Abstract
A ferrocene bis(triazole) macrocycle was synthesised in good yield by the Eglinton coupling of an acyclic bis(alkyne) precursor and characterised in the solid state by X-ray crystallography. Alkylation gives the corresponding triazolium macrocycle, which binds chloride and benzoate strongly in CD3CN solution through favourable charge-assisted C–H···anion interactions, as evidenced by 1H NMR titration experiments. Preliminary electrochemical studies reveal that the redox-active macrocycle is capable of sensing chloride in CH3CN solution.
Keywords: anion binding, C–H···anion interactions, electrochemistry, ferrocene, triazolium
Introduction
The copper(I)-catalysed cycloaddition of alkynes and azides (CuAAC) [1–2] to give the 1,2,3-triazole group is increasingly being exploited in the synthesis of a vast array of materials since its discovery [3]. Several seminal studies have demonstrated the ability of acyclic and macrocyclic bis- and poly(triazole) containing systems to bind anions in organic solvents through triazole C–H···anion interactions [4–7]. More recently, we [8], and others [9–10], have shown that alkylating the triazole group to give the triazolium group increases the strength of anion binding significantly by further polarising the C–H bond of the heterocycle.
With one notable recent exception of an acyclic ferrocene-appended aryl triazole receptor, which selectively senses phosphate species electrochemically in dichloromethane [11], to the best of our knowledge, redox-active triazole and triazolium receptors are unprecedented. Herein we describe the synthesis of a novel ferrocene bis-triazolium macrocyclic receptor and investigate its anion binding and electrochemical-sensing properties.
Results and Discussion
Synthesis
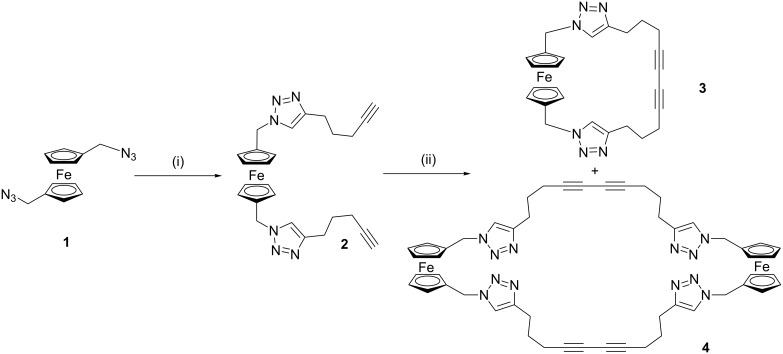
The CuAAC reaction of ferrocene bis(azide) 1 [12], with a large excess of 1,6-heptadiyne afforded 2 in 50% yield. An intramolecular Eglinton cyclisation reaction was used to prepare the ferrocene bis(triazole) macrocycle 3, in surprisingly good yield (54%) after purification by column chromatography, as well as a small amount (12%) of the corresponding larger tetra(triazole) macrocycle, 4 (Scheme 1).
Scheme 1.
Synthesis of bis(triazole) macrocycle 3 and tetra(triazole) macrocycle 4. Conditions and reagents: (i) 1,6-Heptadiyne, [CuI(CH3CN)4](PF6), tris[(benzyltriazol-4-yl)methyl]amine (TBTA), diisopropylamine (DIPEA), CH2Cl2, 50%; (ii) CuII(OAc)2·H2O, CH3CN, reflux, 54% of 3, 12% of 4.
The reaction of macrocycle 3, with an excess of trimethyloxonium tetrafluoroborate gave a crude product, which was difficult to purify. Chromatographic purification was required, but this was complicated by the doubly positively charged macrocycle. Nevertheless, preparative thin-layer chromatography in 17:2:1 acetonitrile:water:saturated KNO3(aq) solution, followed by removal of the organic solvents under reduced pressure and precipitation of the product as its bis(hexafluorophosphate) salt from the remaining aqueous solution (using NH4PF6), gave the desired bis(triazolium) macrocycle 5, in moderate yield (Scheme 2). Alternatively, 2 could be readily alkylated to give bis(triazolium)-bis(alkyne), 6, in good yield; however, attempts to cyclise under analogous conditions to those used to produce 3 were unsuccessful, resulting in decomposition of the ferrocene motif.
