Abstract
Objective:
The DahlS.Z-Leprfa/Leprfa (DS/obese) rat strain was established from a cross between Dahl salt-sensitive rats and Zucker fatty (fa/fa) rats, the latter of which harbor a missense mutation in the leptin receptor gene (Lepr). We examined whether DS/obese rats might be a suitable animal model of metabolic syndrome in humans.
Methods:
The systemic pathophysiological and metabolic characteristics of DS/obese rats were determined and compared with those of homozygous lean littermates, namely, DahlS.Z-Lepr+/Lepr+ (DS/lean) rats.
Results:
Systolic blood pressure was higher in DS/obese rats fed a normal diet than in DS/lean rats at 11 weeks of age and thereafter. The survival rate of DS/obese rats was significantly lower than that of DS/lean rats at 18 weeks. Body weight, visceral and subcutaneous fat mass, as well as heart, kidney and liver weights, were increased in DS/obese rats at 18 weeks compared with DS/lean rats. Serum low-density lipoprotein (LDL)-cholesterol, triglyceride and insulin concentrations, as well as the ratio of LDL-cholesterol to high-density lipoprotein-cholesterol levels, were increased in DS/obese rats, whereas serum glucose concentration did not differ significantly between DS/obese and DS/lean rats. Creatinine clearance was decreased and urinary protein content was increased in DS/obese rats, which also manifested lipid accumulation in the liver and elevation of serum alanine aminotransferase levels.
Conclusion:
These results show that the phenotype of DS/obese rats is similar to that of humans with metabolic syndrome, and that these animals may thus be an appropriate model for this condition.
Keywords: metabolic syndrome, animal model, obesity, hypertension, dyslipidemia, insulin resistance
Introduction
The combination of insulin resistance, glucose intolerance, dyslipidemia, hypertension and obesity, in particular abdominal obesity, has been referred to as metabolic syndrome (MetS), the prevalence of which is increasing worldwide as a result of changes in diet and lifestyle. Individuals with MetS are at increased risk for development of diabetes and cardiovascular disease.1, 2
Given that MetS is a multifactorial condition, it has been difficult to establish adequate experimental models for its study. The most representative rat strain available to date for the study of MetS is thought to be the Zucker fatty (fa/fa) rat,3 which harbors a missense mutation (A to C at nucleotide 806) in the gene for the leptin receptor that results in a Gln269Pro substitution in the extracellular domain common to all receptor isoforms.4 This mutation is associated with reduced binding of leptin to the surface of receptor-expressing cells, without a change in the affinity constant for leptin, and with the development of leptin resistance in the brain, leading to hyperphagia, physical inactivity and consequent obesity. However, conflicting data have been presented with regard to whether these animals are hypertensive compared with lean controls.3 Leptin receptor mutations have been detected in humans with severe, early-onset obesity.5
The Dahl salt-sensitive (DS) rat is one of the most widely studied animal models of hypertension. DS rats develop salt-induced hypertension and subsequently manifest a clear transition from cardiac hypertrophy to heart failure.6 We previously characterized early changes in excitation–contraction coupling in the transition from compensated hypertrophy to failure in cardiomyocytes isolated from DS rats.7 The value of these animals for investigations into the pharmacology and pathophysiology of hypertension and cardiovascular disease has been recognized for many years. Indeed, we have examined the effects of various drugs and other interventions on cardiac pathophysiology in this model of hypertension and heart failure.8, 9, 10, 11, 12
We have now evaluated a potential new animal model of MetS, the DahlS.Z-Leprfa/Leprfa (DS/obese) rat, which was established by crossing DS rats and Zucker rats with the missense mutation in the leptin receptor gene (Lepr). We examined the systemic pathophysiology and metabolic changes in DS/obese rats in comparison with their lean littermates, DahlS.Z-Lepr+/Lepr+ (DS/lean) rats, in order to validate their suitability as an animal model of MetS.
