Abstract
Objectives:
Because females have blunted counterregulatory responses to hypoglycemia relative to males, we hypothesized that females would have greater sensitivity to changes in lipid availability.
Design and subjects:
To assess this, we examined the feeding response to glucoprivation (2-deoxyglucose; 2DG) and lipoprivation (mercaptoacetate; MA) in age-matched male and female Long-Evans rats.
Results:
Males versus females had significantly greater food intake after 250 mg kg−1 of 2DG, but there were no sex differences with the 750 mg kg−1 dose of 2DG. Glucose responses to 250 mg kg−1 of 2DG were also significantly greater in males versus females. In contrast, females had a significant increase in food intake with all doses of MA versus saline, and had significantly greater food intake compared with males at the lowest and highest doses of MA with a trend towards significance with the intermediate dose. To determine whether estradiol (E2) is the mechanism underlying this sexual dimorphism, ovariectomized females were injected with vehicle or 2 μg of E2 every fourth day to mimic the variations in across the estrous cycle. Ovariectomized females significantly increased feeding and glucose after 250 mg kg−1 of 2DG over intact females and E2 had no effect on these responses. Although the feeding response to 2DG was not different, the glucose response to 2DG was still significantly greater in males versus ovariectomies females. However, ovariectomized females also did not increase food intake after MA, regardless of E2 treatment.
Conclusions:
These data collectively suggest that males are relatively more sensitive to glucose deprivation and females are relatively more sensitive to lipid deprivation. Further, these data rule out a role for cyclic changes in E2 in these sex differences.
Keywords: sex dimorphism, nutrient sensing, glucoprivation, lipoprivation, estradiol
Introduction
The central nervous system (CNS) responds to hormonal, nutrient and other afferent feedback to regulate energy homeostasis, and data suggest that there are important sex differences in the responses to many of these CNS signals. As an example, relative to males, females are more sensitive to the anorectic effects of intracerebroventricular (ICV) leptin, but less sensitive to the anorectic effects of ICV insulin1 and to the orexigenic effects of ICV ghrelin.2 Hence, at least with regard to the control of food intake, males and females differ in their responses to neuroendocrine signals. There are also data indicating that males and females respond differentially to changes in nutrient status or flux. For example, during a prolonged fast, women have lower plasma glucose and higher plasma free fatty acid levels than men,3 and during hypoglycemia, women also have blunted neural and hormonal responses relative to males.4 These data suggest that women rely more on fats than on carbohydrates when energy stores are low. Perhaps analogously, in times of high nutrient flux, such as during exercise, women tend to rely more than men on fat versus carbohydrate stores as a fuel source.5 Collectively, these data imply important sex differences in responding to changes in nutrient flux.
The mechanism underlying these sex differences are likely because of different levels of gonadal hormones between males and females, and specifically to estrogen levels. Estrogen is responsible for the enhanced sensitivity of females to ICV leptin and the concomitant reduced sensitivity to ICV insulin,1 and consistent with this, exogenous estradiol (E2) replacement renders ovariectomized females or intact males more female-like in their responses to leptin and insulin.1 In regards to nutrient flux, postmenopausal women taking E2 have blunted counterregulatory responses to hypoglycemia compared with those not taking estrogen,6 and the administration of E2 into the brains of male rats blunted their counterregulatory response to hypoglycemia.7 In addition, females also have greater adiposity and greater lipogenic8, 9 and lipolytic capacity3, 5 than males. Therefore, in this study we hypothesized that E2 shifts metabolism to rely more on fats than on carbohydrates. To test this hypothesis, we challenged male and female rats by selectively interfering with either fat or carbohydrate metabolism and determined the effects on behavior and glycemic control and studied the influence of estrogen on these effects.
Materials and methods
Animal preparation
Age-matched (∼10-weeks old) male (∼225 g) and female (∼175 g) Long-Evans rats were purchased from Harlan (Indianapolis, IN, USA) and singly housed according to sex in the University of Cincinnati Laboratory Animals for Medical Science Facility at the Genome Research Institute under controlled conditions (12:12 light–dark cycle, 50–60% humidity, 25 °C) with free access to standard rodent chow diet and water. All procedures for animal use were approved by The University of Cincinnati Institutional Animal Care and Use Committee.
