.
Table 2.
Modified New York criteria for ankylosing spondylitis[16]
| Low back pain for at least 3 mo duration improved by exercise and not relieved by rest |
| Limitation of lumbar spine motion in sagittal and frontal planes |
| Chest expansion decreased relative to normal values for age and sex |
| Unilateral sacroiliitis grade 3–4 |
| Bilateral sacroiliitis grade 2–4 |
| Definite ankylosing spondylitis if (4a or 4b) and any clinical criterion (1–3) |
Magnetic resonance imaging can visualize active inflammation at sacroiliac joints and spine in established or in early pre-radiological axial disease, regardless of disease stage[19]. The mNY, ESSG criteria and the Amor criteria do not contain MRI as an imaging tool. Actually, MRI of the sacroiliac joints was defined however it was not well established or standardized, when these criteria were developed.
ASAS classification criteria for axial SpA have imaging and clinical arms. The imaging arm includes either sacroiliitis on conventional radiography or sacroiliitis on MRI, which is highly important for recognition of pre-radiographic changes in early SpA[4].
Regarding spondylitis, which may also occur before sacroiliitis, a definition of a “positive MRI” for the spinal inflammation is also needed[20]. However, there is insufficient data for the use of spinal MRI and little is yet known about the specificity of spinal features in the axial SpA[21].
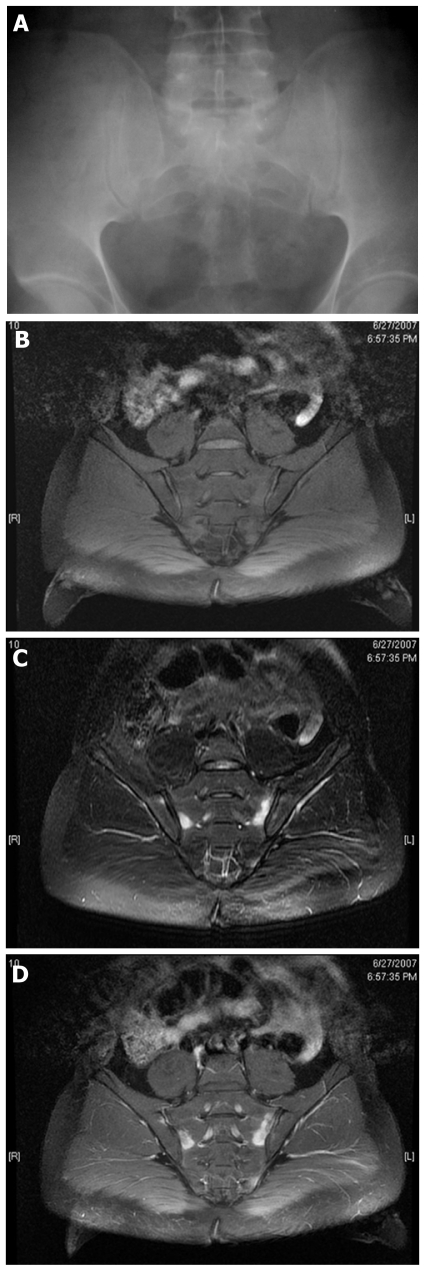
Active inflammatory lesions such as bone marrow edema/osteitis, synovitis, enthesitis and capsulitis associated with SpA can be detected by MRI. Also structural damage such as sclerosis, erosions, fat deposition and ankylosis can be detected by MRI. ASAS/OMERACT imaging group defined minimum amount of bone marrow edema (one lesion at least two adjacent slices or more than one lesion at least one slice) which is required for the definitive diagnosis sacroiliitis[22]. Figure 1A-D represents a normal radiograph of the pelvis and early changes on sacroiliac MRI of a male patient at the early stages of the disease (pre-radiographic stage). Figure 2A-C represents inflammatory changes and structural damage on spinal MRI.
Figure 1.
Normal radiograph of the pelvis and early changes on sacroiliac magnetic resonance imaging of a male patient at the early stages of the disease at the pre-radiographic stage. A: Thirty-five year old male, normal anterior posterior pelvis radiograph; B: T1-weighted Fast Spin Echo semi-oblique coronal scans of the sacroiliac joints; C: T2-weighted fat suppressed images shows bone edema at both sacral and iliac bones; D: T1-weighted post-contrast image shows enhancement of the contrast media revealing acute inflammation.