Materials and methods
Derivation and genetic characterization of DS/obese rats
DahlS.Z-Leprfa/Slc rats were generated as the result of an initial mating of a male Zucker rat (Japan SLC, Hamamatsu, Japan) heterozygous for the fa allele of Lepr with a female DahlS/Jr Sea rat (Seac Yoshitomi, Fukuoka, Japan), followed by multiple rounds of backcrossing of the resulting progeny to the DS strain.13 The fa locus was genotyped by polymerase chain reaction-restricted fragment length polymorphism assay as follows. A primer set to detect the fa allele was designed to cover a 111-bp region containing the fa mutation site in the Lepr with the following sequences: 5′-TATGGAAGTCACAGATGATGG-3′ and 5′-CTTACGATTGTAGAATTCTCTAA-3′. The MspI digestion of the fa allele produces two fragments of 78 and 33 bp. In each generation, male rats in which the fa allele was detected were selected in backcrossing to produce the subsequent generation. Eight to twelve backcrosses were performed to eliminate the undesired genes of the Zucker strain. The final congenic strain was generated by M Nishimura and M Mori (Hamamatsu University School of Medicine) and was established under specific pathogen-free conditions by Japan SLC. Mating of heterozygotes yielded three genotypes but only two phenotypes: homozygous (fa/fa) obese, heterozygous (fa/+) lean and homozygous (+/+) lean, in the ratio of 1:2:1. Our preliminary observations revealed that obese homozygotes, unlike their lean littermates, manifest hyperphagia and develop central (abdominal) obesity beginning at 5 weeks of age.
Animals and experimental protocols
Ten-week-old male inbred DS/obese rats were obtained from Japan SLC and were handled in accordance with the guidelines of Nagoya University Graduate School of Medicine, as well as with the Guide for the Care and Use of Laboratory Animals (US NIH publication no. 85-23, revised 1996). Rats were fed normal laboratory chow containing 0.36% NaCl. Both the diet and tap water were provided ad libitum throughout the experimental period. At 18 weeks of age, rats were placed in metabolic cages to collect 24-h urine specimens and a blood sample was collected from the right carotid artery of the rats fasted overnight. They were subsequently killed by intraperitoneal injection of an overdose of sodium pentobarbital (50 mg per kg body weight), and the heart, liver, kidney, and both visceral (retroperitoneal) and subcutaneous (inguinal) fat were removed and subjected to analysis. Age-matched male homozygous lean littermates of DS/obese rats, DahlS.Z-Lepr+/Lepr+ (DS/lean), served as control animals.
Physiological and metabolic analyses
Systolic blood pressure (SBP) and heart rate were measured weekly in conscious animals by tail-cuff plethysmography (BP-98A; Softron, Tokyo, Japan). SBP was measured five times at each time point for each rat and the average value was calculated. Urine volume, urinary protein and the ratio of creatinine clearance to kidney weight or body weight were determined by analysis of urine specimens. Creatinine clearance was calculated using the standard formula UV/P, where U is the urinary creatinine concentration, V is the 24-h urine volume and P is the serum creatinine concentration. Blood was collected from the right carotid artery of rats deprived of food overnight and was centrifuged at 3000 r.p.m. (1400 × g) for 10 min at room temperature. The resultant serum supernatant was kept frozen at −80 °C until analysis. Serum glucose and blood urea nitrogen levels, as well as serum levels of triglyceride, total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, creatinine, aspartate aminotransferase and alanine aminotransferase were measured by routine enzymatic assays. The concentrations of leptin and insulin in serum were measured using mouse/rat enzyme-linked immunosorbent assay kits (Morinaga Bioscience Institute, Yokohama, Japan).
Histology
Liver and kidney tissue samples were fixed in ice-cold 4% paraformaldehyde for 48–72 h, embedded in paraffin and processed for histology as described.12 The former was sectioned for staining with hematoxylin–eosin and the latter was sectioned for staining with periodic acid–Schiff solution.