Surgeries
In one cohort of female rats (n=41), ovariectomies (OVX) were performed at least 7 days after arrival in the laboratory. Rats were anesthetized with 1 ml kg−1 intraperitoneal (IP) injections of a mixture of 70 mg kg−1 ketamine and 2 mg kg−1 xylazine. To perform the OVX, after a midline laparotomy the ovaries were retracted at the distal end of the oviduct and were removed using surgical blades. The distal end of the oviduct and surrounding tissue were clamped with hemostats to avoid bleeding. Minimal adipose tissue was removed during the procedure.
Before surgery, female animals were weight-matched and assigned to either the E2 (n=21) or vehicle (VEH; n=20) treatment groups. β-estradiol benzoate (Sigma-Aldrich, St Louis, MO, USA) was dissolved in sesame oil at a concentration of 0.02 mg ml−1. E2 or VEH was injected subcutaneously (0.1 ml) every fourth day for 8 weeks to simulate the endogenous fluctuations of E2. This dose and injection procedure have been described previously and closely mimics, in both timing and volume, the E2 peaks across the estrous cycle.10 Food intake and metabolite studies were performed 1 month after the ovariectomy surgery.
Food intake studies
Because we expected both 2-deoxyglucose (2DG) and mercaptoacetate (MA) to increase food intake, all studies were completed when lights were on, and baseline food intake minimal to maximize the experimental signal. To determine the role of sex on response to glucoprivation, animals were weighed and food was removed ∼6 h before lights out and 1 h before the study. Animals were weight-matched within each sex and given an IP dose of 2DG (80, 250 or 750 mg kg−1) or saline VEH (n=12 per group) and food intake was measured hourly for 3 h immediately following injection. All food measures were in the light cycle when baseline food intake is lowest. Assessment of food intake after lipoprivation was assessed with MA (Sigma-Aldrich) as described previously.11 Briefly, animals maintained on a chow diet were weighed 1 h before IP injections and 4 h before lights out. Before 3 h of lights out, animals were weight-matched within each sex and MA (115, 200 and 355 μmol kg−1) or saline (n=12 per group) was injected and food was removed at the same time. One hour later (2 h before lights out), food was replaced and food intake assessed over the following hour (1 h before lights out). OVX animals were studied under similar protocols using the 250-mg kg−1 dose of 2DG and 200 μmol kg−1 dose of MA seen in intact females. Although previous studies have found it necessary to maintain animals on a high-fat diet in order to increase feeding in response to MA, we are using a protocol that has been shown to increase feeding in chow fed animals.11 This allows us to isolate the potential sex differences, independent of diet, on feeding and metabolite responses to gluco- and lipoprivation. Because repeated exposure to 2DG can blunt feeding and counterregulatory responses to subsequent 2DG,12 no animal received two doses of 2DG or MA within a 2 week period.
Metabolite responses to 2DG and MA
Animals were moved to a procedure room ∼2 h before the study. Animals were weight-matched within each sex and surgical intervention to determine 2DG or MA and saline groups. After baseline blood samples (∼200 μl) were taken via tail vein, animals were injected with 2DG (250 mg kg−1) versus saline (n=8 per group males and n=10 per group females) or MA (200 μmol kg−1) versus saline (n=7 per group males and females). However, because we observed minimal changes in metabolites in response to MA in males and intact females, in the OVX females the metabolite response to MA was not assessed. After the IP injections, blood was sampled (∼200 μl whole blood) every 30 min for 120 min. In a separate set of studies, females were injected with the same average absolute dose of 2DG (130 mg; n=12 per group) given previously to the males. In this way, we could compare both the absolute and relative (to body mass) responses to 2DG. For the metabolite studies we chose to assess glucose and glycerol responses to each drug. Triglycerides are composed of glycerol joined to three fatty acids. Lipolysis breaks these bonds to generate free glycerol and fatty acids. However, fatty acids can be reesterified within the adipose tissue while glycerol is released into the plasma. Because of this, plasma glycerol levels are more representative of whole body lipolysis than fatty acid levels.
Analytical methods
Plasma glucose and glycerol were measured using the GM7 Micro-Stat analyzer (Analox Instruments, Lunenberg, MA, USA). Body composition (fat and lean mass) was determined using nuclear magnetic resonance technology (Echo NMR, Waco, TX, USA) on unanaesthetized rats as previously described.13
Statistical analysis
The data were analyzed using mixed-model ANOVAs. A Tukey's post-hoc test was performed to assess differences in individual groups. Statistical significance was set at P<0.05 for all analyses. Data are presented as mean±s.e.