Scheme 2.
Synthesis of bis(triazolium) macrocycle, 5. Conditions and reagents: (i) (a) (Me3O)(BF4), CH2Cl2, (b) chromatography with 17:2:1 CH3CN:H2O: sat. KNO3(aq), then NH4PF6(aq), 12%; (ii) (Me3O)(BF4), CH2Cl2, 77%.
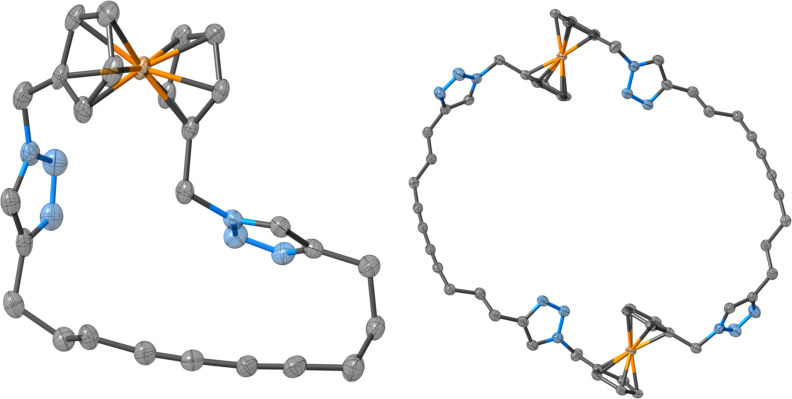
All new compounds were characterised by 1H and 13C NMR spectroscopy and high-resolution ESI mass spectrometry, as well as by 19F and 31P NMR spectroscopy where appropriate. Single crystals of 3 were grown by the vapour diffusion of diethyl ether into an acetone solution of the macrocycle, and of macrocycle 4 by vapour diffusion of pentane into a chloroform/methanol solution. The solid-state structures were determined by synchrotron X-ray crystallography (Figure 1). The structure of 3 shows that the diyne unit appears to enforce a reasonably rigid geometry on the rest of the macrocycle, due to its sterically demanding nature, which may help to constrain the anion binding cleft and lead to selective anion binding. In contrast, the larger size of 4 gives a much more open structure, with a diameter of approximately 17 Å (Figure 1). Despite this open structure, no solvent cocrystallises with the macrocycle, with the structure being tightly packed due to a series of intermolecular hydrogen bonds between triazole C–H donors and triazole N-acceptors on adjacent molecules.
Figure 1.
Solid-state structure of 3 (left) and 4 (right). Hydrogen atoms omitted for clarity, ellipsoids are shown at 50% probability; only one of the two independent molecules in the asymmetric unit of 3 is shown. Colour scheme: grey = carbon, blue = nitrogen, orange = iron.