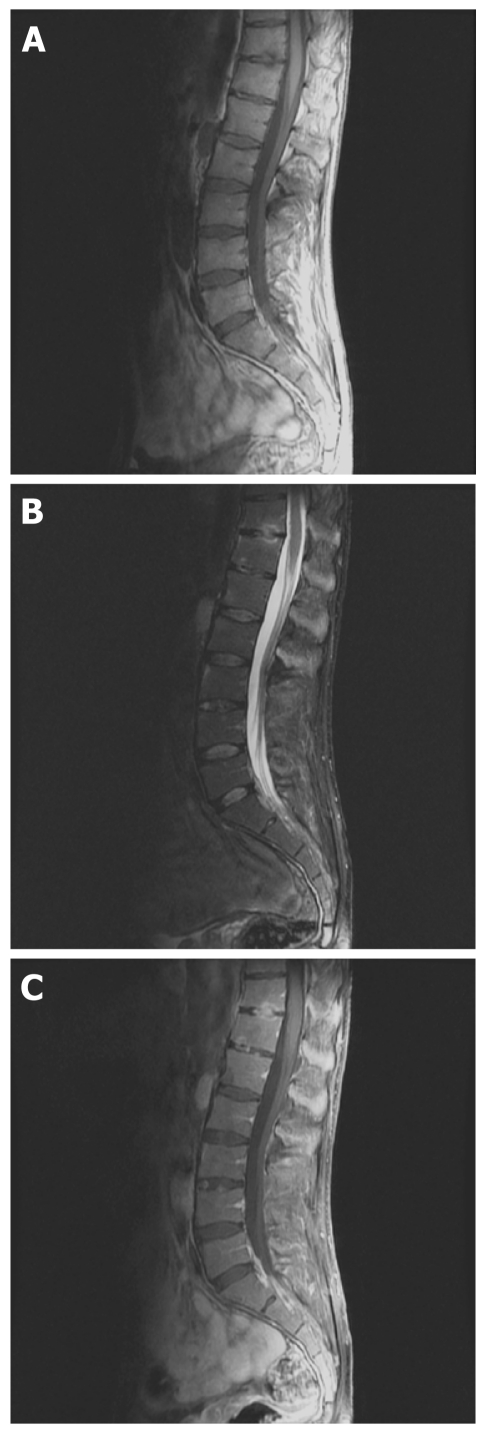
Figure 2.
Inflammatory changes and structural damage on spinal magnetic resonance imaging. A: T1-weighted fast spin echo sagittal magnetic resonance scan of the lumbar spine shows hypointense lesion on end plates of thoracic 11 and 12 vertebrae; B: T2-weighted fat suppressed sagittal image shows hyperintense signals at the lesion and also at the upper anterior of the L3 and lower anterior of L2 vertebra; C: T1-weighted post-contrast images shows enhancement of the contrast media at the borders of the lesion revealing acute spondylodisciitis.
HLA B-27
HLA B-27 positivity is extremely relevant to the early diagnosis of SpA. Five to 10% of the population are HLA B-27 positive and in patients with AS and SpA the positivity of HLA B-27 changes to 70% to 95% and nearly 70%, respectively[23].
SPECTRUM OF SPONDYLOARTHROPATHIES
Ankylosing spondylitis
Ankylosing spondylitis is the most common and most typical form of SpA. It is two to three times more common in men than women. Ankylosing spondylitis usually begins with back pain and stiffness at a young age but various presentations, such as peripheral arthritis and enthesopathy may antedate back symptoms in some patients. Late onset after the age of 45 is uncommon in AS however some patients may reasonably be diagnosed late. Inflammatory low back pain is one of the presenting features but not solely specific to AS. History of uveitis, positive family history for AS, impaired spinal mobility or chest expansion supports the diagnosis[1].
Axial involvement is one of the characteristics of the disease and 90% of patients have radiographic sacroiliitis during the course of the disease. The first classification criteria for AS were proposed in 1963 at the European Congress of Rheumatology in Rome, based on the clinical experience of rheumatologists. Later in 1966, thoracic pain and uveitis were removed from the criteria set because of low specificity and low sensitivity. This preceded the framework of New York criteria which was modified in 1984 by using inflammatory back pain components reported by Calin et al[13]. A patient can be classified as having definite AS if at least one clinical criterion (IBP, limitation of lumbar spine or limitation of chest expansion) plus radiologic criterion (bilaterally grade 2 or unilateral grade 3-4 sacroiliitis) are fulfilled[16]. These classification criteria are inevitably used for the diagnosis of AS by most clinicians (Table 2).
All these criteria included presence of spinal/thoracic pain, restriction of spinal mobility and radiological sacroiliitis. Restriction of spinal mobility and radiological sacroiliitis may reflect structural damage and spinal/thoracic pain may reflect active inflammation and structural damage as well. It is obvious that these criteria do not perform well in patients with early/pre-radiographic phase of AS.
