Abstract
Traditionally performed by a small group of highly trained specialists, bedside sonographic procedures involving the musculoskeletal system are often delayed despite the critical need for timely diagnosis and treatment. Due to this limitation, a need evolved for more portability and accessibility to allow performance of emergent musculoskeletal procedures by adequately trained non-radiology personnel. The emergence of ultrasound-assisted bedside techniques and increased availability of portable sonography provided such an opportunity in select clinical scenarios. This review summarizes the current literature describing common ultrasound-based musculoskeletal procedures. In-depth discussion of each ultrasound procedure including pertinent technical details, indications and contraindications is provided. Despite the limited amount of prospective, randomized data in this area, a substantial body of observational and retrospective evidence suggests potential benefits from the use of musculoskeletal bedside sonography.
Keywords: Musculoskeletal ultrasound-guided procedures, Arthrocentesis, Tendon injection, Articular injection, Fluid collection, Abscess drainage, Foreign body removal
INTRODUCTION
Bedside procedures involving the musculoskeletal system have traditionally been performed by highly trained specialists. Due to reliance on a select group of practitioners, many procedures may be delayed despite their often urgent nature. As a result, a need arose for more portable and accessible means to allow performance of perform emergent musculoskeletal procedures by adequately trained emergency surgical and non-surgical personnel. The emergence of ultrasound-assisted bedside techniques and increased availability of portable sonography provided such an opportunity in select clinical scenarios. The purpose of this review is to summarize the current literature for the most common ultrasound-based musculoskeletal procedures. A thorough discussion of each ultrasound procedure including pertinent technical details and procedural indications/contraindications is included. Although there is a limited number of prospective, randomized studies in this clinical area, there is a significant amount of observational and retrospective evidence that demonstrates potential benefits that stem from ultrasound use in musculoskeletal bedside sonographic applications.
This review will be presented as a series of focused, clinical procedure-oriented sections, each of which is further sub-divided into procedural rationale (including indications and contraindications) and technical overview. Due to the limited scope of this review, the reader is referred to primary literature sources throughout the manuscript for further information pertaining to each topic/procedure.
ARTHROCENTESIS
Rationale
Arthrocentesis involves the aspiration of a synovial joint space, for both therapeutic and diagnostic indications[1]. It is a commonly performed procedure, with an estimated 50%-62% of general medicine physicians utilizing information from arthrocentesis to guide patient management[2]. Given the relative simplicity of the procedure and the overall prevalence of joint problems, a general level of comfort with arthrocentesis should be attainable among a variety of medical and surgical specialists. Major clinical indications include: (1) undiagnosed effusion; (2) undiagnosed arthritis; (3) septic arthritis; and (4) symptomatic relief of effusion. Contraindications to arthrocentesis include: (1) active infection overlying the puncture site; (2) tumor/mass overlying the site; and (3) rash overlying the sampling site (relative contraindication).
Adequate anatomic characterization of the intended joint space must be performed prior to arthrocentesis. Physical examination and knowledge of anatomy are crucial to a safe and effective performance of arthrocentesis. With the advent of modern imaging modalities, the practitioner now has multiple methods of anatomic characterization and pre-procedural planning (magnetic resonance imaging, computed tomography, and ultrasound). It is important to note that the physical exam, when compared to ultrasound of the knee, had only a 59% sensitivity and 65% specificity for detection of knee effusions[3]. This may be due to the finding that the minimal volume of fluid needed for detection on knee ultrasound is approximately 7-10 mL[4]. Having said that, when compared to other imaging modalities, joint ultrasonography is of uncertain value for purely diagnostic purposes. Thus, the most practical use would be for guidance in diagnostic and therapeutic arthrocentesis[5].
Current evidence suggests that ultrasound-guided arthrocentesis may be less technically difficult for emergency physicians, less time consuming, and produce less pain than the traditional “blind” arthrocentesis[6]. Specifically, cadaver-derived evidence shows that ultrasound-guided arthrocentesis has a higher success rate compared to traditional blind arthrocentesis, particularly in the smaller joints (metatarsophalangeal, metacarpophalangeal, and proximal interphalangeal joints)[7,8]. This highlights potential advantages of ultrasound-guided arthrocentesis over traditional methods, especially given the ability of sonography to provide direct visualization of pertinent anatomic structures and confirm accurate entrance of the needle into the joint space (Figures 1 and 2).
Figure 1.
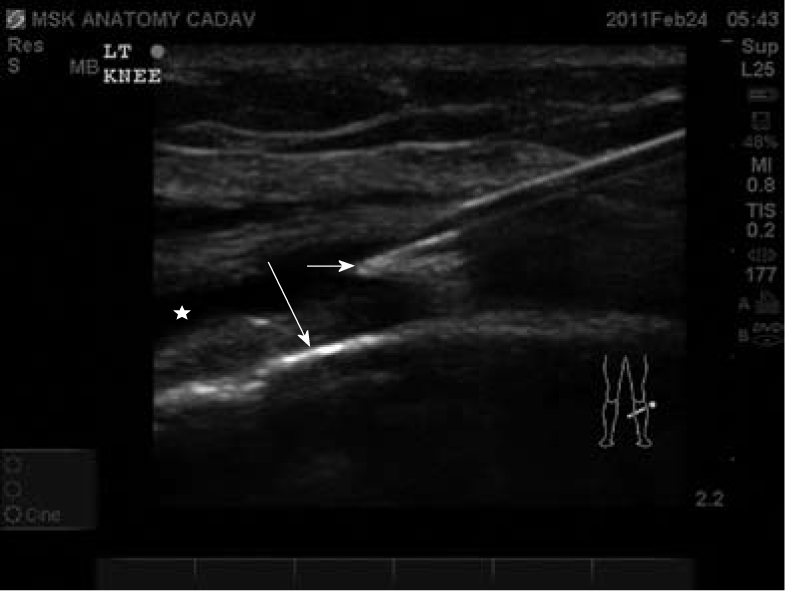
Ultrasound-guided arthrocentesis allows confirmation of the needle within the articular space and real-time visualization of fluid withdrawal. Flow can be noted within the articular space by using color or doppler flow while compressing the joint space. This technique prevents inaccurately inserting the needle within solid masses. Note the needle is best visualized when the probe is perpendicular to the needle. Long arrow indicates tibia cortical bone. Short arrow indicates needle tip. Star indicates joint space.
Figure 2.