Anion-binding investigations
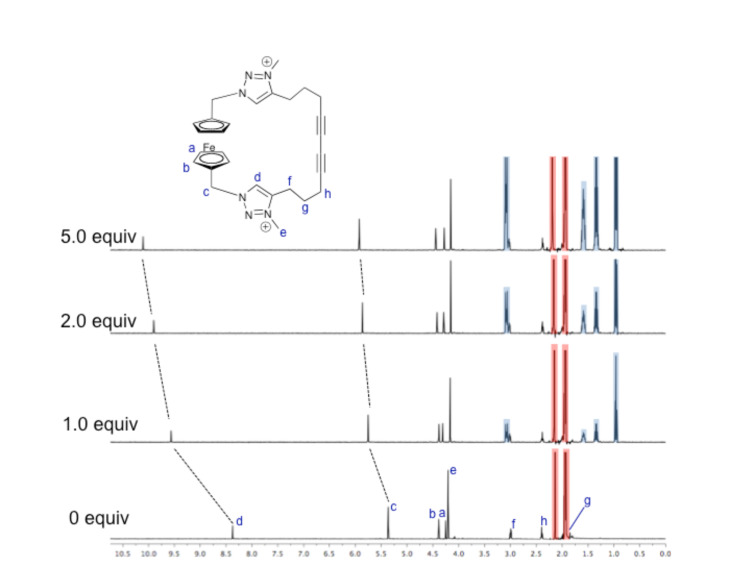
The anion-binding properties of 5·2PF6 were investigated in CD3CN solution by 1H NMR titration experiments. Aliquots of anions as their N-tetrabutylammonium (TBA) salts were added to 5·2PF6 and the chemical shift of the (chemically equivalent) triazolium protons was monitored. As shown in Figure 2, the addition of chloride to the macrocyclic receptor resulted in large downfield shifts of the triazolium signal, d, concomitant with relatively smaller perturbations of proton c, which suggests that halide binding occurs in proximity to the ferrocene group.
Figure 2.
1H NMR spectra of 5·2PF6 after the addition of 0, 1.0, 2.0 and 5.0 equiv of TBA·Cl (500 MHz, 293 K, CD3CN). Red-shaded peaks correspond to solvent signals (H2O and CH3CN); blue-shaded peaks correspond to signals from TBA cations.
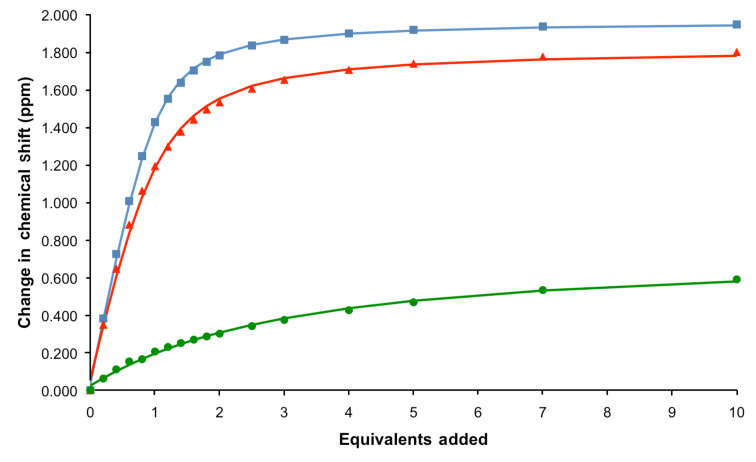
WINEQNMR2 [13] analysis of the titration data (Figure 3) shows that the cyclic receptor, 5·2PF6, binds both chloride and benzoate strongly with 1:1 stoichiometries in CD3CN (association constants are shown in Table 1), with the more basic carboxylate anion forming the strongest complex. The larger halide, iodide, is only weakly bound. The addition of small amounts (<1 equiv) of dihydrogen phosphate caused precipitation and, hence, no association constant could be calculated.
Figure 3.
Titration data (solid points) and fitted binding isotherms (curves) monitoring the triazolium proton, d, for titration of benzoate, chloride and iodide into a solution of 5·2PF6 (500 MHz, 293 K, CD3CN).
Table 1.
Anion association constants for 5·2PF6 calculated by using WINEQNMR2 [13] based on 1H NMR titration data (500 MHz, 293 K, CD3CN).
| Aniona | Ka (M−1)b |
| Benzoate | 4.6(2) × 103 |
| Chloride | 2.5(2) × 103 |
| Iodide | 2.0(2) × 102 |
| Dihydrogen phosphate | Precipitation |
aAll anions added as TBA salts. bEstimated standard errors given in parentheses.