Axial spondyloarthritis
As mentioned above, sacroiliitis on plain radiographs takes years from the onset IBP and the symptoms of IBP alone are not diagnostic in many patients.
Berlin criteria were developed to assist physicians for early diagnosis of SpA. In this criterion set, the clinical, laboratory (HLA B-27) and imaging (MRI of sacroiliac joints) features were included. The diagnosis of recent-onset axial SpA (pre-radiographic SpA) can be established in patients who have clinical features without radiographic changes but sacroiliitis on MRI. This study also analyzed the role of MRI as a diagnostic tool[24]. The performance of Berlin criteria has been tested and showed that the diagnostic capacity in patients with axial undifferentiated SpA in the Chinese population was similar to ESSG and Amor criteria[25].
In 2004, ASAS decided to improve current SpA criteria particularly to apply to patients in the early disease stages. It was proposed that SpA patients with predominantly axial symptoms but without radiographic sacroiliitis could be considered as patients with pre-radiographic phase of AS. The need for an early diagnosis in all patients with AS and axial SpA is put forward[26].
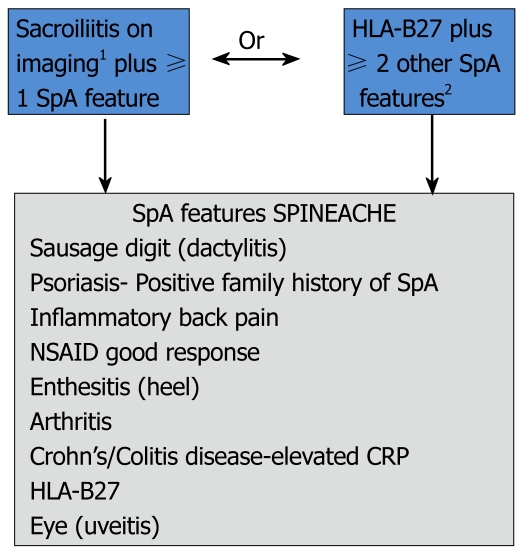
In 2009, ASAS developed two candidate criteria sets for classification of axial SpA that include patients without definite radiographic sacroiliitis[27]. The candidate sets were tested in the entire cohort of 649 patients from 25 centers in 16 countries. The new criteria consisted of a ‘clinical arm’ and ‘imaging arm’ (Figure 3). The entire set had 82.9% sensitivity and 84.4% specificity and for the ‘imaging arm’ alone sensitivity was 66.2% and specificity was 97.3%. The specificity of the new criteria was much better than ESSG criteria modified by adding MRI and slightly better than Amor criteria modified by adding MRI[27]. The sensitivity is almost the same for the three criteria set. ASAS criteria are quite simple and easily applicable in daily clinical practice and a mnemonic is proposed to facilitate its use[17] (Figure 3).
Figure 3.
Assessment in SpondyloArthritis international Society classification criteria for axial spondyloarthritis and mnemonic for assessment of spondyloarthritis international society classification criteria[4,17]. 1Sacroiliitis on imaging active (acute) inflammation on magnetic resonance imaging highly suggestive of sacroiliitis associated with SpA or definitive radiographic sacroiliitis according to modified New York criteria; 2Elevated CRP is considered a SpA feature in the context of chronic back pain. SpA: Spondyloarthropathies; CRP: C-reactive protein; NSAID: Nonsteroidal antiinflammatory drugs; HLA: Human leukocyte antigen.
Peripheral spondyloarthritis
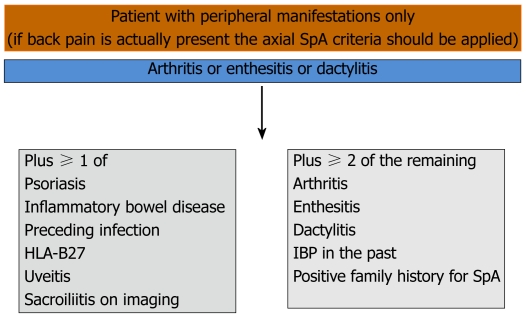
After the development of ASAS criteria for axial SpA, ASAS experts developed criteria for patients with SpA with predominant peripheral manifestations and compared these with ESSG and Amor criteria which were generated for the entire SpA group including peripheral SpA[5]. Patients with peripheral manifestations including peripheral arthritis, dactylitis and enthesitis and without back pain were included. The sensitivity of the criteria was 77.8% and the specificity was 82.2% (Figure 4). The new ASAS classification criteria for peripheral arthritis would seem to perform better than ESSG and Amor criteria.
Figure 4.
Assessment in spondyloarthritis international society classification criteria for peripheral spondyloarthritis or spondyloarthritis in general[5]. SpA: Spondyloarthropathies; IBP: Inflammatory back pain; HLA: Human leukocyte antigen.
