Abstract
Objective:
Along with the increasing prevalence of obesity and related diseases, particularly atherosclerotic diseases, metabolic syndrome (MetS) is now a common and major public health issue in many countries around the world. Adiponectin, a protein secreted by the adipose tissue, has become recognized as a key player in the development of MetS. These days, not only MetS but also borderline metabolic/physiological abnormalities, such as impaired fasting glucose, high normal blood pressure and high normal plasma cholesterol, have been reported to be risk factors for atherosclerotic disease. Therefore, we undertook this study to determine the relationship between adiponectin and borderline metabolic/physiological abnormalities, as well as MetS.
Design:
A cross-sectional study performed from April 2007 to November 2009.
Subjects:
In 16 892 Japanese adults (10 008 men and 6884 women), we examined the relationship between the serum adiponectin concentration and borderline metabolic/physiological abnormalities or MetS by a questionnaire survey about medical treatment, body size measurement and measurement of laboratory parameters including the serum adiponectin concentration.
Results:
Adiponectin showed a significant negative correlation with the number of MetS components. In subjects without overt diabetes mellitus, hypertension or dyslipidemia, the adiponectin concentration also showed a significant negative correlation with the number of borderline metabolic abnormalities.
Conclusion:
The decrease of circulating adiponectin may start before the development of diabetes mellitus, hypertension, dyslipidemia or MetS. Adiponectin is an important biomarker for reflecting the adverse influence of visceral fat in persons with MetS, and also in these subclinical states.
Keywords: adiponectin, visceral fat, metabolic syndrome, borderline metabolic abnormalities, arteriosclerosis
Introduction
In recent years, the increased prevalence of obesity has led to an increase of related diseases, especially atherosclerotic diseases such as cardiovascular and cerebrovascular disease, which are now responsible for high levels of morbidity and mortality.1 Many of these patients have metabolic syndrome (MetS), which arises from the accumulation of visceral fat.2 Thus, MetS has become a major public health issue in many countries around the world.
To identify the molecular mechanisms underlying diseases related to visceral obesity, the biological characteristics of the adipose tissue were investigated by analysis of the gene expression profile in fat, and revealed a gene named adiponectin.3 Adiponectin is found at a relatively high concentration in human plasma, accounting for approximately 0.01% of total plasma protein,4 and its level is inversely correlated with visceral adiposity.5 Many studies have been performed on adiponectin and various components of the MetS. As a result, adiponectin has been reported to exhibit insulin-sensitizing, antiatherogenic and antiinflammatory effects.6, 7 Conversely, low adiponectin levels are related to insulin resistance, an increased risk of type 2 diabetes,8 hypertension,9 dyslipidemia10 and low plasma levels of high-density lipoprotein cholesterol.10 Because of these documented associations, adiponectin is considered to have a key role in the development of MetS and its consequence, cardiovascular and cerebrovascular disease,5, 11 and the regulation of adiponectin might be considered as an efficient therapeutic procedure.2, 12
Several borderline metabolic and physiological abnormalities, such as impaired fasting glucose, high normal blood pressure (BP) and a high normal plasma level of low-density lipoprotein cholesterol, have also been reported to increase the risk of atherosclerotic disease.13, 14, 15, 16, 17 However, there have been no studies of adiponectin levels in such subclinical populations. Therefore, we undertook this study to determine the relationship between adiponectin and borderline metabolic/physiological abnormalities, as well as MetS.
Subjects and methods
The subjects of this study were 16 892 adults (10 008 men and 6884 women) who had complete medical checkup at the Physical Check up Center in Sumitomo Hospital from April 2007 to November 2009. In Japan, people often had their medical checkup by their own will at a physical checkup center attached to some hospitals, in order to evaluate their health condition. The subjects of this study were those who were not patients visited to our hospital, and all of them had no apparent disease that required hospital admission. A questionnaire about medical treatment was completed, followed by the measurement of body size and blood tests (including the serum adiponectin concentration). BP was measured while they are sitting. All people who received medication of diabetes mellitus, hypertension and dyslipidemia were excluded. All participants signed informed consent to provide medical information and blood samples before undergoing the medical check up, and they had the right to refuse use of their data. The ethical committee of Sumitomo Hospital approved this study and it was conducted according to the principles of the Declaration of Helsinki.