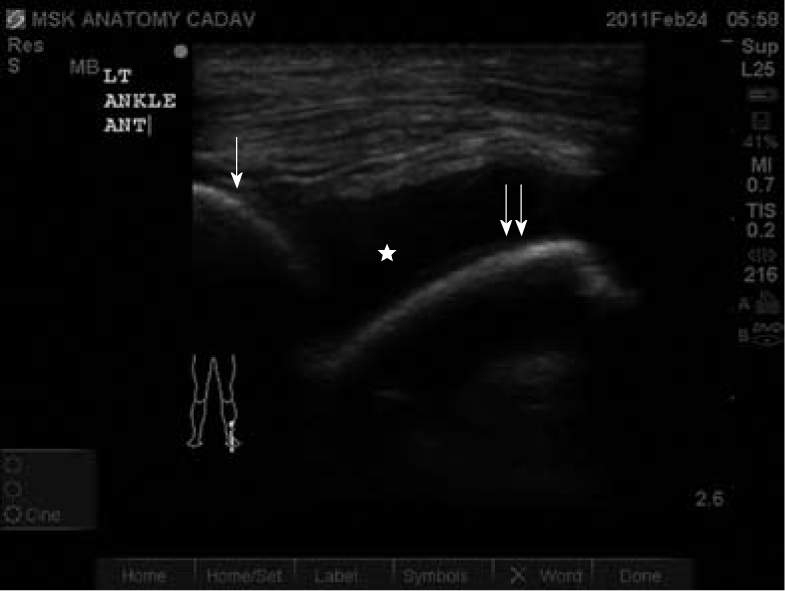
Ultrasound-guided arthrocentesis should be performed by first assessing the joint space for an effusion followed by direct observation of the needle entering the effusion. Use of ultrasound-guided arthrocentesis is highly accurate compared to blind or landmark-techniques for smaller joint spaces such as the tibiotalar joint demonstrated in the image above. Landmarks within the ultrasound image include the bone appearing as a hyperechoic region with superficial tissues including tendons and muscle appearing as heterogenous echoic regions. Fluid within the joint space appears as hypoechoic shapes that conform to the space. Single white arrow indicates the tibia; double white arrow indicates the talus. Star indicates the tibiotalar effusion.
Technique overview
The ultrasound-guided arthrocentesis is performed under standard precautions with appropriate draping of the joint and sterile procedure site preparation. Mandatory procedure site and laterality verification is performed. The ultrasound probe of choice will be determined by the joint of interest. In general, an appropriate probe choice is the linear probe (5-10 MHz) which provides good visualization of most superficial joints[7,8]. If the joint of interest is deep and the linear probe is unable to provide adequate visualization of the space, a curvilinear probe may be necessary. The ultrasound probe is placed in a sterile cover with ultrasound gel within the probe cover or sterile ultrasound gel placed over the joint space in order to obtain adequate quality images. To help determine the intended joint space, the following recommended sonographic criteria may be helpful: (1) anechoic or hypoechoic space; (2) no evidence of flow under color doppler or power doppler; (3) compressible space under direct probe pressure; (4) hyperechoic region deep to the space of interest indicating the cartilage; and (5) hyperechoic region relative to hyaline cartilage, indicating bone[7]. After verifying the site of interest, the ultrasound probe should be placed such that the aspirating needle will be directly visualized as it enters the intended fluid space. The needle is inserted into the space under direct observation and the fluid is aspirated with or without direct visualization.
TENDON AND ARTICULAR INJECTIONS
Rationale
Muscle and tendon injections are utilized for various musculoskeletal complaints. One common indication for tendon injections is tendinopathy. Tendinopathies affect over 500 000 people in the United States alone[9]. Efficacy and safety of injections for management of tendinopathies vary based on the affected site[10], with the most promising results in the treatment of first annular pulley tendinitis[11]. Conversely, injections at other sites including the Achilles tendon are controversial as some studies have shown potential adverse effects on biomechanical properties and incidences of tendon rupture[12,13]. Injections of articular surfaces of joints have been used as a therapy for arthritides and other inflammatory joint conditions. A brief summary of the clinical indications and contraindications are listed below in Table 1. The role of medication injections in the symptomatic and therapeutic treatment of musculoskeletal disease is beyond the scope of this review[14,15].
Table 1.
Ultrasound-guided tendon and articular injections: indications and contraindications
| Indications | Contraindications |
| Tendinopathy | Rash over injection site |
| Achilles tendinitis | |
| Trigger finger | |
| Carpal tunnel syndrome | |
| Lateral epicondylitis | |
| Rotator cuff tendinopathy | |
| Dequervain tenosynovitis | |
| Bursitis | Infection over injection site or obstructing injection path |
| Trochanteric bursitis | |
| Olecranon bursitis | |
| Tumor over injection site or obstructing injection path |
Injection of tendons and articular surfaces requires a thorough knowledge of the anatomy as well as a detailed physical examination to determine the optimal injection site and placement of the injection agent. Given the great number of anatomic structures surrounding tendons and articular surfaces, as well as the lack of true physical feedback during needle placement, ensuring safe and appropriate placement may be extremely difficult. The use of advanced imaging techniques such as magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound has allowed more precise visualization of these structures. Due to inherent limitations of real-time MRI and CT scanning for symptomatic injections of the musculoskeletal system, this approach seems to be less useful than sonography.
The use of ultrasound as a real-time imaging modality to directly visualize the needle placement into the tendon or articular surface is practical and safe. Evidence has demonstrated that ultrasound-guided tendon injection reduces pain both during and after the injection, decreases overall patient discomfort, and improves joint or muscle mobility more than traditional blind injections[16-19]. Furthermore, ultrasound-guided intra-articular injections enable the practitioner to localize fluid collections and perform simultaneous arthrocentesis[17] (Table 2).
Table 2.
Ultrasound-guided tissue biopsy: indications and contraindications
| Indications | Contraindications |
| Solitary bone lesion with indeterminate imaging characteristics | Infection on overlying site |
| New bone lesion in patient with known primary tumor | Rash on overlying site (relative) |
| Determine tumor recurrence | Uncorrected bleeding diathesis (relative) |
| Evaluate etiology of vertebral body compression fracture | Decreased platelet count (relative) |
| Determine infectious organism in chronic wound | Inaccessible site (relative) |
| Determine infectious organism in osteomyelitis |
TECHNIQUE OVERVIEW
Ultrasound-guided tendon or articular injection is performed under standard sterile precautions and appropriate preparation/draping of the site. The ultrasound probe of choice will be determined by the intended tendon or articular surface. In general, a good initial probe choice is the linear probe (10-15 MHz) which provides adequate visualization of most superficial structures/spaces. High-frequency transducers provide the best resolution for near-field tendons, although a curvilinear probe may be needed to visualize deep joint articular surfaces. The ultrasound probe is placed within a sterile cover with ultrasound gel within the cover or sterile ultrasound gel placed directly over the intended site. After procedure site and laterality are confirmed, the site is scanned in order to inspect regional anatomy and identify any nearby neuro-vascular structures. In the case of tendon injections, the muscle and tendon should be scanned throughout their course to determine the safest and most optimal injection site. For articular injections, the joint space should be scanned in all dimensions to determine the safest/optimal injection site. Whenever possible, the probe is placed so that the tendon is seen in longitudinal section, as a higher success rate for tendon injections has been noted in this view. Otherwise, a transverse section can be utilized[18]. The needle is inserted such that it is seen at all times and can be directly visualized entering the tendon or articular space. The authors recommend injection of the agent under direct visualization to prevent inadvertent application into the peri-tendinous or peri-articular structures. Representative ultrasound images can be seen in Figures 3 and 4.