Spondyloarthritis in general
Spondyloarthropathies were formally classified in Amor criteria in 1990. Amor’s criteria are a list of signs based on a scoring system of laboratory, radiologic and clinical features and do not require an entry criterion[28] . The signs in the criteria contribute 1 point, 2 points or 3 points; a score of 6 or more classifies a patient as having SpA. Although sacroiliitis is not mandatory for the diagnosis of SpA, it had the highest score (3 points) and is considered to be very specific for SpA (Table 3).
Table 3.
Amor criteria for the classification of spondyloarthropathies[28]
| Amor criteria | |
| Clinical symptoms or history of scoring | Points |
| Lumbar or dorsal pain at night or morning stiffness of lumbar or dorsal pain | 1 |
| Asymmetrical oligoarthritis | 2 |
| Buttock pain | 1 |
| If alternate buttock pain | 2 |
| Sausage like toe or digit | 2 |
| Heel pain or other well-defined enthesopathy | 2 |
| Iritis | 1 |
| Nongonococcal urethritis or cervicitis within 1 mo before the onset of arthritis | 1 |
| Acute diarrhea within one month before the 1 mo onset of arthritis | 1 |
| Psoriasis, balanitis, or inflammatory bowel disease (ulcerative colitis or Crohn’s disease) | 2 |
| Radiological findings | |
| Sacroiliitis (bilateral grade 2 or unilateral grade 3) | 3 |
| Genetic background | |
| Presence of HLA-B27 and/or family history of ankylosing spondylitis, reactive arthritis, uveitis, psoriasis, or inflammatory bowel disease | 2 |
| Response to treatment | |
| Clear-cut improvement within 48 h after NSAIDs intake or rapid relapse of the pain after their discontinuation | 2 |
| A patient is considered as suffering from a pondyloarthropathy if the sum is ≥ 6 |
NSAID: Nonsterodial anti-inflammatory drug; HLA: Human leukocyte antigen.
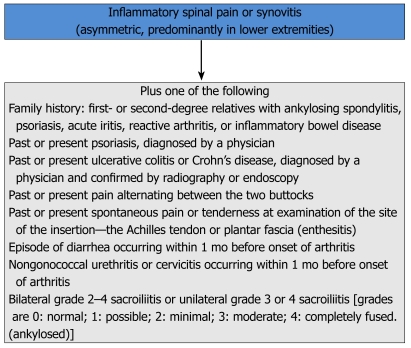
ESSG criteria were proposed in 1991. In ESSG criteria IBP and/or peripheral arthritis are required as entry criteria. Patients with at least one entry criterion and one minor criterion are classified as having SpA[29] (Figure 5). The aim of ESSG criteria is to include undifferentiated SpA which was not been proposed in Amor criteria. Both of these criteria were considered to be helpful for the diagnosis of SpA and had a broader definition of the spectrum however, they have low sensitivity particularly for the early diagnosis of SpA. For example, some of the leading symptoms like uveitis may be omitted by ESSG criteria but captured by Amor criteria.
Figure 5.
European Spondyloarthropathy Study Group Criteria for the classification of spondyloarthropathies[29].
Both sets of criteria were evaluated in a multicenter cross-sectional study including 124 patients with SpA and 1964 controls. Overall performance of both sets was similar and the performance was better in patients with a definite diagnosis[30]. These criteria were evaluated for a Turkish population in 157 patients with SpA and in 127 patients with various rheumatic diseases. Results showed that both criteria had a similar value for classification of SpA and were comparable in terms of specificity and sensitivity[31].
In a newly published study, performance of ESSG criteria, ASAS criteria and mNY criteria were compared in patients with SpA. The ASAS criteria had the highest sensitivity compared to ESSG criteria and mNY criteria 98.4%, 83.6% and 71.9%, respectively[32]. In other studies of different ethnicities, lower sensitivity for mNY but similar sensitivity for ESSG was reported[33-35].
Recently, the French Society of Rheumatology presented the DESIR cohort. Patients were recruited if they had IBP more than 3 mo and less than 3 years. A total of 708 patients were recruited and the mNY criteria, Amor criteria, ESSG criteria and axial ASAS criteria were fulfilled by 26%,77%, 76% and 67% at entry, respectively[36].
The diagnostic accuracy of the ESSG criteria, Amor criteria and the combination of them was analyzed in 24 patients who were misdiagnosed as SpA. The ratio of the misdiagnosed patients who fulfilled ESSG criteria, Amor criteria and combination were 45.8%, 16.7%, 16.7%, respectively. This study suggests that ESSG criteria may not be absolutely secure for the diagnosis of SpA[37].