MetS was diagnosed according to the Japanese criteria,18 which are a waist circumference of at least 85 cm for men or 90 cm for women and at least two of the following: glucose intolerance (fasting plasma glucose⩾110 mg dl−1), high BP (systolic/diastolic BP⩾130/85 mm Hg) and dyslipidemia (triglycerides⩾150 mg dL−1 and high-density lipoprotein cholesterol<40 mg dl−1). Borderline metabolic abnormalities were defined as follows: impaired fasting glycemia was fasting plasma glucose of 110–125 mg dl−1, high normal BP was a systolic BP of 130–139 mm Hg and/or diastolic BP of 85–89 mm Hg, and high normal low-density lipoprotein cholesterol was an low-density lipoprotein cholesterol of 120–139 mg dl−1, according to the criteria of the Japan Diabetes Society,19 Japanese Society of Hypertension20 and Japan Atherosclerosis Society,21 respectively. Diagnosis of diabetes mellitus (fasting plasma glucose⩾126 mg dl−1 or hemoglobin A1c (HbA1c) ⩾6.5%(National Glycohemoglobin Standardization Program)), hypertension (systolic BP⩾140 mm Hg or diastolic BP⩾90 mm Hg) and dyslipidemia (low-density lipoprotein cholesterol⩾140, triglycerides⩾150 and high-density lipoprotein cholesterol<40) was also done according to the criteria of these three societies.
The serum adiponectin level was used to divide the subjects into five groups, which were less than 4 μg ml−1 (hypoadiponectinemia),22 more than 4 μg ml−1 and less than 7 μg ml−1, more than 7 μg ml−1 and less than 10 μg ml−1, more than 10 μg ml−1 and less than 13 μg ml−1 and more than 13 μg ml−1. Then the relationship between the adiponectin level and the number of MetS components, the presence/absence of borderline metabolic abnormalities and the waist circumference was examined. When investigating the number of borderline metabolic abnormalities, patients with clinically overt conditions such as diabetes, hypertension or dyslipidemia (5937 men and 2215 women) were excluded.
The serum adiponectin level was measured by a latex particle-enhanced turbidimeric immunoassay (Human adiponectin latex kit, Otsuka Pharmaceutical Co. Ltd, Tokyo, Japan) as reported previously.23 HbAlc (%) was converted to a National Glycohemoglobin Standardization Program (National Glycohemoglobin Standardization Program) equivalent value (%) by the formula HbA1c (National Glycohemoglobin Standardization Program) (%)=HbA1c (Japan Diabetes Society) (%)+0.4%, based on the relationship previously determined between HbA1c (Japan Diabetes Society) (%) measured with the previous Japanese standard substance and measurement method and HbA1c (National Glycohemoglobin Standardization Program).19
Statistical analysis was performed with Windows-based SPSS software (version 19.0, SPSS, Chicago, IL, USA). Non-normally distributed variables were log transformed before the analysis. Descriptive statistics were determined and the student's t-test or χ2-test was conducted for comparison between MetS and non-MetS subjects among men and among women separately. One-way analysis of variance was performed to compare continuous variables. Post-hoc multiple comparisons were made by using Tukey's test. Multiple linear regression analysis was employed to examine the relationship between the number of MetS components or high normal states and variables listed in Table 1 except for the marker of diabetes mellitus, hypertension, dyslipidemia and waist circumference. Statistical significance was accepted at P<0.05.