Results
Sex differences in response to 2DG
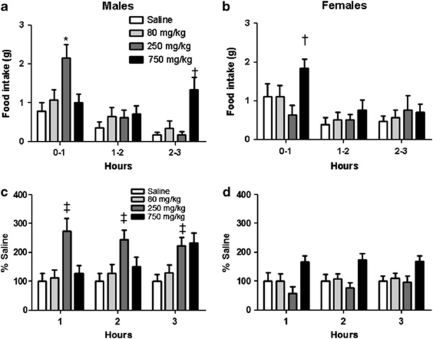
To determine the response to glucoprivation, we used 2DG, a glucose analog that inhibits the enzyme phosphohexose isomerase, and thus blocks the first reaction in glycolysis (conversion of glucose-6-phosphate to fructose-6-phosphate). In response to the 250 and 750 mg kg−1 dose of 2DG, and whether analysis was performed on absolute or relative to saline values, males had significantly greater food intake the first and third hour after injection, respectively, compared with females (P<0.05; Figures 1a–d; significant three-way sex by dose by time interaction). The post hoc analysis revealed that females significantly increased food intake compared with saline only with the 750 mg kg−1 dose (P<0.05; Figure 1b). The higher dose of 2DG has been reported to cause a conditioned taste aversion in male rats,14 which, independent of nutrient availability, could reduce food intake and explain the delayed hyperphagic response to the highest dose of 2DG in the males.
Figure 1.
Feeding response to 2DG. (a, b) Food intake was significantly increased after the 250 and 750 mg kg−1 doses of 2DG versus saline at 0–1 and 2–3 h after injection, respectively, in males versus females (*P<0.05). † within each sex, 750 mg kg−1 dose versus saline (P<0.05). (c, d) Percentage of saline feeding responses were significantly greater in males versus females at the 250 mg kg−1 dose (‡P<0.05). Data are mean±s.e.m.
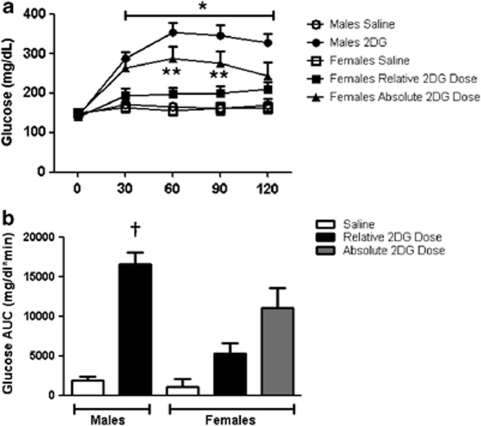
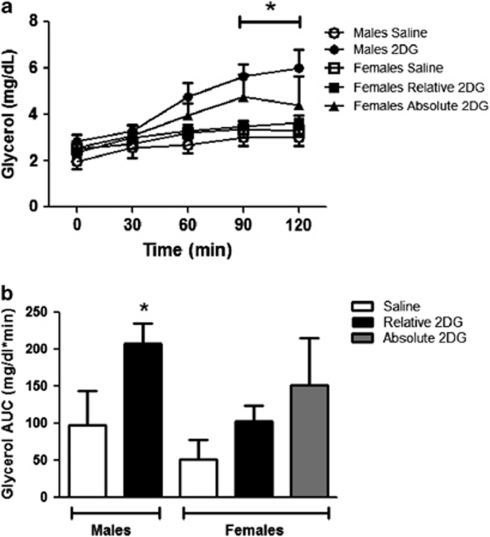
The plasma glucose response to 2DG was significantly increased over baseline in the males and was greater when compared with saline and also to females from 60 to 120 min (P<0.05; Figures 2a and b). The plasma glycerol response to 2DG was also significantly greater in males versus females at 90 and 120 min and when expressed as AUC (P<0.05; Figures 3a and b). In this series of experiments, the 2DG was dosed relative to body weight. Because age-matched females are significantly smaller than males, this led to the possibility that the reduced metabolic response to 2DG in the females could be secondary to receiving a lower dose. Therefore, we subsequently gave the females a fixed dose of 2DG (130 mg) that was similar to the average absolute dose given to the males (130±2.7 mg). This dose of 2DG significantly increased blood glucose over baseline and compared with saline in the females (P<0.05; Figure 2a). However, the achieved hyperglycemia was intermediate between the glucose levels seen in the males and the response seen with the lower dose in the females (P>0.05; Figures 2a and b). Thus, the same absolute dose of 2DG still failed to increase glucose to the same level as seen in the males. The males also had a significantly greater integrated glucose area under the curve (iAUC) in response to 2DG relative to both groups of females, and the females had no significant differences in glucose response between the relative and absolute doses of 2DG (Figure 2b). The fixed dose of 2DG also elicited an intermediate plasma glycerol response (Figures 3a and b) and was not statistically different from either that of the males or of females given the smaller 2DG dose.