Performance of mNY criteria, ESSG criteria, Amor criteria and Berlin criteria in patients with IBP of a maximum of 2 years duration was evaluated. Fourteen of the 68 patients had AS according to mNY and all fulfilled three of SpA criteria sets. The highest classification rate was found with the ESSG criteria (84%), followed by the Amor criteria (71%) and the Berlin criteria (65%). The ESSG criteria were the most sensitive and the mNY criteria for AS appeared to be most specific sets of criteria[38].
Psoriatic arthritis
Psoriatic arthritis (PsA) is defined as an inflammatory arthritis associated with cutaneous psoriasis. Patients may have peripheral arthritis (oligoarthritis or polyarthritis), enthesitis, dactylitis or sacroiliitis/spondylitis[39]. At the beginning of the century PsA was thought to coincidentally occur with rheumatoid arthritis (RA) and psoriasis. Psoriatic arthritis was adopted as a distinct disease for the first time in 1964. The distinction between RA and PsA was made based on the clinical and radiological features[40].
In 1973 Moll and Wright[41] reported a proposal for the classification of PsA. When a patient with psoriasis has inflammatory arthritis and is negative for rheumatoid factor (RF) PsA can be classified in five distinct clinical subsets as: (1) oligoarticular asymmetric arthritis (< 5 tender and swollen joints); (2) polyarticular arthritis; (3) distal interphalangeal joint predominant; (4) spondylitis predominant; and (5) arthritis mutilans predominant.
Over the passing years minor modifications have been made on these criteria. Gladman et al[42] suggested that there is no need to insist on seronegativity for RF, since it can be positive in healthy subjects and in their series, 12% of cases were RF (+) even when the patients who had a characteristic sign of RA, like rheumatoid nodules and extra-articular manifestations were excluded. It is also possible to differentiate seronegative RA from PsA by using other antibodies, anti-cyclic citrullinated peptide which has much higher specificity than RF for the diagnosis of RA.
Psoriasis is a common disease affecting nearly 1%-2% of the population. In some forms of arthritis coincidental psoriasis may also occur. Psoriasis may precede, simultaneously occur or appear many years after the onset of arthritis. In latter cases patients may be misdiagnosed with other types of arthritis like seronegative RA or reactive arthritis; however, positive family history for psoriasis may be helpful in these cases. Patients with arthritis should be carefully examined for existence of “hidden” psoriatic lesions which may be located under the breasts, around the umbilicus or anus, over the hairline, nasal cleft or nails[41].
Patients with PsA tend to have inflammatory axial involvement similar to AS. There are several differences from the classical AS[41]: (1) asymmetrical sacroiliitis; (2) non-marginal syndesmophytes; (3) asymmetrical syndesmophytes; and (4) more frequent involvement of the cervical spine.
Bennett thought that Moll and Wright criteria tend to over diagnosing PsA and suggested new criteria in 1979. In these new set of criteria, clinical and radiological features were combined with synovial fluid analysis and histology. These criteria have not been widely used in prospective studies since synovial fluid analysis and histology are not practical. Psoriatic skin or nail involvement plus either peripheral joint or axial disease were required[41]. Simplification of Bennett’s criteria has been made by Vasey and Espinoza[42].
ESSG criteria were also valid for PsA. For the first time skin or nail involvement was not mandatory in these criteria. Cases in which arthritis precedes psoriasis are well recognized and family history of psoriasis can help the diagnosis[29].
A definition of PsA based on enthesopathy has been proposed by McGonagle et al[43]. There is a significant problem with these criteria because of MRI requirements. It is not practical to use MRI in epidemiological research. MRI appearance shows both features of enthesopathy and synovitis and so the discrimination capacity would be markedly attenuated in established disease. Fournie et al[44] proposed criteria from actual patient data to diagnose PsA which requires a score of 11 points for diagnosis.
There are few studies that compare different criteria for the diagnosis of PsA. A study which compared performance of the criteria revealed that the sensitivity of Vasey and Espinoza, McGonagle and Gladman were 99% whereas Bennett and ESSG criteria were significantly less sensitive. The specificity of the criteria was as high as 93% and 99%, and there were no statistically significant difference between criteria. Fournie criteria were the most difficult to use and Vasey and Espinoza, and Moll and Wright were the easiest. Vasey and Espinoza, Gladman or McGonagle are the most accurate and feasible in distinguishing RA from PsA[45].
