Abstract
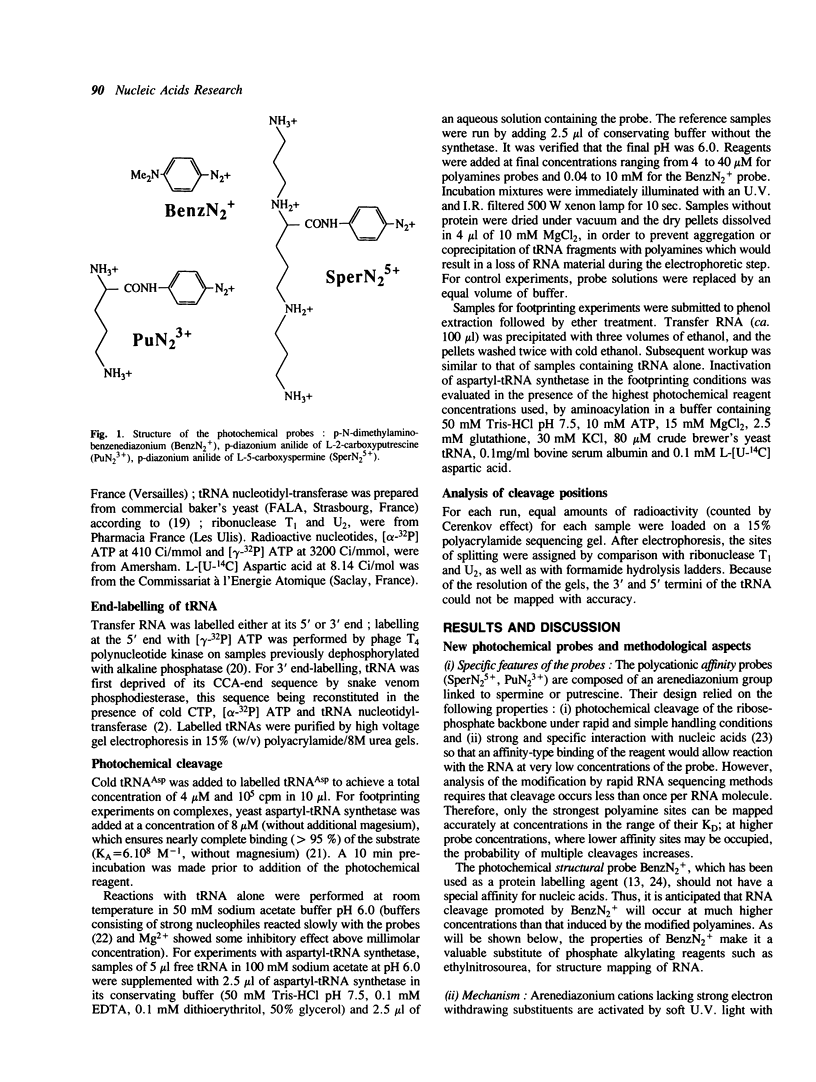
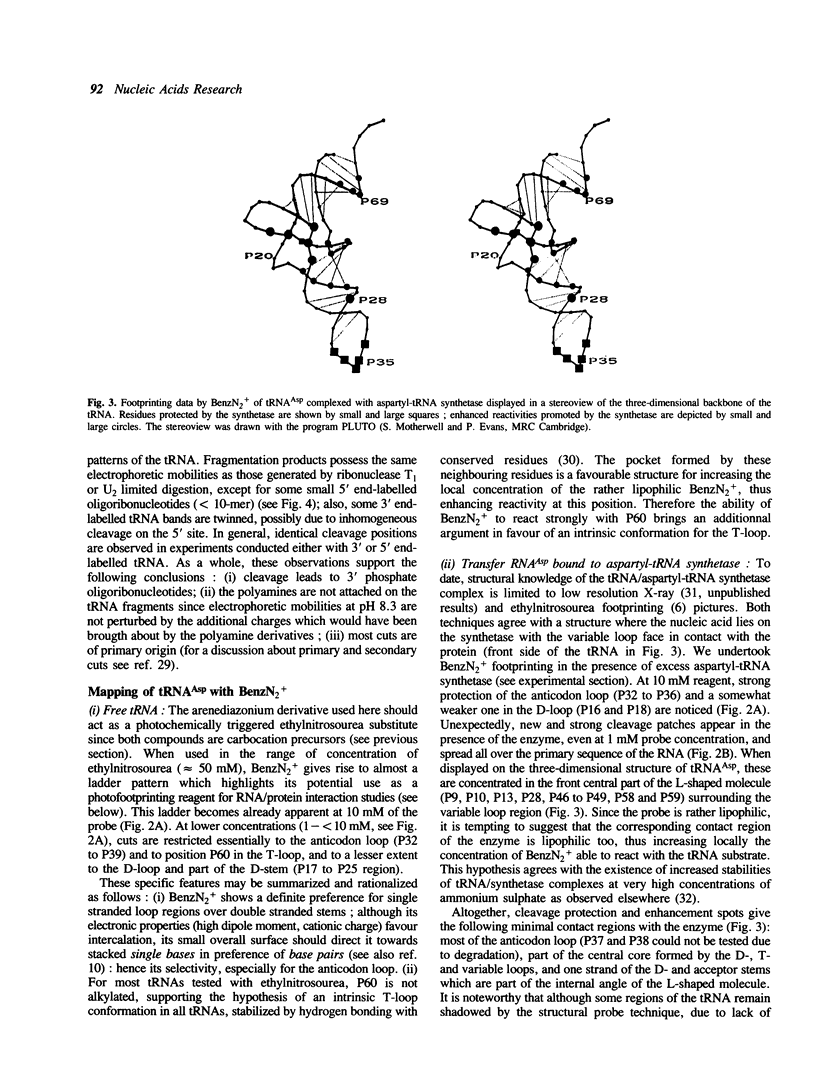
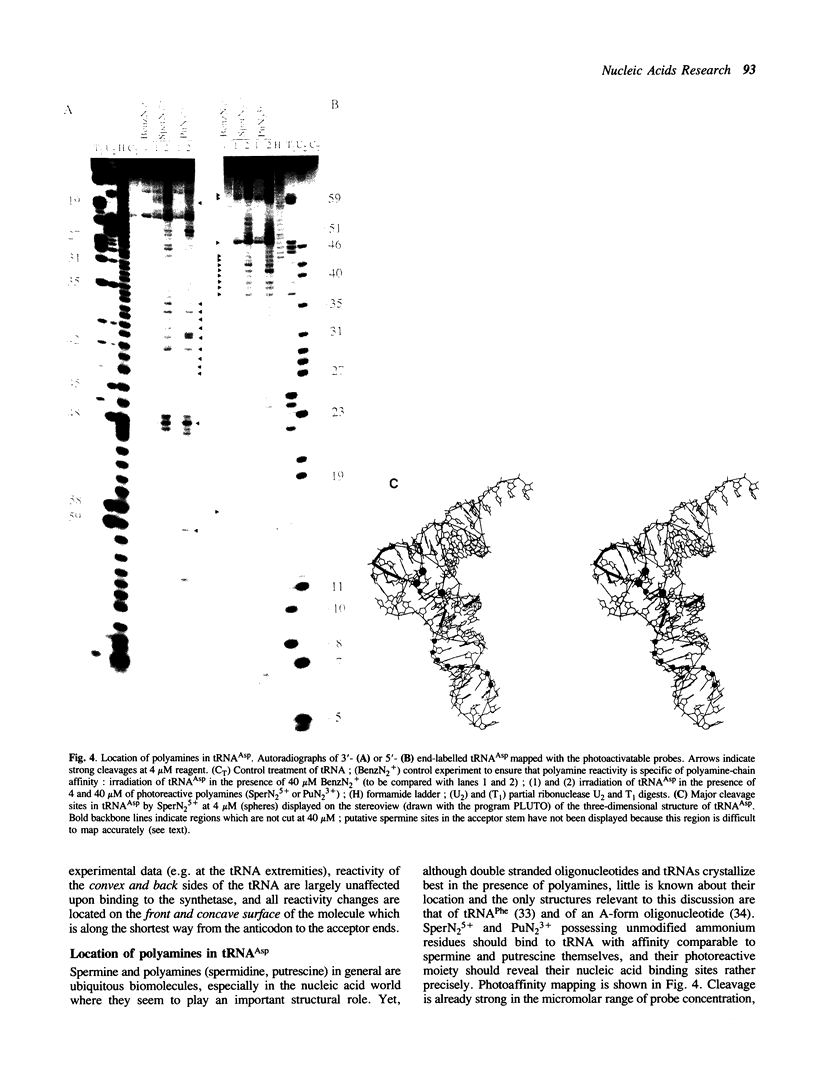
Aryldiazonium salts are shown to be useful as phototriggered structural probes for RNA mapping as well as for footprinting of RNA/protein interaction. In particular the yeast tRNA(Asp)/aspartyl-tRNA synthetase complex is shown to involve the variable loop face and the concave side of the L-shaped nucleic acid bound to a lipophilic area of the enzyme. When chemically linked to spermine, the photoactive group cleaves RNA at polyamine binding sites; 3-4 spermines have been located in the tRNA(Asp), stabilizing the central part of the molecule in regions where two ribose-phosphate strands are close to each other.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnet J., Ebel J. P. Influence of various factors on the recognition specificity of tRNAs by yeast valyl-tRNA synthetase. Eur J Biochem. 1975 Oct 1;58(1):193–201. doi: 10.1111/j.1432-1033.1975.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Anderson J. E., Harrison S. C., Ptashne M. Ethylation interference and X-ray crystallography identify similar interactions between 434 repressor and operator. Nature. 1985 Aug 15;316(6029):651–653. doi: 10.1038/316651a0. [DOI] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P. Fractionnement des tRNA de levure de bière par distribution en contre-courant. Bull Soc Chim Biol (Paris) 1967;49(12):1679–1687. [PubMed] [Google Scholar]
- Ehresmann C., Baudin F., Mougel M., Romby P., Ebel J. P., Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987 Nov 25;15(22):9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorova O. O., Fasiolo F., Keith G., Vassilenko S. K., Ebel J. P. Partial digestion of tRNA--aminoacyl-tRNA synthetase complexes with cobra venom ribonuclease. Biochemistry. 1981 Feb 17;20(4):1006–1011. doi: 10.1021/bi00507a055. [DOI] [PubMed] [Google Scholar]