Figure 2.
Glucose response to 2DG. (a) Male rats had significantly greater glucose responses to 2DG compared with females given the same relative (250 mg kg−1) dose of 2DG at 30–120 min (a; *P<0.05). The glucose response to 130 mg of 2DG was significantly increased versus when given at a dose that was relative to body weight in the females (**P<0.05). (b) The integrated AUC curve response to 2DG was significantly greater in males compared with all other groups (†P<0.05). Data are mean±s.e.m.
Figure 3.
Glycerol responses to 2DG. (a) Male rats had significantly greater glycerol responses to 2DG compared with females given the same relative (250 mg kg−1) dose of 2DG at 90–120 min (a; *P<0.05) and when expressed as AUC (b; *P<0.05), respectively. Data are mean±s.e.m.
Sex differences in response to MA
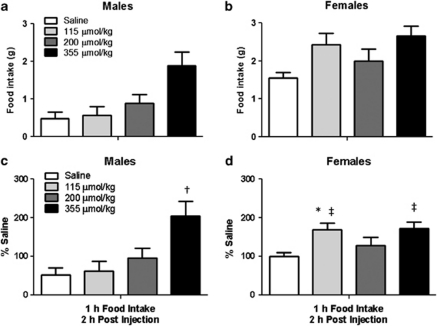
MA inhibits the enzyme fatty acid CoA dehydrogenase, which blocks the first step in fatty acid oxidation. When analyzing absolute amounts of food intake in response to MA across the sexes we saw no significant sex by dose interaction (P=0.08; Figures 4a and b). However, despite the fact that feed efficiency (food intake in Kcal per change in body weight after a 24-h fast) increased similarly in response to fasting between male and female rats (males: 8±2% vs 23±1% females: 8±3% vs 21±2% in fed vs fasted conditions) in this particular study, which takes place just before the light cycle, the females had greater food intake overall compared with the males (main effect of sex). In order to account for these baseline differences we examined the data as a percent of saline. With this analysis we see a significant sex by dose interaction with the females having greater food intake than the males at the 115 and 355 μmol kg−1 doses (P<0.05; Figures 4c and d). The post hoc analysis also revealed that the males significantly increased food intake over saline only in response to the highest dose of MA used (355 μmol kg−1; Figure 4c). This dose is similar to what others have used to elicit a feeding response in male rats on chow11 or medium-fat diets.15 In contrast, compared with saline, females had significantly greater food intake at 115 and 355 μmol kg−1, and a strong trend (P=0.06) at the 200 μmol kg−1 doses of MA (Figure 4d). There were no significant changes in glucose or glycerol response to peripherally administered MA (200 μmol kg−1; data not shown).
Figure 4.
Food intake response to MA in male and female Long-Evans rats. (a, b) There was a significant effect of sex (females >males) and dose (355 μmol kg−1 versus all, P<0.05) but no sex by dose interactions. (c, d) When the data are expressed as a percentage of saline, the feeding response to MA was significantly greater in female versus male rats at the 115 μmol kg−1 (*P<0.05). † within males the 355 versus all other doses (P<0.05). ‡ within the females 155 and 355 μmol kg−1 versus saline (P<0.05) and a tendency for increased feeding at the 200 μmol kg−1 versus saline dose (P=0.06). Data are mean±s.e.m.