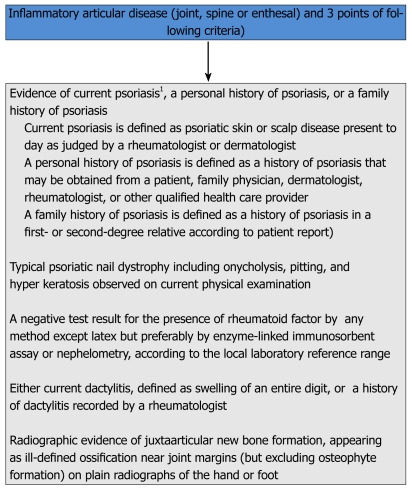
The classification of psoriatic arthritis (CASPAR) study group is an international group of investigators, all of whom have records of research in PsA. They proposed new data-driven classification criteria for PsA and collected prospective clinical and radiological data of 588 patients with PsA and 536 patients with other inflammatory arthritis, at least half of them with RA (Figure 6). The performance of the new criteria were also compared to other existing data[46]. The sensitivity and specificity of the CASPAR criteria in the original study were 91.4% and 98.7%, respectively. These criteria were more specific but less sensitive than Vasey and Espinoza criteria.
Figure 6.
Classification of psoriatic arthritis study group criteria for the classification of psoriatic arthritis[46]. 1Current psoriasis is assigned a score of 2; all other features are assigned a score of 1.
The main limitation of the CASPAR criteria is the applicability to recent-onset disease. Very high sensitivity of CASPAR criteria in early and late PsA was also demonstrated in a study[47]. This study analyzed patients referred to a special tertiary referral clinic and did not have a control population. It seems likely that only patients with secure clinical diagnoses are referred and enrolled into this clinic, possibly leading to an overestimation of the sensitivity of the criteria[48].
Family history of psoriasis is the advantage of CASPAR criteria over Vasey and Espinoza as well as Moll and Wright criteria. It is also possible to make a diagnosis of PsA for patients who are RF positive and have polyarticular symmetric arthritis. The CASPAR, as a simple and user-friendly criteria set, has high potential to be introduced as the universal classification criteria for PsA[42].
CONCLUSION
Chronic low back pain is a common and important problem and patients with this disorder are seen by a variety of specialists including rheumatologists, orthopedic surgeons, physiatrists, family physicians etc. Inflammatory low back pain is usually the leading symptom of spondyloarthropathies and physicians should always be aware. For a correct diagnosis IBP should be differentiated from mechanical back pain. A detailed screening of signs and symptoms in terms of insidious onset, morning stiffness, pain at night, improvement with exercise and favorable response to NSAIDs may ease the discrimination. Other common features of SpA like dactylitis, enthesitis, arthritis and history of preceding infections should also be checked. Imaging has an important role in the early diagnosis of SpA and the very early phase of sacroiliitis or spondylitis could be detected by documenting active inflammatory lesions like bone marrow edema, enthesitis, capsulitis or synovitis on MRI. HLA B-27 positivity is extremely relevant to the early diagnosis of SpA.
Footnotes
Peer reviewers: Thomas J Kishen, Dr., Spine Service, Sparsh-Hospital for Advanced Surgeries 146, Infantry Road, Bangalore 560001, Karnataka, India; Kanji Mori, MD, PhD, Assistant Professor, Department of Orthopaedics Surgery, Shiga University of Medical Science, Tsukinowa-cho, Seta, Otsu 520-2192, Japan; Wen-Bao Wang, MD, Department of Surgery, Harlem Hospital, 106 Fort Washington Avenue, Apt 3H, New York, NY 10032, United States
S- Editor Yang XC L- Editor Roemmele A E- Editor Yang XC
References
- 1.Khan MA. Update on spondyloarthropathies. Ann Intern Med. 2002;136:896–907. doi: 10.7326/0003-4819-136-12-200206180-00011. [DOI] [PubMed] [Google Scholar]
- 2.Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006;20:401–417. doi: 10.1016/j.berh.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Braun J, Sieper J. Building consensus on nomenclature and disease classification for ankylosing spondylitis: results and discussion of a questionnaire prepared for the International Workshop on New Treatment Strategies in Ankylosing Spondylitis, Berlin, Germany, 18-19 January 2002. Ann Rheum Dis. 2002;61 Suppl 3:iii61–iii67. doi: 10.1136/ard.61.suppl_3.iii61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 5.Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 6.Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–66. doi: 10.1007/s00296-002-0237-4. [DOI] [PubMed] [Google Scholar]