Electrochemical Investigations
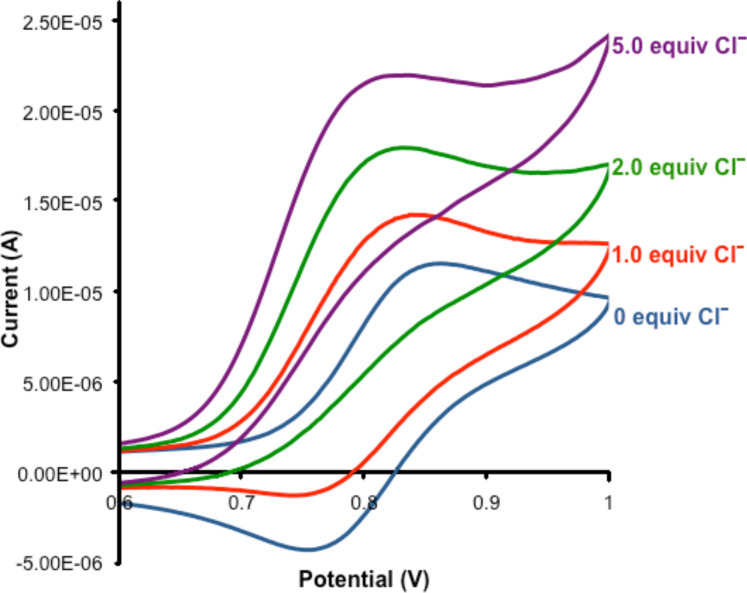
The ability of 5·2PF6 to sense anions electrochemically was investigated by cyclic voltammetry in 0.1 M TBA·PF6 in CH3CN. The macrocycle displays a quasi-reversible oxidation of the ferrocene/ferrocenium couple, which is shifted to a more positive potential by 0.38 V relative to ferrocene by virtue of the positively charged triazolium groups of the receptor. Addition of one equiv of TBA·Cl caused a cathodic shift of the oxidation potential of 25 mV and a loss of reversibility, which may be indicative of an EC mechanism [14] (Figure 4). Further addition of TBA·Cl led to increasing cathodic perturbations of the Epa oxidation wave of up to 40 mV after five equiv. As noted with other ferrocene based anion receptors [15–17], chloride-anion binding stabilises the ferrocenium oxidation state, which results in the cathodic perturbation observed. Analogous electrochemical experiments with benzoate caused an immediate disappearance of the redox signal, which indicates precipitation of an insoluble ferrocenium complex upon oxidation.
Figure 4.
CV of 5·2PF6 upon the addition of TBA·Cl (electrolyte: 0.1 M TBA·PF6/CH3CN, [5·2PF6] = 0.50 mM, 293 K. Potential vs. Ag/AgCl reference).
Conclusion
A new ferrocene bis(triazole) macrocycle was readily synthesised by intramolecular Eglinton coupling of an acyclic 1,1’-bis(triazolylalkyne) ferrocene precursor. Alkylation of this macrocycle gave the dicationic bis(triazolium) macrocycle, which was demonstrated by 1H NMR titration experiments to bind chloride and benzoate in CD3CN, solely through charge-assisted C–H···anion hydrogen-bonding interactions. The redox-active macrocycle was also shown to sense chloride electrochemically via a cathodic shift of the Epa wave of the ferrocene/ferrocenium redox couple.
Experimental
General Remarks
Bis(azide) 1 [12], and TBTA [18] were prepared as described in the literature. Dry dichloromethane and acetonitrile were purged with nitrogen and passed through a MBraun MSP-800 column. Water was deionised and microfiltered in a Milli-Q® Millipore machine. Tetrabutylammonium salts were stored in a vacuum desiccator under reduced pressure. All other compounds were bought commercially and used as received. Routine NMR spectra were recorded on a Varian Mercury 300 spectrometer with 1H NMR operating at 300 MHz, 13C at 75.5 MHz. Spectra for anion-binding titrations were recorded on a Varian Unity Plus 500 spectrometer with 1H operating at 500 MHz. Mass spectra were recorded on a Bruker microTOF spectrometer.