Statistical analysis
Data are presented as means±s.e.m. Differences between groups of rats at 18 weeks of age were assessed using the Mann–Whitney U-test. The time courses of SBP or body weight were compared between groups using two-way repeated-measures analysis of variance. Survival rate was analyzed by the standard Kaplan–Meier method with the log-rank test. A P value of <0.05 was considered statistically significant.
Results
Physiological analysis and survival of DS/obese rats
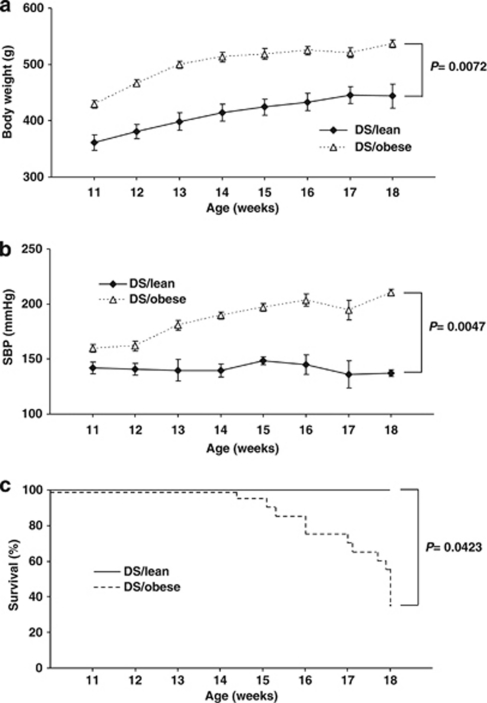
Body weight and visceral (4.7-fold) and subcutaneous (5.0-fold) fat mass were increased in DS/obese rats compared with DS/lean rats (Figure 1a, Table 1). SBP was significantly higher in DS/obese rats than in DS/lean rats at 11 weeks of age and thereafter (Figure 1b, Table 1). In total, 13 (65%) of 20 DS/obese rats died during the experimental period (7 from renal failure, 2 from cerebrovascular events and 4 from sudden cardiac death). Kaplan–Meier analysis confirmed that the survival rate of DS/obese rats was significantly lower than that of DS/lean rats (Figure 1c, Table 1). The ratio of left ventricular (LV) weight to tibial length and the ratio of LV weight to right ventricular weight, both of which are indices of LV hypertrophy, were increased in DS/obese rats compared with DS/lean rats (Table 1). The ratios of kidney or liver weight to tibial length were also significantly increased in DS/obese rats compared with DS/lean rats (Table 1). Tibial length of DS/obese rats was significantly shorter than that of DS/lean rats (Table 1).
Figure 1.
Time course of body weight (a), SBP (b) and survival (c) of DS/obese and DS/lean rats. Data for body weight and SBP are given as means±s.e.m. for surviving animals (n=6 and 20 for DS/obese and DS/lean rats, respectively, at 11 weeks and n=6 and 7 for DS/obese and DS/lean rats, respectively, at 18 weeks).
Table 1. Physiological parameters of DS/lean and DS/obese rats at 18 weeks of age.
| Parameter | DS/lean | DS/obese | P |
|---|---|---|---|
| Body weight (g) | 443.75±21.62 | 536.92±6.98 | 0.0072 |
| Tibial length (mm) | 40.27±0.24 | 36.47±0.12 | 0.0036 |
| SBP (mm Hg) | 137.7±03.04 | 210.62±2.54 | 0.0047 |
| Heart rate (beats min−1) | 417.32±13.71 | 400.73±15.40 | 0.1792 |
| Heart weight/tibial length (mg/mm) | 32.71±1.43 | 42.22±0.29 | 0.0019 |
| LV weight/tibial length (mg/mm) | 24.22±1.02 | 32.69±0.37 | 0.0036 |
| LV weight/RV weight | 4.21±0.05 | 4.74±0.12 | 0.0301 |
| Atrial weight/tibial length (mg/mm) | 2.06±0.23 | 2.55±0.14 | 0.0896 |
| Kidney weight/tibial length (mg/mm) | 78.41±6.36 | 128.89±3.79 | 0.0025 |
| Liver weight/tibial length (mg/mm) | 312.19±25.29 | 600.11±42.14 | 0.0021 |
| Visceral fat weight/tibial length (mg/mm) | 178.02±31.55 | 839.28±33.00 | 0.0036 |
| Subcutaneous fat weight/tibial length (mg/mm) | 158.77±27.34 | 795.02±41.33 | 0.0036 |
| Survival (%) | 100 | 35 | 0.0423 |
Abbreviations: DS, Dahl salt-sensitive; LV, left ventricular; RV, right ventricular; SBP, systolic blood pressure.