- 7.Ozgocmen S, Ardicoglu O, Kamanli A, Kaya A, Durmus B, Yildirim K, Baysal O, Gur A, Karatay S, Altay Z, et al. Pattern of disease onset, diagnostic delay, and clinical features in juvenile onset and adult onset ankylosing spondylitis. J Rheumatol. 2009;36:2830–2833. doi: 10.3899/jrheum.090435. [DOI] [PubMed] [Google Scholar]
- 8.Sieper J. Developments in the scientific and clinical understanding of the spondyloarthritides. Arthritis Res Ther. 2009;11:208. doi: 10.1186/ar2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson SR, Goek ON, Singh-Grewal D, Vlad SC, Feldman BM, Felson DT, Hawker GA, Singh JA, Solomon DH. Classification criteria in rheumatic diseases: a review of methodologic properties. Arthritis Rheum. 2007;57:1119–1133. doi: 10.1002/art.23018. [DOI] [PubMed] [Google Scholar]
- 10.Dougados M, Gossec L. Classification criteria for rheumatic diseases: why and how? Arthritis Rheum. 2007;57:1112–1115. doi: 10.1002/art.23015. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum. 2006;54:569–578. doi: 10.1002/art.21619. [DOI] [PubMed] [Google Scholar]
- 12.Sieper J, van der Heijde D, Landewé R, Brandt J, Burgos-Vagas R, Collantes-Estevez E, Dijkmans B, Dougados M, Khan MA, Leirisalo-Repo M, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS) Ann Rheum Dis. 2009;68:784–788. doi: 10.1136/ard.2008.101501. [DOI] [PubMed] [Google Scholar]
- 13.Calin A, Porta J, Fries JF, Schurman DJ. Clinical history as a screening test for ankylosing spondylitis. JAMA. 1977;237:2613–2614. [PubMed] [Google Scholar]
- 14.Gran JT. An epidemiological survey of the signs and symptoms of ankylosing spondylitis. Clin Rheumatol. 1985;4:161–169. doi: 10.1007/BF02032287. [DOI] [PubMed] [Google Scholar]
- 15.Van der Linden SM, Fahrer H. Occurrence of spinal pain syndromes in a group of apparently healthy and physically fit sportsmen (orienteers) Scand J Rheumatol. 1988;17:475–481. doi: 10.3109/03009748809098810. [DOI] [PubMed] [Google Scholar]
- 16.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 17.Ozgocmen S, Akgul O, Khan MA. Mnemonic for assessment of the spondyloarthritis international society criteria. J Rheumatol. 2010;37:1978. doi: 10.3899/jrheum.100477. [DOI] [PubMed] [Google Scholar]
- 18.Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker-Hermann E, Zeidler H, Braun J, Sieper J. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60:717–727. doi: 10.1002/art.24483. [DOI] [PubMed] [Google Scholar]
- 19.De Rycke L, Maas M, Tak PP, Baeten D. 'MRI-tis' in the early diagnosis of axial SpA: issues and limitations. Nat Rev Rheumatol. 2010;6:666–669. doi: 10.1038/nrrheum.2010.161. [DOI] [PubMed] [Google Scholar]
- 20.Braun J, Baraliakos X. Imaging of axial spondyloarthritis including ankylosing spondylitis. Ann Rheum Dis. 2011;70 Suppl 1:i97–103. doi: 10.1136/ard.2010.140541. [DOI] [PubMed] [Google Scholar]
- 21.van der Heijde D, Rudwaleit M, Landewé RB, Sieper J. Justification for including MRI as a tool in the diagnosis of axial SpA. Nat Rev Rheumatol. 2010;6:670–672. doi: 10.1038/nrrheum.2010.160. [DOI] [PubMed] [Google Scholar]
- 22.Rudwaleit M, Jurik AG, Hermann KG, Landewé R, van der Heijde D, Baraliakos X, Marzo-Ortega H, Ostergaard M, Braun J, Sieper J. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–1527. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 23.Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108–114. doi: 10.1016/j.jbspin.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–543. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z, Gu J, Huang F, Lin Z, Zhao L, Yu B. Verification of Berlin algorithm for diagnosing undifferentiated spondyloarthropathy patients in Chinese population. Joint Bone Spine. 2009;76:146–149. doi: 10.1016/j.jbspin.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;52:1000–1008. doi: 10.1002/art.20990. [DOI] [PubMed] [Google Scholar]
- 27.Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, Burgos-Vargas R, Collantes-Estevez E, Davis J, Dijkmans B, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68:770–776. doi: 10.1136/ard.2009.108217. [DOI] [PubMed] [Google Scholar]
- 28.Amor B, Dougados M, Mijiyawa M. [Criteria of the classification of spondylarthropathies] Rev Rhum Mal Osteoartic. 1990;57:85–89. [PubMed] [Google Scholar]