Bis(alkyne) 2
Bis(azide) 1 (0.148 g, 0.500 mmol) and 1,6-heptadiyne (0.69 mL, 0.55 g, 6.0 mmol) were dissolved in dichloromethane (50 mL). DIPEA (0.17 mL, 0.13 g, 1.0 mmol), TBTA (0.053 g, 0.10 mmol) and [CuI(CH3CN)4](PF6) (0.037 g, 0.10 mmol) were added, and the yellow solution was stirred at room temperature under a nitrogen atmosphere for three days. It was then taken to dryness under reduced pressure and purified by column chromatography (silica, 2% methanol in dichloromethane) to give 2 as a yellow solid. Yield: 0.120 g (50%). 1H NMR (CDCl3) δ 7.24 (s, 2H, trz-H), 5.19 (s, 4H, Fc-CH2-trz), 4.23 (t, 3J = 1.6 Hz, 4H, Fc-H), 4.19 (t, 3J = 1.6 Hz, 4H, Fc-H), 2.80 (t, 3J = 7.6 Hz, 4H, trz-CH2-CH2), 2.22 (dt, 3J = 7.0 Hz, 4J = 2.6 Hz, 4H, CH2-C≡CH), 1.95 (t, 4J = 2.6 Hz, 2H, C≡CH), 1.87 (m, 4H, trz-CH2-CH2); 13C NMR (CDCl3) δ 147.3, 120.6, 83.9, 82.7, 69.9, 69.6, 69.0, 49.5, 28.1, 24.6, 18.0; HRMS–ESI (m/z): [M + Na]+ calcd for C26H28N6Fe·Na, 503.1617; found, 503.1614.
Bis(triazole) macrocycle 3
The bis(alkyne) 2 (0.120 g, 0.250 mmol) and copper(II) acetate monohydrate (0.125 g, 0.625 mmol) were heated under reflux in acetonitrile (150 mL) under a nitrogen atmosphere overnight. The green solution was cooled to room temperature and then taken to dryness under reduced pressure. It was taken up in water (20 mL) and extracted with dichloromethane (4 × 20 mL). The combined organic fractions were dried (magnesium sulfate), taken to dryness under reduced pressure and then purified by column chromatography (silica, gradient: 3% methanol in dichloromethane) to give 3 as an orange powder. Yield: 0.065 g (54%). 1H NMR (CDCl3) δ 7.44 (s, 2H, trz-H), 5.06 (s, 4H, Fc-CH2-trz), 4.15 (t, 3J = 1.9 Hz, 4H, Fc-H), 4.10 (t, 3J = 1.9 Hz, 4H, Fc-H), 2.91 (t, 3J = 6.6 Hz, 4H, trz-CH2-CH2), 2.31 (t, 3J = 6.6 Hz, 4H, CH2-C≡C), 1.92–2.01 (m, 4H, trz-CH2-CH2); 13C NMR (CDCl3) δ 146.9, 121.9, 110.9, 84.0, 69.3, 69.1, 66.6, 49.1, 26.6, 24.2, 18.4; HRMS–ESI (m/z): [M + Na]+ calcd for C26H26N6Fe·Na, 501.1461; found, 501.1467.
Tetra(triazole) macrocycle 4
The same procedure as for the synthesis of 3 but switching to 7% methanol in dichloromethane as eluent gave 4 as an orange powder. Yield: 0.014 g (12%). 1H NMR (CDCl3) δ 7.33 (s, 4H, trz-H), 5.11 (s, 8H, Fc-CH2-trz), 4.14 (t, 3J = 1.7 Hz, 8H, Fc-H), 4.11 (t, 3J = 1.7 Hz, 8H, Fc-H), 2.74 (t, 3J = 6.6 Hz, 4H, trz-CH2-CH2), 2.23 (t, 3J = 6.6 Hz, 4H, CH2-C≡C), 1.76–1.86 (m, 8H, trz-CH2-CH2); 13C NMR (DMSO-d6) δ 145.8, 121.9, 83.6, 77.6, 69.3, 69.0, 65.7, 48.3, 27.7, 24.0, 17.9; HRMS–ESI (m/z): [M + Na]+ calcd for C52H52N12Fe2·Na, 979.3029; found, 979.3038.