- Giegé R., Lorber B., Ebel J. P., Moras D., Thierry J. C., Jacrot B., Zaccai G. Formation of a catalytically active complex between tRNAAsp and aspartyl-tRNA synthetase from yeast in high concentrations of ammonium sulphate. Biochimie. 1982 May;64(5):357–362. doi: 10.1016/s0300-9084(82)80440-9. [DOI] [PubMed] [Google Scholar]
- Goeldner M. P., Hirth C. G. Specific photoaffinity labeling induced by energy transfer: application to irreversible inhibition of acetylcholinesterase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6439–6442. doi: 10.1073/pnas.77.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Zon G., Sundaralingam M. Base only binding of spermine in the deep groove of the A-DNA octamer d(GTGTACAC). Biochemistry. 1989 Mar 21;28(6):2360–2364. doi: 10.1021/bi00432a002. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Buc H., Spassky A., Wang J. C. Mapping of single-stranded regions in duplex DNA at the sequence level: single-strand-specific cytosine methylation in RNA polymerase-promoter complexes. Proc Natl Acad Sci U S A. 1983 May;80(9):2544–2548. doi: 10.1073/pnas.80.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B., Kern D., Dietrich A., Gangloff J., Ebel J. P., Giegé R. Large scale purification and structural properties of yeast aspartyl-tRNA synthetase. Biochem Biophys Res Commun. 1983 Nov 30;117(1):259–267. doi: 10.1016/0006-291x(83)91569-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moras D., Comarmond M. B., Fischer J., Weiss R., Thierry J. C., Ebel J. P., Giegé R. Crystal structure of yeast tRNAAsp. Nature. 1980 Dec 25;288(5792):669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Blankenship J. W., Matthews H. R. Association constants for the interaction of double-stranded and single-stranded DNA with spermine, spermidine, putrescine, diaminopropane, N1- and N8-acetylspermidine, and magnesium: determination from analysis of the broadening of thermal denaturation curves. Arch Biochem Biophys. 