Figure 3.
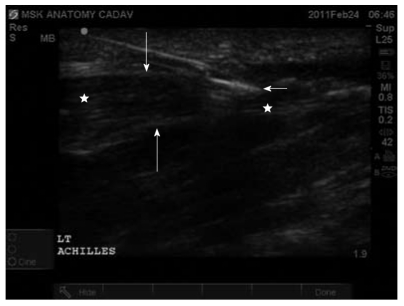
Percutaneous tenotomy or dry-needling can be performed under ultrasound-guidance to provide ideal visualization of the needle. Use of the short-axis plane should be to localize neighboring structures and visualize complete disruption of the tendon fibers. Use of the long-axis plane should be to confirm complete disruption of the tendon from anterior to posterior. However, the actual procedure should be performed within the short-axis plane as maintaining the needle in long-axis is difficult and unreliable to prevent neighboring structure damage. Confirmation of the structure as tendon fibers should rely on noting anisotropy which is characteristic of tendons. Short arrow indicates needle tip. Long arrows indicate Achilles tendon sheath. Stars indicate Achilles tendon fibers in long-axis plane.
Figure 4.
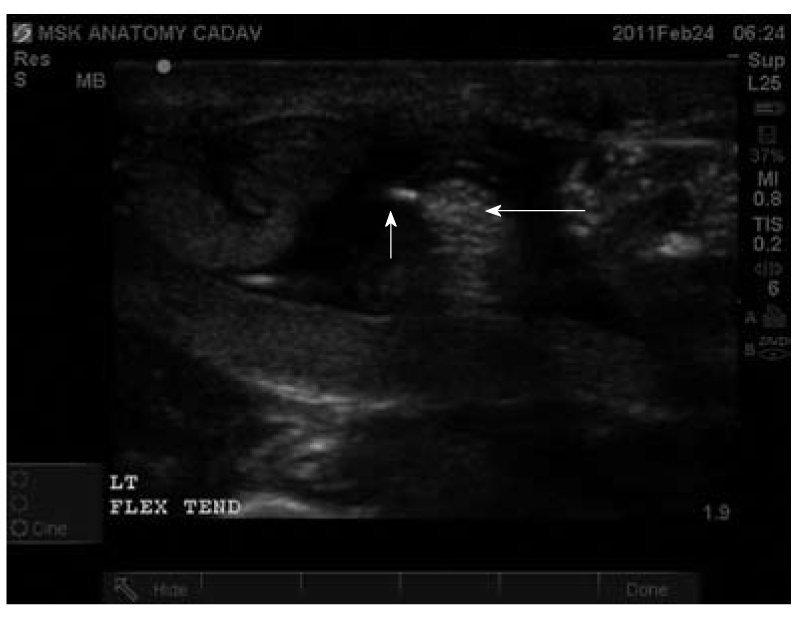
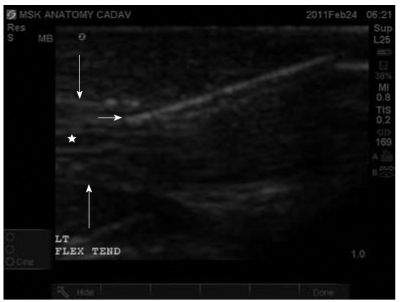
Ultrasound-guided injection of the left flexor tendon in transverse plane. Tendon injection under ultrasound (US)-guidance allows improved accuracy in tendon injection. Furthermore, US-guidance allows visualization of the fluid forming a complete peri-tendon fluid collection as noted by the hypoechoic space surrounding the heterogenous tendon appearance. Short arrow indicates the needle in transverse. Long arrow indicates flexor tendon in transverse section.
TISSUE BIOPSY
Rationale
In certain clinical situations, a diagnostic biopsy may be necessary before pursuing a more definitive treatment course. The ability to rapidly diagnose and initiate treatment may help improve outcomes. The advent of ultrasound-guided biopsies of the musculoskeletal system allows an appropriately trained clinician to readily obtain important diagnostic information in situations where a rapidly progressive disease process is beingconsidered. Furthermore, in situations where trained interventional radiologists are either unavailable or unable to perform timely biopsies, the ability of a general clinician to perform bedside biopsies may be invaluable in conserving medical resources. In the musculoskeletal system the differential diagnosis can include a large number of pathologies (i.e. primary bone tumors, bony tumor metastases, infections, and chronic inflammatory changes). A brief listing of clinical indications and contraindications is listed below in Table 3. More comprehensive discussion of this topic is beyond the scope of this review[20]. It should be noted that a suspected primary tumor of bone or soft tissue in the musculoskeletal system should only be biopsied by a physician trained in orthopaedic oncology. Also, biopsy performed by general clinicians or at the referring facility (rather than definitive treatment center) may increase both diatnostic errors and complication rates (i.e. need for wider tumor resection at time of surgery, skin complications requiring flap coverage, increased risk of amputation)[21].
Table 3.
Ultrasound-guided drainage and catheter insertion: indications and contraindications
| Indications | Contraindications |
| Undiagnosed soft tissue collection | Infection on overlying site |
| Cyst | |
| Abscess | |
| Hematoma | |
| Diagnosis of Abscess | Rash on overlying site (relative) |
| Obtain fluid for determination of causative organism | |
| Treatment of known abscess | Tumor on overlying site (relative) |
| Aspiration | |
| Placement of drainage catheter (if feasible) | |
| Aspiration of Cyst | |
| Ganglion cyst | |
| Synovial cyst | |
| Determination of causative organism for osteomyelitis |
Biopsy of the musculoskeletal system includes a broad grouping of procedures that may be divided into open and percutaneous procedures. While certain clinical scenarios preclude percutaneous biopsy and require an open procedure, percutaneous biopsy should be attempted, if possible and safe, to decrease patient discomfort and diatnostic costs[22]. Percutaneous biopsies can be further grouped by the type of imaging guidance used to aid the clinician performing the biopsy. Traditional percutaneous biopsy consists of utilizing physical exam findings and knowledge of anatomy to place the needle within the lesion of interest, a method utilized infrequently when the depth of the lesion is beyond a few centimeters of tissue. The availability of CT-guided and fluoroscopically-guided biopsies allows the clinician to perform highly accurate needle placement into lesions that are located near critical/sensitive (i.e. neuro-vascular) structures or in deeper locations[20]. Advances in ultrasound technology and clinical implementation have made ultrasound-guided musculoskeletal biopsies both feasible and accurate[23-28]. Ultrasound-guided needle and core biopsy sensitivities in obtaining the tissue of interest range from 80%-98.4%[23-28]. Core-needle biopsy has been demonstrated to have a higher sensitivity in obtaining diagnosis with estimated sensitivity of 81%-95% compared to 76%-80% for fine-needle aspiration[25-27,29]. Additionally, a method of creating a portal to enable forceps to perform a comprehensive biopsy of synovium has been described[30]. While there may be a perception that ultrasound is less facilitating when performing a diagnostic biopsy of bone lesions, evidence shows sampling accuracy for such lesions of 92%-98% for ultrasound compared to 87% for CT-guided biopsies of similar lesions[23,25]. Conventional biopsy performed under ultrasound-guidance relies on the echogenicity of the needle to localize it during the procedure - not always an easy task. Recent improvements may further aid the clinician in visualizing the needle. For example, biopsy needles are available that have been coated with echogenic surface markers (Teflon, etched tips, and an echogenic polymer) or feature a vibration system[29,31]. While there is limited data supporting the use of these types of needles, the use of polymer coated needles may be the most beneficial for technically difficult biopsies[31]. While further discussion on fine-needle aspiration vs core-needle biopsy in musculoskeletal lesions is beyond the scope of this review, it is important to note that the suspected etiology may dictate the type of percutaneous biopsy required Table 2.