Bis(triazolium) macrocycle 5·2PF6
The neutral macrocycle 3 (0.048 g, 0.10 mmol) was dissolved in dry dichloromethane (10 mL). Trimethyloxonium tetrafluoroborate (0.032 g, 0.22 mmol) was added and the orange solution stirred at room temperature under a nitrogen atmosphere overnight, during which time it took on a brown tinge. Methanol (1 mL) was added to quench the alkylating agent, and the mixture was evaporated to dryness under reduced pressure. The crude mixture was purified by preparative thin layer chromatography (silica, eluent: 17:2:1 CH3CN/H2O/sat. KNO3(aq)). The band containing the product was scraped from the plate and washed off the silica with the same solvent mixture, and the acetonitrile was removed under reduced pressure to give an orange aqueous solution. Addition of NH4PF6 (0.15 g in 0.5 mL H2O) caused the precipitation of a yellow solid. This was extracted into dichloromethane (2 × 15 mL), washed with water (2 × 15 mL) and thoroughly dried in vacuo to give 5 as a glassy orange solid. Yield: 0.0098 g (12%). 1H NMR (CD3CN) δ 8.32 (s, 2H, Hd), 5.34 (s, 6H, Hc), 4.37 (t, 3Ja,b = 1.9 Hz, 4H, Hb), 4.24 (t, 3Ja,b = 1.9 Hz, 4H, Ha), 4.19 (s, 6H, He), 2.99 (t, 3Jf,g = 6.6 Hz, 4H, Hf), 2.40 (t, 3Jg,h = 6.5 Hz, 4H, Hh), 1.95–2.03 (m, partially obscured by residual acetonitrile solvent peak, 4H, Hg). Compound lettering shown in Figure 2. 13C NMR (CD3CN) δ 145.1, 129.1, 81.6, 77.6, 71.2, 70.3, 66.6, 53.6, 38.7, 25.6, 22.7, 18.4; 19F NMR (CD3CN) δ −144.6 (heptet, JP,F = 707 Hz); 31P NMR (CD3CN) δ −73.4 (d, JP,F = 707 Hz); HRMS–ESI (m/z): [M + PF6]+ calcd for C28H32N6Fe·PF6, 653.1674; found, 653.1693
Bis(triazolium)-bis(alkyne) 6·2BF4
The neutral bis(alkyne) 2 (0.096 g, 0.20 mmol) was dissolved in dichloromethane (15 mL). Trimethyloxonium tetrafluoroborate (0.065 g, 0.44 mmol) was added and the orange solution stirred at room temperature under a nitrogen atmosphere overnight. Methanol (1 mL) was added to quench the alkylating agent, and the mixture was evaporated to dryness under reduced pressure. Purification by preparative thin-layer chromatography (silica, eluent: 10% methanol in dichloromethane) gave 6·2BF4 as an orange powder. Yield: 0.106 g (77%). 1H NMR (acetone-d6) δ 8.60 (s, 2H, trz+-H), 5.75 (s, 4H, Fc-CH2-trz+), 4.64 (t, 3J = 1.9 Hz, 4H, Fc-H), 4.42 (t, 3J = 1.9 Hz, 4H, Fc-H), 4.34 (s, 6H, trz+-CH3), 3.08 (t, 3J = 7.7 Hz, 4H, trz+-CH2-CH2), 2.41 (t, 4J = 2.6 Hz, 2H, C≡CH), 2.35 (dt, 3J = 6.8 Hz, 4J = 2.6 Hz, 4H, CH2-C≡CH), 1.93–2.02 (m, 4H, CH2-CH2-CH2-C≡CH); 13C NMR (acetone-d6) δ 145.0, 128.6, 83.5, 80.9, 71.6, 71.5, 71.2, 53.7, 38.1, 26.5, 22.8, 18.0; 19F NMR (acetone-d6) δ −150.9 (m); HRMS–ESI (m/z): [M + BF4]+ calcd for C28H34N6Fe·BF4, 597.2218; found, 597.2239.