Table 1. Clinical and biological characteristics of participants.
|
Men (n=10 008) |
Pa |
Women (n=6884) |
Pa | |||
|---|---|---|---|---|---|---|
| Non-MetS (n=8722) | MetS (n=1286) | Non-MetS (n=6799) | MetS (n=85) | |||
| Age (year) | 51.3±11.0 | 52.4±10.0 | <0.001 | 48.7±10.9 | 56.2±10.7 | <0.001 |
| Height (cm) | 170.3±6.0 | 171.7±6.0 | <0.001 | 158.2±5.5 | 156.8±5.3 | 0.016 |
| Body weight (kg) | 66.6±9.0 | 77.5±9.9 | <0.001 | 51.8±7.3 | 69.2±12.7 | <0.001 |
| BMI (kg m−2) | 22.9±2.7 | 26.3±2.9 | <0.001 | 20.7±2.8 | 28.1±4.4 | <0.001 |
| Waist (cm) | 83.7±7.4 | 93.5±6.4 | <0.001 | 76.5±8.2 | 97.8±7.4 | <0.001 |
| Smoking (no/yes) | 5935/2787 | 825/461 | 0.007 | 6253/546 | 73/12 | 0.041 |
| Systolic BP (mm Hg) | 122.7±14.0 | 138.0±12.5 | <0.001 | 117.1±15.3 | 144.8±14.7 | <0.001 |
| Diastolic BP (mm Hg) | 77.2±9.5 | 87.0±8.6 | <0.001 | 72.5±9.9 | 87.9±9.4 | <0.001 |
| T-CHO (mg dl−1) | 210.4±32.1 | 223.2±33.4 | <0.001 | 215.1±36.0 | 245.3±43.2 | <0.001 |
| LDL-C (mg dl−1) | 126.4±30.5 | 136.7±32.0 | <0.001 | 118.8±31.6 | 154.1±38.6 | <0.001 |
| HDL-C (mg dl−1) | 60.0±13.9 | 52.5±11.1 | <0.001 | 73.1±16.3 | 58.7±13.9 | <0.001 |
| TG (mg dl−1) | 122.6±85.2 | 219.9±124.8 | <0.001 | 83.2±46.5 | 192.3±81.4 | <0.001 |
| FPG (mg dl−1) | 94.1±12.9 | 108.9±25.5 | <0.001 | 88.0±9.2 | 112.1±38.8 | <0.001 |
| HbA1c (NGSP) (%) | 5.6±0.5 | 6.0±0.9 | <0.001 | 5.4±0.4 | 6.1±1.3 | <0.001 |
| HOMA-IR | 1.3±0.8 | 2.6±2.0 | <0.001 | 1.1±0.6 | 3.2±2.4 | <0.001 |
| Uric acid (mg dl−1) | 6.1±1.2 | 6.6±1.2 | <0.001 | 4.4±0.9 | 5.5±1.0 | <0.001 |
| Cr (mg dl−1) | 0.83±0.20 | 0.82±0.12 | 0.253 | 0.61±0.10 | 0.58±0.09 | 0.001 |
| Adiponectin (μg ml−1) | 8.3±4.2 | 6.2±2.8 | <0.001 | 13.3±6.0 | 9.3±4.8 | <0.001 |
Abbreviations: BMI, body mass index; BP, blood pressure; Cr, serum creatinine; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; NGSP, National Glycohemoglobin Standardization Program; T-CHO, total cholesterol; TG, triglycerides; HOMA-IR, homeostasis model assessment-insulin resistance.
Waist, waist circumference.
Values are expressed as mean±standard deviations.
Student's t-test or χ2-test at P<0.05.
Results
Clinical and biological characteristics
The clinical and biological characteristics of the participants are shown in Table 1. On average, subjects without MetS were younger, had a smaller body mass index and waist circumference than those with MetS both in men and women. They also had a lower BP, fasting plasma glucose, hemoglobin Alc, homeostasis model assessment-insulin resistance and uric acid than the subjects with MetS, as well as a better lipid profile both in men and women. The mean serum adiponectin concentration was higher in people without MetS than those with MetS, being 8.3 vs 6.2 μg ml−1 in men and 13.3 vs 9.3 μg ml−1 in women, respectively (P<0.001).