Methods
Ultrasound-guided biopsy is performed under standard sterile conditions (i.e. appropriate procedural preparation and draping) over the intended biopsy site. Site and laterality verification is essential. The choice of the ultrasound probe, as described in previous sections, should be guided by the anatomic location of the tissue to be sampled. The linear probe (10-15 MHz) provides appropriate visualization of most superficial sites including joints, superficial muscles, and superficial bones. For sites located deeper, a curvilinear probe (5-10 MHz) may be required. The ultrasound gel is then utilized as needed throughout the course of the procedure.
The first step in performing an adequate tissue biopsy is to confirm the site of the lesion, bone or soft tissue. If the lesion is located within the bone then a larger (i.e. 14-gauge) cutting needle should be used to allow for bone fragments to be contained within the needle sample. If the lesion is located within the soft tissues then a smaller (18 or 20 gauge) needle is usually sufficient[22-28,31,32]. Local analgesia should be used generously for all biopsy procedures and should be performed along the entire anticipated biopsy tract (including periosteum and adjacent muscles) prior to initiation of the procedure. Sedation is not universally required, but may be needed for more extensive procedures and may help facilitate more accurate sampling and improve patient comfort. When utilized, sedation requires additional monitoring (i.e. frequent vital sign and pulse oxymetry assessments) and personnel (i.e. sedation nurse and/or anesthesiologist). When possible, the performance of biopsy under local anesthetic is preferred, with sedation used if the patient is unable to tolerate the pain and/or anxiety associated with the procedure.
Prior to the incision for the biopsy, a sonographic scan of the intended biopsy site should be performed to visualize all critical anatomic structures in the area. The optimal biopsy path should be determined based on avoidance of nearby vessels/nerves, and avoidance of muscles if possible. Again, when primary musculoskeletal malignancy is suspected, it is imperative that the biopsy tract be determined by an orthopaedic oncologist, as biopsy obtained via an improperly planned tract may be a factor in subsequent inability to perform limb salvage surgery[21]. Identification of vascular structures in the area of biopsy using color or power Doppler is encouraged[31]. Detailed recording of the lesion echogenicity, margins, mass size, relation to bone (cortical invasion), and vascularity is an essential part of pre-biopsy evaluation of the intended sampling site because procedural bleeding or even the very presence of a biopsy tract can distort critical sonographic characteristics of the lesion in question[25]. A small stab incision is then made in pre-marked skin and the biopsy needle is inserted into the lesion under direct sonographic visualization. Longitudinal orientation of the needle in relation to the ultrasound probe is preferred. Once the needle is confirmed to be within the lesion of interest, the biopsy is performed and the needle is removed with or without ultrasound visualization. A post-biopsy ultrasound scan of the region should be performed to confirm hemostasis of the sampled area. The biopsy specimen should then be handled according to established pathology guidelines regarding tissue/sample processing.
FLUID COLLECTION ASPIRATION AND DRAINAGE CATHETER INSERTION
Rationale
Tissue fluid collections are common in all areas of medicine. Therefore, practitioners in a variety of medical fields need to be aware of the relevant diagnostic and therapeutic considerations concerning tissue fluid collections. Within the realm of the musculoskeletal system, some specific subtypes of cysts and abscesses have been studied particularly closely. In general, the diagnosis of a fluid collection can be readily made using ultrasound, CT, or MRI. In many situations, the optimal therapy is either to aspirate the contents of the fluid collection or to place a drainage catheter for continuous drainage, depending on the precise character and/or size of the collection in question. A list of indications and contraindications for percutaneous aspiration or drainage catheter placement in the setting of tissue fluid collections is listed in Table 3. More comprehensive review of this topic is beyond the scope of this manuscript.
Ultrasound has been well described as a tool for diagnosis of tissue fluid collections as well as characterization of soft tissue infections[33-35]. A brief listing of types of soft tissue infections where ultrasound can be used for diagnosis can be seen in Table 4[33-35]. Additionally, ultrasound can be used to aid in the diagnosis of osteomyelitis, particularly in pediatric cases[36]. There is also evidence to support the use of ultrasound in the diagnosis and characterization of soft tissue cysts in the musculoskeletal system[37-39]. For example, the reported sensitivity and specificity for ultrasound in the diagnosis of meniscal cysts is 97% and 86%, respectively[37]. However, evidence on the use of ultrasound as a therapeutic aid in aspiration or drainage catheter insertion is still limited. Currently, the most common method to perform fluid collection characterization is by imaging, with aspiration of fluid for analysis in clinically uncertain scenarios.
Table 4.
Soft tissue infections identifiable on sonography
| Cellulitis |
| Necrotizing fasciitis |
| Infective bursitis |
| Infective tenosynovitis |
| Pyomyositis |
| Abscess |
| Hydatid or Tuberculous cysts |
| Septic arthritis |
| Post-operative infection |
| Foreign body |
The use of ultrasound as an image-guidance method in the setting of tissue fluid collections is a relatively new concept. Among musculoskeletal applications, there may be distinct advantages of ultrasound as an image-guidance tool. Firstly, ultrasound-guided aspiration has been identified as an effective method for treating both ganglionic and synovial cysts[40-43]. Given the evidence to support ultrasound as a diagnostic tool and the ease, cost, and lack of ionizing radiation exposure, the use of ultrasound-guidance in aspiration of these fluid collections should be considered as first-line therapy. Although a discussion of the optimal therapy for various ganglion and synovial cysts is beyond the scope of this review, there is some evidence to support the use of guided aspiration prior to or in lieu of surgical therapy[44]. For infectious indications, the use of ultrasound for both diagnostic and therapeutic purposes is also well described[45-52]. Ultrasound-guided aspiration or drainage catheter insertion has been successfully used to obtain fluid samples for microbial cultures as well as therapeutic drainage of collections[46-51]. There is also evidence supporing the use of ultrasound-guided techniques in the critically ill where transporting patients between different hospital locations may be either dangerous or not at all feasible[50]. In the case of multiple abscesses requiring drainage, the use of ultrasound guidance is further supported due to the ability to manipulate the probe rather than the patient, as well as the avoidance of excessive/additional exposure to ionizing radiation seen with CT-guidance[48]. Another potential application of ultrasound is the performance of tissue aspiration for cultures in the diagnosis of suspected osteomyelitis. While data are still limited, the current literature suggests that ultrasound guidance can be particularly helpful when obtaining tissue samples in suspected pediatric osteomyelitis[45].