Effect of gonadal hormones on response to 2DG
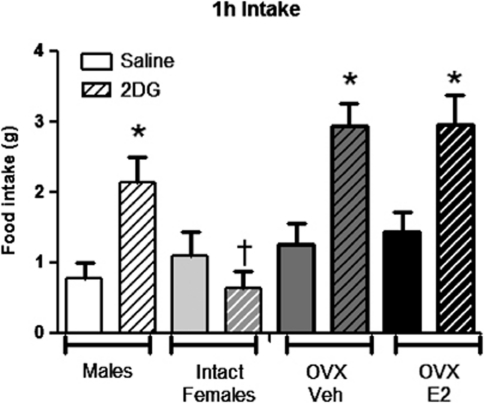
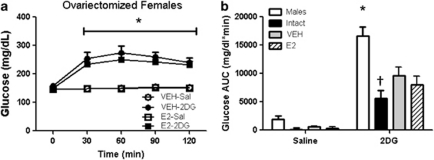
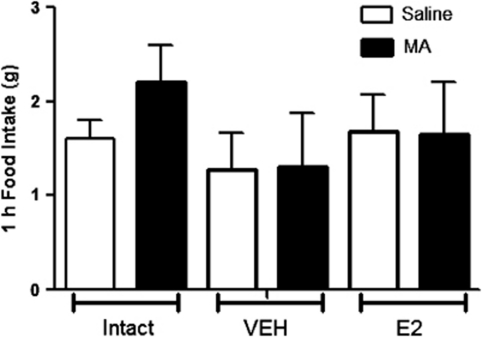
Relative to OVX females treated with VEH (sesame oil), OVX females treated with subcutaneous E2 versus VEH (sesame oil) had a significantly elevated E2 levels (100±20 vs 44±9 pmol l−1) and reduced body weight beginning at 2 weeks and continuing throughout the rest of the study period (Figure 5). E2 treatment also reduced body fat (36.8±2.5 vs 53.5±4.0 g in E2 versus VEH, respectively; P<0.05) while maintaining similar lean mass (data not shown), indicating that the females were responding as anticipated to the E2 treatment (P<0.05). In response to IP 2DG at the same dose (250 mg kg−1) that significantly increased food intake in males, OVX females treated with VEH as well as with E2 had significantly increased food intake the first hour post injection (Figure 6) and both groups had significantly elevated blood glucose responses to IP 2DG versus saline (Figure 7). If we statistically compare the 2DG response between the OVX versus intact females and males studied in the sex comparison studies we see that the feeding was significantly greater in the OVX versus intact females regardless of E2 treatment (group by drug interaction; P<0.05; Figure 6) but there was no statistical difference between the males versus either group of the OVX females (P=0.07; also no significant differences in the 1–2 or 2–3 h periods after injection, data not shown). The glucose AUC response to 2DG was also significantly greater in both groups of OVX animals, regardless of E2 treatment, however, it never reached the level seen in the male rats (Figure 7). Neither of the OVX groups increased food intake in response to MA (Figure 8) nor there were any significant interactions when compared with the intact females at the 200-μmol kg−1 dose.
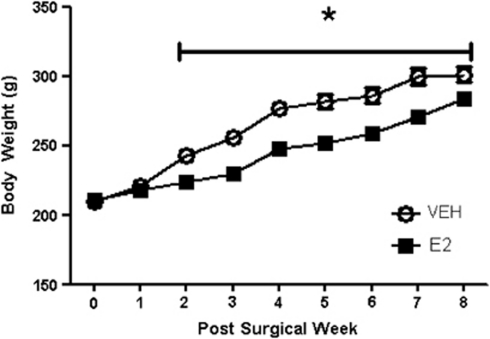
Figure 5.
Ovariectomized females treated with VEH (sesame oil) or E2 for 2 months after ovariectomy. E2 treated rats had significantly lower body mass from 2 to 8 weeks (*P<0.05). Data are mean±s.e.m.
Figure 6.
Food intake response to 250 mg kg−1 of 2DG. 2DG significantly increased the food intake over saline in males and OVX females regardless of treatment (*P<0.05). †Intact females versus all other groups. Data are mean±s.e.m.
Figure 7.
Glucose response to 2DG (250 mg kg−1). (a) 2DG significantly increased glucose over saline in both groups of OVX rats (*P<0.05). (b) Glucose integrated AUC response to 2DG was significantly greater in male rats compared with all other groups (*P<0.05). † intact females less than all other groups in response to 2DG versus saline in ovariectomized females treated with VEH or E2. Data are mean±s.e.m.
Figure 8.
Feeding response to MA. OVX did not significantly increase their feeding response to MA. There were no significant group by drug interactions between intact and OVX animals. Data are mean±s.e.m.