With the exception of survival rate, data are given as means±s.e.m. for surviving animals (n=6 and 7 for DS/lean and DS/obese rats, respectively).
Metabolic and histological analysis of DS/obese rats
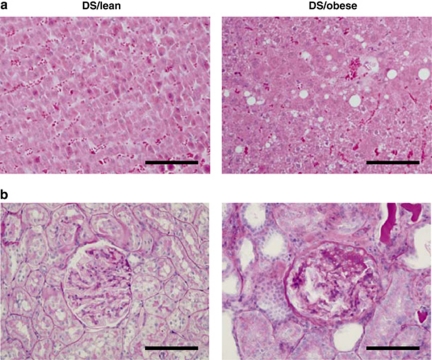
The metabolic parameters of DS/obese and DS/lean rats are summarized in Table 2. The serum leptin concentration in DS/obese rats was 30 times that in DS/lean rats. Although the fasting serum insulin concentration was significantly increased in DS/obese rats compared with DS/lean rats, there was no significant difference in the fasting serum glucose levels between the two strains. However, in another group of DS/obese rats (n=6), the casual serum glucose concentration was increased markedly (mean 309.00 mg per 100 ml; range 208–426 mg per 100 ml). These data indicate that DS/obese rats developed type 2 diabetes mellitus and insulin resistance. Serum levels of total cholesterol, LDL-cholesterol, HDL-cholesterol and triglyceride, as well as the ratio of LDL-cholesterol to HDL-cholesterol levels, were increased in DS/obese rats. The serum alanine aminotransferase level was also increased in DS/obese rats, whereas serum AST levels did not differ significantly between the two strains. Furthermore, DS/obese rats manifested lipid accumulation in the liver, whereas DS/lean rats did not (Figure 2a). Blood urea nitrogen, serum creatinine and urinary protein levels were significantly increased, whereas the ratio of creatinine clearance to either body or kidney weight was significantly decreased in DS/obese rats compared with DS/lean rats. Periodic acid–Schiff-stained micrographs revealed a grossly normal appearance in DS/lean rats (Figure 2b, left). DS/obese rats exhibited advanced renal lesions, such as glomerular basement membrane thickening, mesangial matrix accumulation, glycogen deposition and tubulointerstitial changes (Figure 2b, right).
Table 2. Metabolic parameters of DS/lean and DS/obese rats at 18 weeks of age.
| Parameter | DS/lean | DS/obese | P |
|---|---|---|---|
| Leptin (ng ml−1) | 1.67±0.37 | 53.97±2.34 | 0.0005 |
| Glucose (mg per 100 ml) | 124.50±6.40 | 136.33±5.27 | 0.2864 |
| Insulin (ng ml−1) | 0.55±0.29 | 3.18±1.39 | 0.0209 |
| Triglyceride (mg per 100 ml) | 102.25±25.98 | 1559.93±353.89 | 0.0029 |
| Total cholesterol (mg per 100 ml) | 116.25±11.59 | 371.14±39.21 | 0.0029 |
| LDL-cholesterol (mg per 100 ml) | 24.00±2.94 | 73.85±7.15 | 0.0046 |
| HDL-cholesterol (mg per 100 ml) | 26.75±1.11 | 50.14±2.30 | 0.0029 |
| LDL-cholesterol/HDL-cholesterol | 0.89±009 | 1.59±0.15 | 0.0257 |
| AST (U l−1) | 57.00±1.52 | 67.33±4.67 | 0.1213 |
| ALT (U l−1) | 24.67±0.88 | 39.25±2.63 | 0.0087 |
| Blood urea nitrogen (mg per 100 ml) | 19.98±1.86 | 76.21±13.56 | 0.0029 |
| Creatinine (mg per 100 ml) | 0.32±0.03 | 1.09±0.19 | 0.0029 |
| Urinary protein (mg day−1) | 336.29±19.58 | 735.52±93.97 | 0.0143 |
| Ccr (ml min–1 per g kidney weight) | 1.25±0.12 | 0.20±0.03 | 0.0082 |
| Ccr (ml min–1 per 100 g body weight) | 0.91±0.06 | 0.18±0.02 | 0.0082 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Ccr, creatinine clearance; DS, Dahl salt-sensitive; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Analytes were measured in serum unless indicated otherwise. Data are given as means±s.e.m. for surviving animals (n=6 and 7 for DS/lean and DS/obese rats, respectively).