One-way analysis of variance
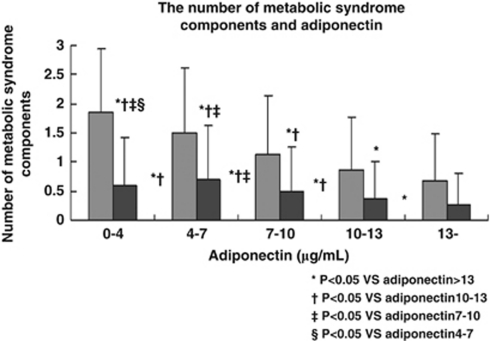
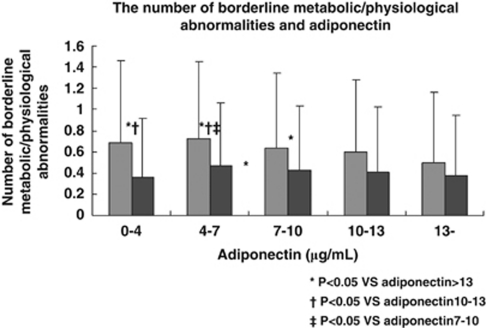
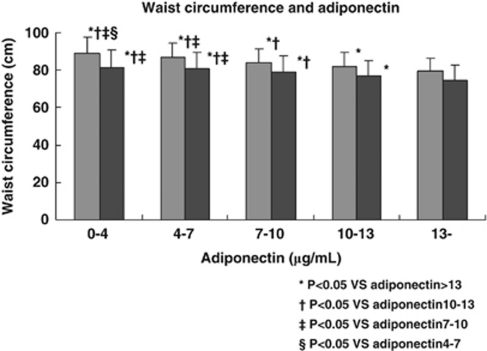
Adiponectin showed a significant negative correlation with the number of MetS components (Figure 1), the number of borderline metabolic abnormalities (Figure 2) and the waist circumference (Figure 3).
Figure 1.
The number of MetS components in relation to the serum adiponectin level. Gray bars (left) show the number of MetS components in men and black bars (right) show the number in women.
Figure 2.
The number of borderline metabolic/physiological abnormalities in relation to the serum adiponectin level among subjects without diabetes, hypertension or dyslipidemia. Gray bars (left) show the number of borderline abnormalities in men and black bars (right) show the number in women.
Figure 3.
Waist circumference and the serum adiponectin level. Gray bars (left) show the waist circumference in men and black bars (right) show it in women.
Multivariable analysis
According to multivariate analysis, the serum adiponectin concentration was negatively correlated with the number of MetS components (Table 2). Body mass index, age, HOMA-R, uric acid and smoking habit showed a significant positive correlation with the number of MetS components, and sex (female) and creatinine showed a significant negative correlation with them. Similarly, after excluding the patients with overt diabetes mellitus, hypertension and dyslipidemia, the serum adiponectin concentration was negatively correlated with the number of borderline metabolic abnormalities (Table 3). Body mass index, age, HOMA-R and uric acid also showed a significant positive correlation with the number of high normal states, and smoking habit, sex (female) and creatinine showed a significant negative correlation with them.
Table 2. The result of multivariable analysis of correlation between the number of metabolic syndrome components and various parameters, including adiponectin.
| B | β | P |
95% CI |
||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Invariable | −2.503 | 0.00 | −2.657 | −2.349 | |
| BMI | 0.117 | 0.362 | 0.00 | 0.112 | 0.122 |
| HOMA-R | 0.249 | 0.226 | 0.00 | 0.234 | 0.263 |
| Age (year) | 0.015 | 0.164 | 0.00 | 0.014 | 0.016 |
| Sex (female) | −0.298 | −0.144 | 0.00 | −0.332 | −0.263 |
| Uric acid (mg dl−1) | 0.081 | 0.110 | 0.00 | 0.07 | 0.091 |
| Adiponectin (μg ml−1) | −0.011 | −0.061 | 0.00 | −0.014 | −0.009 |
| Cr | −0.288 | −0.053 | 0.00 | −0.362 | −0.214 |
| Smoking (yes) | 0.069 | 0.028 | 0.00 | 0.041 | 0.097 |
Abbreviations: BMI, body mass index; CI, confidence interval; Cr, serum creatinine; HOMA-IR, homeostasis model assessment-insulin resistance.