X-ray crystallography
Single crystal X-ray diffraction data for 3 were collected by using synchrotron radiation (λ = 0.6889 Å) at the Diamond Light Source, Beam I19. The diffractometer was equipped with a Cryostream N2 open-flow cooling device [19] and the data were collected at 100(2) K. A series of ω-scans was performed in such a way as to collect a half-sphere of data to a maximum resolution of 0.77 Å. Cell parameters and intensity data (including interframe scaling) were processed with CrysAlis Pro [20].
The structures were solved by charge-flipping methods with SUPERFLIP [21] and refined using full-matrix least-squares on F2 within the CRYSTALS suite [22]. All nonhydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were generally visible in the difference map, and their positions and displacement parameters were refined by using restraints prior to inclusion into the model employing riding constraints [23].
Crystallographic data for the structures have been deposited with the Cambridge Crystallographic Data Centre, CCDC: 859564 and 859565. Copies of these data can be obtained free of charge from The Cambridge Crystallographic Data Centre at http://www.ccdc.cam.ac.uk/data_request/cif.
NMR titration protocols
Initial sample volumes were 0.50 mL and concentrations were 2.0 mmol L−1 of host. Solutions (100 mmol L−1) of anions as their tetrabutylammonium salts were added in aliquots, the samples thoroughly shaken and spectra recorded. Spectra were recorded at 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.5, 3.0, 4.0, 5.0, 7.0 and 10 equivalents. Stability constants were obtained by analysis of the resulting data with the WinEQNMR2 [13] computer program, following the triazolium C–H protons in all cases.
Electrochemistry protocols
Cyclic voltammetry was performed on an Autolab Potentiostat/Galvanostat model PG-STAT 12, controlled by General Purpose Electrochemical System Software v. 4.9 (Eco Chemie). A standard one-compartment three-electrode electrochemical cell, located inside a Faraday cage, was used with a glassy carbon solid-disc working electrode, a platinum-wire auxiliary electrode and an Innovative Instruments, Inc. LF-2 leak-free silver/silver chloride reference electrode. A 0.50 mM ferrocene sample was used in order to check the reference electrode and internal resistance of the equipment. The electrolyte solution used in all experiments was 0.10 M TBA·PF6 in CH3CN. CVs were recorded with a 1 s equilibration time and a step potential of 1 mV.
The receptor 3 was dissolved in 3.0 mL of the electrolyte solution, such that the receptor concentration was 0.50 mM, and cyclic voltammograms were recorded. Scan rates of 25, 50, 100, 200, 300, 400 and 500 mV s−1 were used in order to test for reversibility. Aliquots of TBA·Cl or TBA·BzO (as a 0.50 M solution of electrolyte solution) were added to the receptor solution, stirred and the cyclic voltammograms recorded at a scan rate of 100 mV s−1.
Supporting Information
NMR-spectra of new compounds.
X-ray data of macrocycles 3 and 4.
Acknowledgments
We thank the Clarendon Fund and Trinity College, Oxford for funding and Diamond Light Source for the award of beam time on Beamline I19. Ben Mullaney is thanked for assistance with electrochemistry experiments.
This article is part of the Thematic Series "Supramolecular chemistry II".