- 29.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 30.Amor B, Dougados M, Listrat V, Menkes CJ, Dubost JJ, Roux H, Benhamou C, Blotman F, Pattin S, Paolaggi JB. [Evaluation of the Amor criteria for spondylarthropathies and European Spondylarthropathy Study Group (ESSG). A cross-sectional analysis of 2,228 patients] Ann Med Interne (Paris) 1991;142:85–89. [PubMed] [Google Scholar]
- 31.Ertürk M, Alaca R, Tosun E, Duruöz MT. Evaluation of the Amor and ESSG classification criteria for spondylarthropathies in a Turkish population. Rev Rhum Engl Ed. 1997;64:293–300. [PubMed] [Google Scholar]
- 32.Chung HY, Lau CS, Wu KP, Wong WS, MOK MY. Comparison of performance of the Assessment of SpondyloArthritis International Society, the European Spondyloarthropathy Study Group and the modified New York criteria in a cohort of Chinese patients with spondyloarthritis. Clin Rheumatol. 2011;30:947–953. doi: 10.1007/s10067-011-1693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyer GS, Templin DW, Goring WP. Evaluation of the European Spondylarthropathy Study Group preliminary classification criteria in Alaskan Eskimo populations. Arthritis Rheum. 1993;36:534–538. doi: 10.1002/art.1780360414. [DOI] [PubMed] [Google Scholar]
- 34.Cury SE, Vilar MJ, Ciconelli RM, Ferraz MB, Atra E. Evaluation of the European Spondylarthropathy Study Group (ESSG) preliminary classification criteria in Brazilian patients. Clin Exp Rheumatol. 1997;15:79–82. [PubMed] [Google Scholar]
- 35.Baddoura R, Awada H, Okais J, Habis T, Attoui S, Abi Saab M. Validation of the European Spondylarthropathy Study Group and B. Amor criteria for spondylarthropathies in Lebanon. Rev Rhum Engl Ed. 1997;64:459–464. [PubMed] [Google Scholar]
- 36.Dougados M, d'Agostino MA, Benessiano J, Berenbaum F, Breban M, Claudepierre P, Combe B, Dargent-Molina P, Daures JP, Fautrel B, et al. The DESIR cohort: A 10-year follow-up of early inflammatory back pain in France: Study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine. 2011;78:598–603. doi: 10.1016/j.jbspin.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Deng XL, Liu XY, Xu N. Comparative study on low back pain misdiagnosed as spondyloarthropathy. Clin Rheumatol. 2009;28:893–898. doi: 10.1007/s10067-009-1198-8. [DOI] [PubMed] [Google Scholar]
- 38.Heuft-Dorenbosch L, Landewé R, Weijers R, Houben H, van der Linden S, Jacobs P, van der Heijde D. Performance of various criteria sets in patients with inflammatory back pain of short duration; the Maastricht early spondyloarthritis clinic. Ann Rheum Dis. 2007;66:92–98. doi: 10.1136/ard.2006.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantini F, Niccoli L, Nannini C, Kaloudi O, Bertoni M, Cassarà E. Psoriatic arthritis: a systematic review. Int J Rheum Dis. 2010;13:300–317. doi: 10.1111/j.1756-185X.2010.01540.x. [DOI] [PubMed] [Google Scholar]
- 40.Rudwaleit M, Taylor WJ. Classification criteria for psoriatic arthritis and ankylosing spondylitis/axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2010;24:589–604. doi: 10.1016/j.berh.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis. 2005;64 Suppl 2:ii3–ii8. doi: 10.1136/ard.2004.032318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Congi L, Roussou E. Clinical application of the CASPAR criteria for psoriatic arthritis compared to other existing criteria. Clin Exp Rheumatol. 2010;28:304–310. [PubMed] [Google Scholar]
- 43.McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum. 1999;42:1080–1086. doi: 10.1002/1529-0131(199906)42:6<1080::AID-ANR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Fournié B, Crognier L, Arnaud C, Zabraniecki L, Lascaux-Lefebvre V, Marc V, Ginesty E, Andrieu V, Dromer C, Fournié A. Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev Rhum Engl Ed. 1999;66:446–456. [PubMed] [Google Scholar]
- 45.Taylor WJ, Marchesoni A, Arreghini M, Sokoll K, Helliwell PS. A comparison of the performance characteristics of classification criteria for the diagnosis of psoriatic arthritis. Semin Arthritis Rheum. 2004;34:575–584. doi: 10.1016/j.semarthrit.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 47.Chandran V, Schentag CT, Gladman DD. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2007;57:1560–1563. doi: 10.1002/art.23104. [DOI] [PubMed] [Google Scholar]
- 48.Coates LC, Helliwell PS. Classification and categorisation of psoriatic arthritis. Clin Rheumatol. 2008;27:1211–1216. doi: 10.1007/s10067-008-0947-4. [DOI] [PubMed] [Google Scholar]