Figure 2.
Histology of the liver and kidney of DS/lean and DS/obese rats at 18 weeks of age. Sections of the liver were stained with hematoxylin–eosin (a) and those of the kidney were stained with periodic acid–Schiff (PAS) solution (b). Scale bar, 100 μm.
Discussion
We have shown that DS/obese rats fed a normal diet develop obesity, as well as hypertension, dyslipidemia, insulin resistance and type 2 diabetes mellitus. In addition, these animals develop cardiac hypertrophy as well as renal and liver damage, which may be responsible for their premature death. Our results thus indicate that this rat strain may be a suitable model of human MetS.
Leptin is an adipocyte-derived hormone that has a pivotal role in regulation of food intake, energy expenditure, body weight and neuroendocrine function.14, 15, 16 The serum concentration of leptin was markedly increased in DS/obese rats compared with DS/lean rats, suggesting that the obesity of DS/obese rats is the result of leptin resistance. Hyperleptinemia and leptin resistance occur in animals with diet-induced obesity, as well as in obese humans.17, 18 We also found that the serum concentration of insulin was increased in DS/obese rats compared with DS/lean rats, but that serum glucose levels did not differ between the two strains, which is indicative of the development of insulin resistance in the former animals. Leptin suppresses insulin secretion through both central actions and direct effects on pancreatic beta cells.19, 20 Leptin opposes insulin resistance, in part, through activation of AMP-activated protein kinase, which results in a reduction in lipid deposition in insulin-sensitive tissues.21 A lack of effective leptin signaling thus promotes the development of insulin resistance and results in severe obesity at an early age. Indeed, leptin resistance has been shown to induce hyperinsulinemia and insulin resistance in both humans and animals.22, 23 Furthermore, there is an interdependent relation between insulin sensitivity and salt sensitivity of blood pressure in DS rats.24 DS/obese rats may therefore develop insulin resistance as a result of a failure to respond to leptin (because of the mutation in the leptin receptor) and the increased salt sensitivity of blood pressure.
Leptin regulates blood pressure through the sympathetic nervous system.25 Blood pressure in genetically obese animals with leptin resistance, including obese Zucker rats, has been shown to be reduced or at least not elevated compared with that in their lean littermates.26, 27 In the present study, DS/obese rats fed a normal diet manifested increases in both SBP and body weight compared with DS/lean rats at 11 weeks of age and thereafter. It is possible that the presence of the fa allele of Lepr is associated with increased salt sensitivity of blood pressure. Whereas SBP was relatively constant in DS/lean rats during the entire experimental period, it increased progressively, together with body weight, in DS/obese rats. Given that the ratios of both LV weight to tibial length and LV weight to right ventricular weight were significantly increased in DS/obese rats, LV hypertrophy was induced in association with hypertension. Tibial length was significantly shorter in DS/obese rats than in DS/lean rats. Given that leptin contributes to the central regulation of bone metabolism,28 the growth of bone may be inhibited in DS/obese rats because of the leptin receptor mutation.