Technique
Ultrasound-guided aspiration or drainage catheter insertion is performed under standard sterile conditions. Procedure site/laterality confirmation is essential. The ultrasound probe of choice is determined by the characteristics of the tissue in question. The linear probe (10-15 MHz) provides appropriate visualization of most soft tissue sites. For deeper lesions, a curvilinear probe (5-10 MHz) may be preferred/necessary. After placing the probe in a sterile cover and applying ultrasound gel, a brief scan through the site of interest should be performed prior to any definitive procedural intervention(s). Critical structures such as vessels and nerves should be identified. The fluid collection in question should be clearly identified and described with regards to the type (i.e. cyst), size, and overall characteristics (i.e. simple vs complex).
The ultrasound probe is then placed over the region of interest, with visualization of the needle passage in longitudinal section being preferred. The needle (typically 20- or 22-gauge) is inserted under direct sonographic visualization. Once the needle is within the fluid collection, an aspirate is obtained for analysis. If there is evidence of purulence or other signs of infection, then the placement of a drainage catheter should be considered. This involves the use of a larger needle with a guide-wire being inserted through the needle into the fluid space[50]. After removing the needle with the guide-wire left in place, a dilator is placed over the guide-wire and the insertion site is expanded to accommodate an appropriately-sized catheter[50]. The catheter is then inserted over the guide-wire and placement of the catheter within the fluid collection confirmed by direct vizualisation. After removing the guide-wire, the catheter is sutured in place and connected to an appropriate drainage system.
PERCUTANEOUS TENOTOMY
Rationale
Tenotomy is the complete or incomplete surgical division of a tendon for therapeutic purposes. The procedure has been described for a myriad of purposes with some of the original descriptions relating to the treatment of foot deformities[53]. The procedure can be performed using either an open or percutaneous method. The open version of the procedure was the first described and allows direct visualization of all para-tendon structures and the pathologic region of the tendon to confirm the diagnosis[53]. However, with the advent of percutaneous techniques, shorter procedural times and improved aesthetic outcomes became possible. Furthermore, there is evidence to suggest that percutaneous tenotomy is as safe and effective as the open procedure.It is notable that the extent of the tendinous portion divided in the muscle of interest appears directly correlated with increased postoperative mobility[54,55]. In animal studies, ultrasound-guided percutaneous tenotomy has been shown to increase complete tendon transection and decrease damage to surrounding structures compared to palpation-guided tenotomy (63). A brief listing of clinical indications and contraindications for percutaneous tenotomy are listed in Table 5. A comprehensive discussion of this topic is beyond the scope of this review[53-63].
Table 5.
Percutaneous ultrasound-guided tenotomy: indications and contraindications
| Indications | Contraindications |
| Chronic tendinosis refractory to conservative therapy | Infection on overlying site |
| Common extensor tendinosis | |
| Achilles tendinopathy | |
| Patellar tendinopathy | |
| Iliotibialis tendinopathy | |
| Trigger finger | |
| Symptomatic tendon release | Tumor on overlying site |
| Developmental dysplasia of the hip | |
| Spastic cerebral palsy | |
| Deformities of the foot | |
| Rash on overlying site (relative) |
Percutaneous tenotomy is ideally performed at the bedside or in an outpatient setting. The percutaneous approach was first described in the setting of tenotomy of the common extensor tendon for “tennis elbow” with symptomatic improvement equivalent to other surgical procedures[64,65]. The procedure has since been implemented for a variety of tendinopathies. While the initial descriptions of percutaneous tenotomy involved blind palpation and determination of the site using anatomic landmarks alone, recent advances in imaging modalities have significantly enhanced the anatomic accuracy of tenotomy procedures. Although CT- and MRI-guided tenotomy is possible, the literature focuses heavily on ultrasound-guidance for percutaneous procedures. Although MRI and other advanced imaging modalities offer an accurate method of diagnosing tendinopathy and/or other tendon abnormalities, the reported sensitivity of ultrasound in tendinopathies of 67%-100% is sufficient to recommend it as a screening exam based, given cost and time requirements compared to the other imaging modalities[66,67]. There is also evidence from animal models that, compared to surface anatomy/palpation-based techniques, ultrasound-guided tenotomy may be more accurate, faster, and associated with less morbidity[68].
Technique
Ultrasound-guided percutaneous tenotomy is performed under full sterile precautions and standard draping over the site of interest. Procedural site/laterality confirmation is essential. The ultrasound probe of choice will be determined by anatomic considerations. Most practitioners choose a high frequency (10-15 MHz) linear probe when approaching most superficial structures. If the tendon of interest is located deeper or cannot be visualized anterior to bone or cartilage, then a lower frequency (5-10 MHz) curvilinear prove may be more appropriate. Using a sterile ultrasound cover and gel, a brief scan of the site of interest should be performed prior to any invasive intervention(s). In addition to identifying any important neuro-vascular structures, the preliminary scan may help better characterize the region of interest for the tenotomy. Typical findings of tendinopathy on ultrasound include hypoechoic or anechoic regions within a tendon. Calcifications may appear as hyperechoic regions with clean shadows deep to the region, and tenderness to transducer pressure over the affected area may be present[66,67]. Most ultrasound machines can also facilitate color or power Doppler imaging to evaluate for vascularity of the region and help guide the procedure to minimize the potential for bleeding.
Although there are different ways to perform a tenotomy, we will focus on techniques that use ultrasound-guidance. Specifically, we will discuss partial tenotomy using a needle (needling) and complete tenotomy using a scalpel[56-61]. The anatomic region of interest is first injected with local anesthetic. There is currently no evidence to support the routine use of general anesthesia for this procedure[56-61]. Subsequent to achieving adequate analgesia, the ultrasound probe is positioned parallel to the tendon of interest in order to help guide the procedure, preferably in the longitudinal view.
Needle-based percutaneous tenotomy is performed by using a narrow (20- or 22-gauge) needle, which is inserted under direct ultrasound guidance and penetrates the abnormal tendon region while avoiding neighboring structures. Any calcifications are disrupted during needle passes through the region. This is repeated under direct ultrasound visualization until the entire tendon has been disrupted by the needle.
Section tenotomy using scalpel is performed using a number 11 blade scalpel. The scalpel is inserted parallel to the tendon fibers under direct ultrasound visualization and penetrates the fibers[57,58]. The cutting edge of the blade is initially pointed proximally on the tendon[57,58]. The joint is then passively flexed and extended under visualization. The scalpel is then withdrawn and rotated so that the cutting edge is pointed distally on the tendon. Joint flexion and extension is then repeated[57,58]. This produces a disruption of a single region within the tendon fibers. The procedure is then repeated by angling the scalpel so that the blade penetrates a series of tendon fibers lateral to the original incision. This is then repeated until the tendon is completely disrupted along the region of interest. The skin incision should be minimized to a single entry point, thus decreasing the chance of any additional tissue injury. A representative sonographic example of tenotomy can be seen in Figure 5.