B, partial regression coefficient; β, standard partial regression coefficient; P, significance probability.
Objective variable, the number of metabolic syndrome components stepwise multiple regression analysis.
Table 3. The result of multivariable analysis of the relationship between the number of high normal components and other variables, including adiponectin.
| B | β | P |
95% CI |
||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Invariable | −0.863 | 0.000 | −1.049 | −0.677 | |
| Age (year) | 0.013 | 0.226 | 0.000 | 0.012 | 0.015 |
| BMI | 0.033 | 0.138 | 0.000 | 0.027 | 0.039 |
| HOMA-R | 0.131 | 0.126 | 0.000 | 0.108 | 0.154 |
| Uric acid (mg dl−1) | 0.025 | 0.050 | 0.000 | 0.012 | 0.039 |
| Sex (female) | −0.066 | −0.050 | 0.001 | −0.106 | −0.026 |
| Adiponectin (μg ml−1) | −0.004 | −0.039 | 0.001 | −0.007 | 0.002 |
| Smoking (yes) | −0.051 | −0.030 | 0.005 | −0.086 | −0.016 |
| Cr | −0.091 | −0.027 | 0.033 | −0.175 | −0.007 |
Abbreviations: BMI, body mass index; CI, confidence interval; Cr, serum creatinine; HOMA-IR, homeostasis model assessment-insulin resistance.
B, partial regression coefficient; β, standard partial regression coefficient; P, significance probability.
Objective variable: the number of metabolic syndrome components stepwise multiple regression analysis.
Discussion
MetS is now well known to be a risk factor for the cardiovascular and cerebrovascular disease, and many studies have demonstrated an association between serum adiponectin levels and MetS. In recent years, borderline metabolic/physiological abnormalities, such as impaired fasting glucose, high normal BP and high normal plasma LDL cholesterol, have also been reported to be the risk factors for atherosclerotic disease. However, there has been no investigation of the relationship between the serum adiponectin level and such high normal states. Accordingly, this is the first large-scale investigation of the relationship between adiponectin and borderline metabolic/physiological abnormalities.
The present study revealed several important findings. First, it confirmed a significant negative correlation between adiponectin and the number of MetS components, as previously reported.24 Second, this study also showed a significant negative correlation between adiponectin and the number of borderline metabolic/physioligical abnormalities in a population from which overt diabetes, hypertension and dyslipidemia had been excluded. Although the association was not significant in women from the lowest adiponectin group (Figure 2), this was probably due to the small number of women in the hypoadiponectinemia group (n=53). Third, the adiponectin level decreased significantly as the waist circumference increased. Though waist circumference is not the optimal measure of visceral fat, this result shows the association between serum adiponectin level and the amount of visceral fat, as it has been reported.2
The decrease of adiopnectin is mainly caused by the accumulation of visceral fat, as well as genetic characteristics, and the present study showed that adiponectin starts to decline and its lack has adverse effects even in people with borderline metabolic/physiological abnormalities. It has been already reported that the adiponectin level is decreased in patients with excess visceral fat who have overt diabetes mellitus, hypertension, dyslipidemia or MetS.8, 9, 10, 11 In addition, the present study suggests the possibility that the decrease of adiponectin may begin at an early stage when there is less accumulation of visceral fat with borderline abnormalities, on the way to MetS.