Serum levels of total cholesterol, LDL-cholesterol, HDL-cholesterol and triglyceride, as well as the ratio of LDL-cholesterol to HDL-cholesterol concentrations, were significantly increased in DS/obese rats compared with DS/lean rats. The serum of most DS/obese rats was also milky in appearance as a result of their triglyceride and total cholesterol levels being increased 15- and 3-fold, respectively. The marked increase in serum alanine aminotransferase level and lipid accumulation in the liver of DS/obese rats is indicative of fat-induced liver damage. Pronounced renal damage was also apparent in these animals, as revealed by their proteinuria and a decrease in creatinine clearance and by abnormal periodic acid–Schiff staining. Several metabolic, functional and structural renal changes in diabetic rodents have fundamental similarities to those occurring in diabetic patients.29 These diabetic rodents develop renal enlargement, glomerular hypertrophy and renal hyperfiltration within weeks after diabetes debut and, subsequently, increased albumin excretion rate, glomerular extracellular matrix accumulation, increased glomerular basement membrane thickness and mesangial proliferation within months. Moreover, tubulointerstitial fibrosis in diabetes mellitus is an important pathological feature of advanced diabetic nephropathy, particularly in those patients with associated renal insufficiency.30 Thus, the renal pathological findings observed in DS/obese rats were in good agreement with those in overt diabetic nephropathy.
Several rat strains could be potentially used to study MetS.3 Although Zucker fatty rats are a widely used model of insulin resistance and type 2 diabetes mellitus, they cannot be considered as a clear experimental model of hypertension. The JCR:LA-corpulent rat, if homozygous for the cp gene, exhibits signs and symptoms characteristic of MetS, but they are not hypertensive.31 The spontaneously hypertensive rats (SHRs), a well-known experimental model of hypertension, have also been proposed as a model of insulin resistance. In the background of SHRs, different strains of corpulent SHRs, such as obese SHRs/Koletsky rats, SHRs/N-corpulent rats and SHRs/NDmc-corpulent rats, seem to be more appropriate to study MetS than the SHRs. However, these strains slowly develop moderate hypertension with a normal diet and therefore require a long period to exhibit the characteristics of cardiovascular disease.32 In contrast, DS/obese rats used in the present study become markedly hypertensive even with a normal diet, reflecting increased salt sensitivity of blood pressure, and cause cardiovascular complications and premature death within a relatively short period.
In conclusion, our results show that DS/obese rats fed a normal diet develop obesity as well as hypertension, dyslipidemia and insulin resistance. The phenotype of DS/obese rats is thus similar to that of humans with MetS. The newly established DS/obese rat may therefore be a good animal model of human MetS, and further studies of this model should improve our understanding of the interaction between metabolic and cardiovascular phenotypes in MetS.
Acknowledgments
This work was supported by unrestricted research grants from Nippon Boehringer Ingelheim Co. Ltd. (Tokyo, Japan) and Kyowa Hakko Kirin Co. Ltd. (Tokyo, Japan), as well as by Management Expenses Grants from the Japanese government to Nagoya University.
The authors declare no conflict of interest.