Figure 5.
Tendon injection can be performed in either transverse or longitudinal planes. Insertion of the needle in long-axis allows excellent visualization of the tendon sheath and tendon fibers. Prior to injection the needle tip should be well-visualized between the tendon sheath and the tendon fibers. An important method to ensure needle visualization is to angle the probe to make the angle of ultrasound wave as close to perpendicular as possible. Additionally, confirmation of a structure as a tendon involves angling the probe along the tendon to note tendon anisotropy characteristic of a tendon and not noted in nerves or vessels. Short arrow indicates needle tip in long-axis section. Long arrows indicate tendon sheath. Star indicates tendon fibers in long-axis section.
OTHER POTENTIAL USES
Foreign body removal
Ultrasound has been utilized in diagnosis and treatment of various types of foreign bodies within the soft tissue including wood, plastic, and other radiolucent objects[69-72]. There is evidence to support the use of ultrasound as a screening tool for foreign bodies and identification of critical neighboring structures that may present difficulty during the removal the object in question. Compared to imaging techniques such as plain radiography or computed tomography, modern ultrasound equipment is capable of rapidly producing a 3-dimensional image of the area in question and allows the physician to quickly and efficiently plan a surgical or percutaneous removal of the foreign object. In situations where the exact nature of the foreign object is unknown, imaging methods such as MRI may be contraindicated due to the migration risk of metallic objects. Although the evidence is still limited, the use of ultrasound guidance during the removal of foreign bodies should be considered in appropriately selected cases[69-73].
CONCLUSION
The ultimate goal of all image-guided procedures is to maximize patient safety, improve procedural accuracy, and optimize clinical outcomes. In addition to facilitating these objectives, ultrasound-guidance also offers the benefit of eliminating ionizing radiation exposure during procedures. Ultrasound-guided musculoskeletal procedures described in this review demonstrate the growing trend of using ultrasound as first-line modality in selected bedside musculoskeletal applications, among both specialist and generalist physicians. While additional information is needed to refine the utilization of ultrasound-guided bedside musculoskeletal procedures, there is sufficient evidence to support their increasing use in everyday clinical practice, as outlined in this review. The authors emphasize the need for adequate training, accreditation, and maintenance of skills among those who perform ultrasound-based procedures described herein, regardless of their specialty.
Footnotes
Peer reviewer: Nahum Rosenberg, MD, Orthopaedics A, Rambam Medical Center, POB 9602, Haifa 31096, Israel
S- Editor Sun H L- Editor Hughes D E- Editor Zheng XM
References
- 1.Bianchi S, Zamorani MP. US-guided interventional procedures. In: Bianchi S, Martinoli C, editors. Ultrasound of the musculoskeletal system. Berlin: Springer-Verlag; 2007. pp. 891–917. [Google Scholar]
- 2.Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25:448–452. doi: 10.1007/s11606-010-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30:966–971. [PubMed] [Google Scholar]
- 4.Delaunoy I, Feipel V, Appelboom T, Hauzeur JP. Sonography detection threshold for knee effusion. Clin Rheumatol. 2003;22:391–392. doi: 10.1007/s10067-003-0759-5. [DOI] [PubMed] [Google Scholar]
- 5.Weybright PN, Jacobson JA, Murry KH, Lin J, Fessell DP, Jamadar DA, Kabeto M, Hayes CW. Limited effectiveness of sonography in revealing hip joint effusion: preliminary results in 21 adult patients with native and postoperative hips. AJR Am J Roentgenol. 2003;181:215–218. doi: 10.2214/ajr.181.1.1810215. [DOI] [PubMed] [Google Scholar]
- 6.Wiler JL, Costantino TG, Filippone L, Satz W. Comparison of ultrasound-guided and standard landmark techniques for knee arthrocentesis. J Emerg Med. 2010;39:76–82. doi: 10.1016/j.jemermed.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002;29:2209–2213. [PubMed] [Google Scholar]
- 8.Raza K, Lee CY, Pilling D, Heaton S, Situnayake RD, Carruthers DM, Buckley CD, Gordon C, Salmon M. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology (Oxford) 2003;42:976–979. doi: 10.1093/rheumatology/keg269. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Petersen M, Cameron L. Prevalence and risk factors of tendinitis and related disorders of the distal upper extremity among U.S. workers: comparison to carpal tunnel syndrome. Am J Ind Med. 2001;39:328–335. doi: 10.1002/1097-0274(200103)39:3<328::aid-ajim1021>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–1767. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 11.Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14:722–727. doi: 10.1016/0363-5023(89)90199-8. [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe D, Achten J, Costa ML. Glucocorticoid injections in lesions of the achilles tendon. Foot Ankle Int. 2009;30:661–665. doi: 10.3113/FAI.2009.0661. [DOI] [PubMed] [Google Scholar]
- 13.Hugate R, Pennypacker J, Saunders M, Juliano P. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86-A:794–801. doi: 10.2106/00004623-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Inês LP, da Silva JA. Soft tissue injections. Best Pract Res Clin Rheumatol. 2005;19:503–527. doi: 10.1016/j.berh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Fredberg U. Local corticosteroid injection in sport: review of literature and guidelines for treatment. Scand J Med Sci Sports. 1997;7:131–139. doi: 10.1111/j.1600-0838.1997.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 16.Naredo E, Cabero F, Beneyto P, Cruz A, Mondéjar B, Uson J, Palop MJ, Crespo M. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31:308–314. [PubMed] [Google Scholar]