References
- 1.Rostovtsev V V, Green L G, Fokin V V, Sharpless K B. Angew Chem, Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Tornøe C W, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 3.Juríček M, Kouwer P H J, Rowan A E. Chem Commun. 2011;47:8740–8749. doi: 10.1039/c1cc10685f. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Flood A H. J Am Chem Soc. 2008;130:12111–12122. doi: 10.1021/ja803341y. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Flood A H. Angew Chem, Int Ed. 2008;47:2649–2652. doi: 10.1002/anie.200704717. [DOI] [PubMed] [Google Scholar]
- 6.Meudtner R M, Hecht S. Angew Chem, Int Ed. 2008;47:4926–4930. doi: 10.1002/anie.200800796. [DOI] [PubMed] [Google Scholar]
- 7.Juwarker H, Lenhardt J M, Castillo J C, Zhao E, Krishnamurthy S, Jamiolkowski R M, Kim K-H, Craig S L. J Org Chem. 2009;74:8924–8934. doi: 10.1021/jo901966f. [DOI] [PubMed] [Google Scholar]
- 8.Mullen K M, Mercurio J, Serpell C J, Beer P D. Angew Chem. 2009;48:4781–4784. doi: 10.1002/anie.200901313. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Pandey P S. Org Lett. 2008;10:165–168. doi: 10.1021/ol702457w. [DOI] [PubMed] [Google Scholar]
- 10.Schulze B, Friebe C, Hager M D, Günther W, Kohn U, Jahn B O, Görls H, Schubert U S. Org Lett. 2010;12:2710–2713. doi: 10.1021/ol100776x. [DOI] [PubMed] [Google Scholar]
- 11.Cao Q-Y, Pradhan T, Kim S, Kim J S. Org Lett. 2011;13:4386–4389. doi: 10.1021/ol201722d. [DOI] [PubMed] [Google Scholar]
- 12.Casas-Solvas J M, Ortiz-Salmerón E, Giménez-Martínez J J, García-Fuentes L, Capitán-Vallvey L F, Santoyo-González F, Vargas-Berenguel A. Chem–Eur J. 2009;15:710–725. doi: 10.1002/chem.200800927. [DOI] [PubMed] [Google Scholar]
- 13.Hynes M J. J Chem Soc, Dalton Trans. 1993:311–318. [Google Scholar]
- 14.Bard A J, Faulkner L R. Electrochemical methods: fundamentals and applications. John Wiley & Sons; 1980. [Google Scholar]
- 15.Evans N H, Serpell C J, Beer P D. Chem Commun. 2011;47:8775–8777. doi: 10.1039/c1cc13247d. [DOI] [PubMed] [Google Scholar]
- 16.Wong W W H, Curiel D, Lai S-W, Drew M G B, Beer P D. Dalton Trans. 2005:774–781. doi: 10.1039/b416851h. [DOI] [PubMed] [Google Scholar]
- 17.Beer P D, Hayes E J. Coord Chem Rev. 2003;240:167–189. doi: 10.1016/S0010-8545(02)00303-X. [DOI] [Google Scholar]
- 18.Lee B-Y, Park S R, Jeon H B, Kim K S. Tetrahedron Lett. 2006;47:5105–5109. doi: 10.1016/j.tetlet.2006.05.079. [DOI] [Google Scholar]
- 19.Cosier J, Glazer A M. J Appl Crystallogr. 1986;19:105–107. doi: 10.1107/S0021889886089835. [DOI] [Google Scholar]
- 20.CrysAlis Pro. Yarnton, U.K.: Oxford Diffraction; 2010. [Google Scholar]
- 21.Palatinus L, Chapuis G. J Appl Crystallogr. 2007;40:786–790. doi: 10.1107/S0021889807029238. [DOI] [Google Scholar]
- 22.Betteridge P W, Carruthers J R, Cooper R I, Prout K, Watkin D J. J Appl Crystallogr. 2003;36:1487. doi: 10.1107/S0021889803021800. [DOI] [Google Scholar]
- 23.Cooper R I, Thompson A L, Watkin D J. J Appl Crystallogr. 2010;43:1100–1107. doi: 10.1107/S0021889810025598. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NMR-spectra of new compounds.
X-ray data of macrocycles 3 and 4.