1986 Apr;246(1):225–232. doi: 10.1016/0003-9861(86)90467-4. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podjarny A., Rees B., Thierry J. C., Cavarelli J., Jésior J. C., Roth M., Lewitt-Bentley A., Kahn R., Lorber B., Ebel J. P. Yeast tRNA(Asp)-aspartyl-tRNA synthetase complex: low resolution crystal structure. J Biomol Struct Dyn. 1987 Oct;5(2):187–198. doi: 10.1080/07391102.1987.10506389. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rether B., Bonnet J., Ebel J. P. Studies on tRNA nucleotidyltransferase from baker's yeast. 1. Purification of the enzyme. Protection against thermal inactivation and inhibition by several substrates. Eur J Biochem. 1974 Dec 16;50(1):281–288. doi: 10.1111/j.1432-1033.1974.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Romby P., Carbon P., Westhof E., Ehresmann C., Ebel J. P., Ehresmann B., Giegé R. Importance of conserved residues for the conformation of the T-loop in tRNAs. J Biomol Struct Dyn. 1987 Dec;5(3):669–687. doi: 10.1080/07391102.1987.10506419. [DOI] [PubMed] [Google Scholar]
- Romby P., Moras D., Bergdoll M., Dumas P., Vlassov V. V., Westhof E., Ebel J. P., Giegé R. Yeast tRNAAsp tertiary structure in solution and areas of interaction of the tRNA with aspartyl-tRNA synthetase. A comparative study of the yeast phenylalanine system by phosphate alkylation experiments with ethylnitrosourea. J Mol Biol. 1985 Aug 5;184(3):455–471. doi: 10.1016/0022-2836(85)90294-3. [DOI] [PubMed] [Google Scholar]
- Romby P., Moras D., Dumas P., Ebel J. P., Giegé R. Comparison of the tertiary structure of yeast tRNA(Asp) and tRNA(Phe) in solution. Chemical modification study of the bases. J Mol Biol. 1987 May 5;195(1):193–204. doi: 10.1016/0022-2836(87)90336-6. [DOI] [PubMed] [Google Scholar]