- 17.Sibbitt WL, Peisajovich A, Michael AA, Park KS, Sibbitt RR, Band PA, Bankhurst AD. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892–1902. doi: 10.3899/jrheum.090013. [DOI] [PubMed] [Google Scholar]
- 18.Luz KR, Furtado RN, Nunes CC, Rosenfeld A, Fernandes AR, Natour J. Ultrasound-guided intra-articular injections in the wrist in patients with rheumatoid arthritis: a double-blind, randomised controlled study. Ann Rheum Dis. 2008;67:1198–1200. doi: 10.1136/ard.2007.084616. [DOI] [PubMed] [Google Scholar]
- 19.Smith J, Finnoff JT, Santaella-Sante B, Henning T, Levy BA, Lai JK. Sonographically guided popliteus tendon sheath injection: techniques and accuracy. J Ultrasound Med. 2010;29:775–782. doi: 10.7863/jum.2010.29.5.775. [DOI] [PubMed] [Google Scholar]
- 20.Gogna A, Peh WC, Munk PL. Image-guided musculoskeletal biopsy. Radiol Clin North Am. 2008;46:455–473, v. doi: 10.1016/j.rcl.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Ward WG, Kilpatrick S. Fine needle aspiration biopsy of primary bone tumors. Clin Orthop Relat Res. 2000:80–87. doi: 10.1097/00003086-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Saifuddin A, Mitchell R, Burnett SJ, Sandison A, Pringle JA. Ultrasound-guided needle biopsy of primary bone tumours. J Bone Joint Surg Br. 2000;82:50–54. doi: 10.1302/0301-620x.82b1.10141. [DOI] [PubMed] [Google Scholar]
- 24.Konermann W, Wuisman P, Ellermann A, Gruber G. Ultrasonographically guided needle biopsy of benign and malignant soft tissue and bone tumors. J Ultrasound Med. 2000;19:465–471. doi: 10.7863/jum.2000.19.7.465. [DOI] [PubMed] [Google Scholar]
- 25.Gil-Sánchez S, Marco-Doménech SF, Irurzun-López J, Fernández-García P, de la Iglesia-Cardeña P, Ambit-Capdevila S. Ultrasound-guided skeletal biopsies. Skeletal Radiol. 2001;30:615–619. doi: 10.1007/s002560100417. [DOI] [PubMed] [Google Scholar]
- 26.Torriani M, Etchebehere M, Amstalden E. Sonographically guided core needle biopsy of bone and soft tissue tumors. J Ultrasound Med. 2002;21:275–281. doi: 10.7863/jum.2002.21.3.275. [DOI] [PubMed] [Google Scholar]
- 27.Yeow KM, Tan CF, Chen JS, Hsueh C. Diagnostic sensitivity of ultrasound-guided needle biopsy in soft tissue masses about superficial bone lesions. J Ultrasound Med. 2000;19:849–855. doi: 10.7863/jum.2000.19.12.849. [DOI] [PubMed] [Google Scholar]
- 28.Rubens DJ, Fultz PJ, Gottlieb RH, Rubin SJ. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med. 1997;16:831–842. doi: 10.7863/jum.1997.16.12.831. [DOI] [PubMed] [Google Scholar]
- 29.Tikkakoski T, Päivänsalo M, Siniluoto T, Hiltunen S, Typpö T, Jartti P, Apaja-Sarkkinen M. Percutaneous ultrasound-guided biopsy. Fine needle biopsy, cutting needle biopsy, or both? Acta Radiol. 1993;34:30–34. [PubMed] [Google Scholar]
- 30.Koski JM, Helle M. Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis. 2005;64:926–929. doi: 10.1136/ard.2004.027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jandzinski DI, Carson N, Davis D, Rubens DJ, Voci SL, Gottlieb RH. Treated needles: do they facilitate sonographically guided biopsies? J Ultrasound Med. 2003;22:1233–1237. doi: 10.7863/jum.2003.22.11.1233. [DOI] [PubMed] [Google Scholar]
- 32.Jones CD, McGahan JP, Clark KJ. Color Doppler ultrasonographic detection of a vibrating needle system. J Ultrasound Med. 1997;16:269–274. doi: 10.7863/jum.1997.16.4.269. [DOI] [PubMed] [Google Scholar]
- 33.Chau CL, Griffith JF. Musculoskeletal infections: ultrasound appearances. Clin Radiol. 2005;60:149–159. doi: 10.1016/j.crad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Koc Z, Ağildere AM, Yalcin O, Pourbagher A, Pourbagher M. Primary hydatid cyst in the anterior thigh: Sonographic findings. J Clin Ultrasound. 2004;32:358–360. doi: 10.1002/jcu.20044. [DOI] [PubMed] [Google Scholar]
- 35.Vastyan AM, MacKinnon EA. Primary psoas abscess in a neonate. Am J Perinatol. 2006;23:253–254. doi: 10.1055/s-2006-939532. [DOI] [PubMed] [Google Scholar]
- 36.Chao HC, Lin SJ, Huang YC, Lin TY. Color Doppler ultrasonographic evaluation of osteomyelitis in children. J Ultrasound Med. 1999;18:729–734; quiz 735-736. doi: 10.7863/jum.1999.18.11.729. [DOI] [PubMed] [Google Scholar]
- 37.Rutten MJ, Collins JM, van Kampen A, Jager GJ. Meniscal cysts: detection with high-resolution sonography. AJR Am J Roentgenol. 1998;171:491–496. doi: 10.2214/ajr.171.2.9694482. [DOI] [PubMed] [Google Scholar]
- 38.Ward EE, Jacobson JA, Fessell DP, Hayes CW, van Holsbeeck M. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol. 2001;176:373–380. doi: 10.2214/ajr.176.2.1760373. [DOI] [PubMed] [Google Scholar]
- 39.Seymour R, Lloyd DC. Sonographic appearances of meniscal cysts. J Clin Ultrasound. 1998;26:15–20. doi: 10.1002/(sici)1097-0096(199801)26:1<15::aid-jcu4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Chiou HJ, Chou YH, Wu JJ, Hsu CC, Tiu CM, Chang CY, Yu C. Alternative and effective treatment of shoulder ganglion cyst: ultrasonographically guided aspiration. J Ultrasound Med. 1999;18:531–535. doi: 10.7863/jum.1999.18.8.531. [DOI] [PubMed] [Google Scholar]
- 41.DeFriend DE, Schranz PJ, Silver DA. Ultrasound-guided aspiration of posterior cruciate ligament ganglion cysts. Skeletal Radiol. 2001;30:411–414. doi: 10.1007/s002560100374. [DOI] [PubMed] [Google Scholar]
- 42.Nakamichi K, Tachibana S. Ganglion-associated ulnar tunnel syndrome treated by ultrasonographically assisted aspiration and splinting. J Hand Surg Br. 2003;28:177–178. doi: 10.1016/s0266-7681(02)00308-x. [DOI] [PubMed] [Google Scholar]
- 43.Macmahon PJ, Brennan DD, Duke D, Forde S, Eustace SJ. Ultrasound-guided percutaneous drainage of meniscal cysts: preliminary clinical experience. Clin Radiol. 2007;62:683–687. doi: 10.1016/j.crad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Stephen AB, Lyons AR, Davis TR. A prospective study of two conservative treatments for ganglia of the wrist. J Hand Surg Br. 1999;24:104–105. doi: 10.1016/s0266-7681(99)90051-7. [DOI] [PubMed] [Google Scholar]
- 45.Azam Q, Ahmad I, Abbas M, Syed A, Haque F. Ultrasound and colour Doppler sonography in acute osteomyelitis in children. Acta Orthop Belg. 2005;71:590–596. [PubMed] [Google Scholar]