Moreover, this study suggested that not only visceral fat but also adiponectin itself can influence metabolic/physiological factors, just as it has been reported that the decrease of adiponectin increases the risk of type 2 diabetes mellitus,8 hypertension,9 dyslipidemia10 and atherosclerotic diseases.7 Therefore, the adiponectin level can be a reliable predictor of the risk of cardiovascular and cerebrovascular disease in people with borderline metabolic/physiological abnormalities, as well as in those who have other obvious risk factors like diabetes, hypertension, dyslipidemia or MetS.
In conclusion, we found that a decrease of circulating adiponectin starts at a very early stage before the onset of overt diabetes, hypertension, dyslipidemia or MetS. Accordingly, in persons with borderline metabolic/physiological abnormalities (as well as overt diseases), adiponectin is an important biomarker for the risk of atherosclerotic disease both independently and as a reflection of the accumulation of visceral fat.
Acknowledgments
We thank Dr Akihiro Nishi, Harvard School of Public Health, for giving statistical advice.
The authors declare no conflict of interest.
References
- Berrington de Gonzalez A, Hartqe P, Cerhan JR, Flint AJ, Hannan L, Maclnnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:131–141. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM 1(adipose most abundant gene transcript-1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006;110:267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- Almeda-Valdes P, Cuevas-Ramos D, Mehta R, Gomez-Perez FJ, Cruz-Bautista I, Arellano-Campos O, et al. Total and high molecular weight adiponectin for the identification of insulin resistance. Cardiovasc Diabetol. 2010;9:26. doi: 10.1186/1475-2840-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cui J, Zhang C. Emerging role of adipokines as mediators in atherosclerosis. World J Cardiol. 2010;2:370–376. doi: 10.4330/wjc.v2.i11.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Möhlig M, Bergmann MH, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Ouchi N, Matsuzawa Y. Adiponectin and hypertension. Am J Hypertens. 2011;24:263–269. doi: 10.1038/ajh.2010.216. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Muruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Prac Cardiovasc Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- Kitada S, Otsuka Y, Kokubu N, Kasahara Y, Kataoka Y, Noguchi T, et al. Post-load hyperglycemia as an important predictor of long-term adverse cardiac events after acute myocardial infarction: a scientific study. Cardiovasc Diabetol. 2010;9:75. doi: 10.1186/1475-2840-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizibash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto T, Okamura T, Kadowaki T, Hayakawa T, Kita Y, et al. Combined cardiovascular risk factors and outcome-nippon data80, 1980-1994. Circ J. 2006;70:960–964. doi: 10.1253/circj.70.960. [DOI] [PubMed] [Google Scholar]
- Kubo M, Hata J, Doi Y, Iida M, Kiyohara Y. Secular trends in the incidence and risk factors of ischemic stroke and its subtypes in the Japanese population. Circulation. 2008;118:2672–2678. doi: 10.1161/CIRCULATIONAHA.107.743211. [DOI] [PubMed] [Google Scholar]
- Committee to Evaluate Diagnostic Standards for Metabolic Syndrome Definition and the diagnostic standard for metabolic syndrome Nippon Naika Gakkai Zasshi 200594794–809.(article in Japanese). [PubMed] [Google Scholar]
- The Committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus Report of the Committee on the classification and diagnostic criteria of diabetes mellitus Journal of the Japan Diabetes Society 201053450–467.(article in Japanese). [Google Scholar]
- Japanese Society of Hypertension Committee The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) 2009. pp. 3–107. [PubMed]
- Committee for Epidemiology and Clinical Management of Atherosclerosis Diagnostic criteria for dyslipidemia-executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler thromb. 2007;14:155–158. doi: 10.5551/jat.e537. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Biochem Biophys Res Commun. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2006;371:163–168. doi: 10.1016/j.cca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Koh SB, Park JK, Yoon JH, Chang SJ, Oh SS, Kim JY, et al. Preliminary report: a serious link between adiponectin levels and metabolic syndrome in a Korean nondiabetic population. Metabolism. 2010;59:325–332. doi: 10.1016/j.metabol.2009.07.031. [DOI] [PubMed] [Google Scholar]