- Ruff M., Cavarelli J., Mikol V., Lorber B., Mitschler A., Giege R., Thierry J. C., Moras D. A high resolution diffracting crystal form of the complex between yeast tRNAAsp and aspartyl-tRNA synthetase. J Mol Biol. 1988 May 5;201(1):235–236. doi: 10.1016/0022-2836(88)90450-0. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Effects of polyamines on the structure and reactivity of tRNA. Prog Nucleic Acid Res Mol Biol. 1976;17:15–42. doi: 10.1016/s0079-6603(08)60064-1. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Onishi T. Binding of transfer RNA to polyamines in preference to Mg-2+. Biochem Biophys Res Commun. 1975 Apr 7;63(3):611–617. doi: 10.1016/s0006-291x(75)80428-1. [DOI] [PubMed] [Google Scholar]
- Theobald A., Springer M., Grunberg-Manago M., Ebel J. P., Giege R. Tertiary structure of Escherichia coli tRNA(3Thr) in solution and interaction of this tRNA with the cognate threonyl-tRNA synthetase. Eur J Biochem. 1988 Aug 15;175(3):511–524. doi: 10.1111/j.1432-1033.1988.tb14223.x. [DOI] [PubMed] [Google Scholar]
- Tometsko A. M., Turula J., Comstock J. Photolytic inhibition and labeling of proteins with aryl diazonium compounds. Int J Pept Protein Res. 1978 Sep;12(3):143–154. doi: 10.1111/j.1399-3011.1978.tb02878.x. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Srivenugopal K. S., Reid B. R., Morris D. R. Nuclear magnetic resonance studies of polyamine binding to a defined DNA sequence. J Mol Biol. 1985 Sep 20;185(2):457–459. doi: 10.1016/0022-2836(85)90418-8. [DOI] [PubMed] [Google Scholar]
- Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol. 1985 Jul 5;184(1):119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Zakrzewska K., Pullman B. Spermine-nucleic acid interactions: a theoretical study. Biopolymers. 1986 Mar;25(3):375–392. doi: 10.1002/bip.360250302. [DOI] [PubMed] [Google Scholar]