- 46.Ormeci N, Idilman R, Akyar S, Palabiyikoğlu M, Coban S, Erdem H, Ekiz F. Hydatid cysts in muscle: a modified percutaneous treatment approach. Int J Infect Dis. 2007;11:204–208. doi: 10.1016/j.ijid.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Dib M, Bedu A, Garel C, Mazda K, Philippe-Chomette P, Rajguru M, Hassan M, Aujard Y. Ilio-psoas abscess in neonates: treatment by ultrasound-guided percutaneous drainage. Pediatr Radiol. 2000;30:677–680. doi: 10.1007/s002470000309. [DOI] [PubMed] [Google Scholar]
- 48.Heneghan JP, Everts RJ, Nelson RC. Multiple fluid collections: CT- or US-guided aspiration--evaluation of microbiologic results and implications for clinical practice. Radiology. 1999;212:669–672. doi: 10.1148/radiology.212.3.r99se25669. [DOI] [PubMed] [Google Scholar]
- 49.Ohara N, Tominaga O, Uchiyama M, Nakano H, Muto T. Primary iliopsoas abscess successfully treated by ultrasonographically guided percutaneous drainage. J Orthop Sci. 1998;3:221–224. doi: 10.1007/s007760050046. [DOI] [PubMed] [Google Scholar]
- 50.Crass JR, Karl R. Bedside drainage of abscesses with sonographic guidance in the desperately ill. AJR Am J Roentgenol. 1982;139:183–185. doi: 10.2214/ajr.139.1.183. [DOI] [PubMed] [Google Scholar]
- 51.Dinç H, Onder C, Turhan AU, Sari A, Aydin A, Yuluğ G, Gümele HR. Percutaneous catheter drainage of tuberculous and nontuberculous psoas abscesses. Eur J Radiol. 1996;23:130–134. doi: 10.1016/0720-048x(96)01045-5. [DOI] [PubMed] [Google Scholar]
- 52.Chao HC, Lin SJ, Huang YC, Lin TY. Sonographic evaluation of cellulitis in children. J Ultrasound Med. 2000;19:743–749. doi: 10.7863/jum.2000.19.11.743. [DOI] [PubMed] [Google Scholar]
- 53.Barwell R On Certain Grave Evils attending Tenotomy and on a new mode of curing Deformities of the Foot. Med Chir Trans 1862; 45: 25-41. doi: 10.1177/095952876204500103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Hage S, Rachkidi R, Noun Z, Haidar R, Dagher F, Kharrat K, Ghanem I. Is percutaneous adductor tenotomy as effective and safe as the open procedure? J Pediatr Orthop. 2010;30:485–488. doi: 10.1097/BPO.0b013e3181df619d. [DOI] [PubMed] [Google Scholar]
- 55.Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86:701–704. doi: 10.1302/0301-620x.86b5.14469. [DOI] [PubMed] [Google Scholar]
- 56.Rajeswaran G, Lee JC, Eckersley R, Katsarma E, Healy JC. Ultrasound-guided percutaneous release of the annular pulley in trigger digit. Eur Radiol. 2009;19:2232–2237. doi: 10.1007/s00330-009-1397-3. [DOI] [PubMed] [Google Scholar]
- 57.Testa V, Capasso G, Maffulli N, Bifulco G. Ultrasound-guided percutaneous longitudinal tenotomy for the management of patellar tendinopathy. Med Sci Sports Exerc. 1999;31:1509–1515. doi: 10.1097/00005768-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34:573–580. doi: 10.1097/00005768-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 59.McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281–1289. doi: 10.7863/jum.2006.25.10.1281. [DOI] [PubMed] [Google Scholar]
- 60.McShane JM, Shah VN, Nazarian LN. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow: is a corticosteroid necessary? J Ultrasound Med. 2008;27:1137–1144. doi: 10.7863/jum.2008.27.8.1137. [DOI] [PubMed] [Google Scholar]
- 61.Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187–1192. doi: 10.7863/jum.2009.28.9.1187. [DOI] [PubMed] [Google Scholar]
- 62.Tamir E, McLaren AM, Gadgil A, Daniels TR. Outpatient percutaneous flexor tenotomies for management of diabetic claw toe deformities with ulcers: a preliminary report. Can J Surg. 2008;51:41–44. [PMC free article] [PubMed] [Google Scholar]
- 63.Lakhey S, Mansfield M, Pradhan RL, Rijal KP, Paney BP, Manandhar RR. Percutaneous extensor tenotomy for chronic tennis elbow using an 18G needle. Kathmandu Univ Med J (KUMJ) 2007;5:446–448. [PubMed] [Google Scholar]
- 64.Rosen MJ, Duffy FP, Miller EH, Kremchek EJ. Tennis elbow syndrome: results of the ”lateral release” procedure. Ohio State Med J. 1980;76:103–109. [PubMed] [Google Scholar]
- 65.Yerger B, Turner T. Percutaneous extensor tenotomy for chronic tennis elbow: an office procedure. Orthopedics. 1985;8:1261–1263. doi: 10.3928/0147-7447-19851001-11. [DOI] [PubMed] [Google Scholar]
- 66.Tran N, Chow K. Ultrasonography of the elbow. Semin Musculoskelet Radiol. 2007;11:105–116. doi: 10.1055/s-2007-1001876. [DOI] [PubMed] [Google Scholar]
- 67.Stewart B, Harish S, Oomen G, Wainman B, Popowich T, Moro JK. Sonography of the lateral ulnar collateral ligament of the elbow: study of cadavers and healthy volunteers. AJR Am J Roentgenol. 2009;193:1615–1619. doi: 10.2214/AJR.09.2812. [DOI] [PubMed] [Google Scholar]
- 68.Esterline ML, Armbrust L, Roush JK. A comparison of palpation guided and ultrasound guided percutaneous biceps brachii tenotomy in dogs. Vet Comp Orthop Traumatol. 2005;18:135–139. [PubMed] [Google Scholar]
- 69.Bray PW, Mahoney JL, Campbell JP. Sensitivity and specificity of ultrasound in the diagnosis of foreign bodies in the hand. J Hand Surg Am. 1995;20:661–666. doi: 10.1016/S0363-5023(05)80287-4. [DOI] [PubMed] [Google Scholar]
- 70.Hill R, Conron R, Greissinger P, Heller M. Ultrasound for the detection of foreign bodies in human tissue. Ann Emerg Med. 1997;29:353–356. doi: 10.1016/s0196-0644(97)70347-0. [DOI] [PubMed] [Google Scholar]
- 71.Crystal CS, Masneri DA, Hellums JS, Kaylor DW, Young SE, Miller MA, Levsky ME. Bedside ultrasound for the detection of soft tissue foreign bodies: a cadaveric study. J Emerg Med. 2009;36:377–380. doi: 10.1016/j.jemermed.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 72.Young AS, Shiels WE, Murakami JW, Coley BD, Hogan MJ. Self-embedding behavior: radiologic management of self-inserted soft-tissue foreign bodies. Radiology. 2010;257:233–239. doi: 10.1148/radiol.10091566. [DOI] [PubMed] [Google Scholar]
- 73.Blyme PJ, Lind T, Schantz K, Lavard P. Ultrasonographic detection of foreign bodies in soft tissue. A human cadaver study. Arch Orthop Trauma Surg. 1990;110:24–25. doi: 10.1007/BF00431361. [DOI] [PubMed] [Google Scholar]