References
- Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the ‘metabolic syndrome' and incidence of type 2 diabetes. Diabetes. 2002;51:3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Aleixandre de Artinano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. Br J Nutr. 2009;102:1246–1253. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, et al. Molecular cloning of rat leptin receptor isoform complementary DNAs—identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun. 1996;225:75–83. doi: 10.1006/bbrc.1996.1133. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko M, Kihara Y, Morii I, Fujiwara H, Sasayama S. Transition from compensatory hypertrophy to dilated, failing left ventricles in Dahl salt-sensitive rats. Am J Physiol. 1994;267 (Part 2:H2471–H2482. doi: 10.1152/ajpheart.1994.267.6.H2471. [DOI] [PubMed] [Google Scholar]
- Nagata K, Liao R, Eberli FR, Satoh N, Chevalier B, Apstein CS, et al. Early changes in excitation-contraction coupling: transition from compensated hypertrophy to failure in Dahl salt-sensitive rat myocytes. Cardiovasc Res. 1998;37:467–477. doi: 10.1016/s0008-6363(97)00278-2. [DOI] [PubMed] [Google Scholar]
- Nagata K, Somura F, Obata K, Odashima M, Izawa H, Ichihara S, et al. AT1 receptor blockade reduces cardiac calcineurin activity in hypertensive rats. Hypertension. 2002;40:168–174. doi: 10.1161/01.hyp.0000026668.50222.1e. [DOI] [PubMed] [Google Scholar]
- Xu J, Nagata K, Obata K, Ichihara S, Izawa H, Noda A, et al. Nicorandil promotes myocardial capillary and arteriolar growth in the failing heart of Dahl salt-sensitive hypertensive rats. Hypertension. 2005;46:719–724. doi: 10.1161/01.HYP.0000185189.46698.15. [DOI] [PubMed] [Google Scholar]
- Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, et al. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension. 2006;47:656–664. doi: 10.1161/01.HYP.0000203772.78696.67. [DOI] [PubMed] [Google Scholar]
- Kato MF, Shibata R, Obata K, Miyachi M, Yazawa H, Tsuboi K, et al. Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. J Hypertens. 2008;26:1669–1676. doi: 10.1097/HJH.0b013e328302f0f7. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, et al. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension. 2009;53:701–707. doi: 10.1161/HYPERTENSIONAHA.108.127290. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Nakama K, Katsuta Y, Zhang XJ, Ohsuga M, Ishizaki M, et al. Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Lepr(fa)) rat. Biochem Biophys Res Commun. 2008;366:556–562. doi: 10.1016/j.bbrc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Hoggard N, Mercer JG, Rayner DV. Leptin: fundamental aspects. Int J Obes Relat Metab Disord. 1999;23 (Suppl 1:22–28. doi: 10.1038/sj.ijo.0800791. [DOI] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274:1185–1188. doi: 10.1126/science.274.5290.1185. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun. 1996;224:522–527. doi: 10.1006/bbrc.1996.1059. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Huang KC, Lin RC, Kormas N, Lee LT, Chen CY, Gill TP, et al. Plasma leptin is associated with insulin resistance independent of age, body mass index, fat mass, lipids, and pubertal development in nondiabetic adolescents. Int J Obes Relat Metab Disord. 2004;28:470–475. doi: 10.1038/sj.ijo.0802531. [DOI] [PubMed] [Google Scholar]
- Zimmet PZ, Collins VR, de Courten MP, Hodge AM, Collier GR, Dowse GK, et al. Is there a relationship between leptin and insulin sensitivity independent of obesity? A population-based study in the Indian Ocean nation of Mauritius. Mauritius NCD Study Group. Int J Obes Relat Metab Disord. 1998;22:171–177. doi: 10.1038/sj.ijo.0800559. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension. 2002;40:83–89. doi: 10.1161/01.hyp.0000022880.45113.c9. [DOI] [PubMed] [Google Scholar]
- Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Leung YM, Yao L, Battell M, Dumont AS, McNeill JH. Hyperinsulinemia superimposed on insulin resistance does not elevate blood pressure. Am J Hypertens. 2001;14 (Part 1:429–432. doi: 10.1016/s0895-7061(00)01261-9. [DOI] [PubMed] [Google Scholar]
- Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17 (Part 2:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A. Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia. 2000;43:1205–1223. doi: 10.1007/s001250051515. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Goldfarb S. The renal tubulointerstitium in diabetes mellitus. Kidney Int. 1991;39:464–475. doi: 10.1038/ki.1991.57. [DOI] [PubMed] [Google Scholar]
- Russell JC, Graham S, Hameed M. Abnormal insulin and glucose metabolism in the JCR:LA-corpulent rat. Metabolism. 1994;43:538–543. doi: 10.1016/0026-0495(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, et al. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008;52:287–294. doi: 10.1161/HYPERTENSIONAHA.108.111815. [DOI] [PubMed] [Google Scholar]