Discussion
The lipostatic theory stated that the body's level of adiposity provided a feedback signal to the CNS, which in turn increased or decreased energy intake and/or expenditure to maintain energy homeostasis.16 In contrast, the glucostatic theory dictated that it was the levels of glucose (and its utilization) that provided the predominant signal to the CNS for regulating energy balance.17 Although it remains unclear the extent to which these various signals influence satiation under normal circumstances, it is clear that acute reductions of glucose or some lipids can elicit feeding.18, 19, 20 Our results add a further degree of complexity to this by suggesting that males and females respond differently to changes in nutrient status in regulation of energy homeostasis. Specifically, we see that males respond to a more mild level of glucopenia whereas females response to a more mild lipopenia. Removal of the ovaries increased the female's response to glucoprivation and decreased the response to lipoprivation. As expected,1, 8, 9 E2 administration to the ovariectomized females significantly reduced body mass, fat mass and food intake; however, it did not impact the feeding response to either gluco- or lipoprivation. Therefore, physiological levels of E2 alone are not responsible for the sexual dimorphism seen in response to 2DG or MA indicating that some other consequence of ovariectomy must regulate these responses.
2DG inhibits the enzyme phospohexose isomerase, which blocks the first step in glycolysis and thus blocks glucose metabolism and thus deprives cells of glucose. It induces a profound CNS glucopenia,21 which initiates endocrine and autonomic responses aimed at increasing circulating glucose levels. Both human and animal model data show that the autonomic and hormonal responses to falling glucose levels are initiated at a lower absolute glucose level in females compared with males.6, 22, 23, 24 Thus, the present finding of right-shifted glucose dose-response to 2DG in female rats is consistent with human and rat data.
Because females are less sensitive to glucose deprivation, we hypothesized that they would be more sensitive to lipoprivation. MA inhibits β-oxidation by inhibiting the enzyme long-chain acyl-coenzyme A dehydrogenase.25 Although some studies report that MA stimulates feeding only in the presence of a high-fat diet,26 using a previously reported protocol,11 we were able to repeatedly (data not shown) duplicate findings that MA significantly stimulates feeding in both male and intact female rats fed a low-fat chow diet. Nonetheless, the dose effect was shifted to suggest that the females are more sensitive to lipoprivation compared with males. One other study has compared the feeding response to MA in males versus females.27 At doses that were higher than what we used here (403 and 605 vs 115, 200 and 355 μmol kg−1), that study found that males but not females, increased feeding response to MA. The reason for this difference is unclear. MA causes conditioned taste aversion and perhaps females are more sensitive to this effect at higher doses. A more simplistic reason is the timing of the study. Swithers et al.27 performed the food intake study 5 h into a shorter light cycle (14:10) compared with our study for which food intake was measured 1 h before the end of a 12-h light cycle, a time when fatty acid oxidation is higher, especially in females.
E2 has widespread metabolic effects, including the stimulation of liploysis and glycogen synthesis, and several studies have suggested that it also modulates CNS glucose sensing.6, 7, 22 Although the OVX females significantly increased feeding and blood glucose response to 2DG and had a lack of feeding response to MA compared with the intact females, physiological replacement of E2 did not restore responses to the levels of intact females. This is despite the fact that our E2 replacement mimics the timing and levels of the rodent cyclic variation in E2.10 Importantly, others have reported a lack of feeding response to MA in ovariectomized females whether treated with E2 or VEH.28 This could suggest inherent sex differences of nutrient sensing that are independent of ovarian hormones.
There was a different time course response to 2DG in the male rats. Males increased feeding within 1 h of injection of the 250-mg kg−1 dose, but did not increase feeding response to the 750-mg kg−1 dose until 3 h after the injection. This higher dose of 2DG has been reported to cause a conditioned taste aversion14 and could also cause lethargy. Either of these effects could initially override the hyperphagic effect of 2DG, an effect that could wane over time.
We did not determine the phase of the estrous cycle during these experiments, therefore, we cannot speak to whether acute fluctuations of reproductive hormones affect the responses to 2DG or MA. However, we administered E2 every fourth day to mimic the levels of E2 seen during the estrous phase of the estrous cycle,10 but this had no effect on the responses to 2DG or MA. It is possible that E2 is only part of the mechanism for the sex differences we observed. We chose not to replace progesterone along with the E2 because previous work suggested that E2 was the underlying mechanism for the differences seen in glucoprivic feeding. However, these hormones work together to regulate reproductive function and could cooperate in the regulation of metabolism as well. For example, estrogen can increase progesterone receptor mRNA29 and replacement of both E2 and progesterone in ovariectomized rats has been found to suppress feeding response to 2DG.30 In the latter study, neither hormone was given alone making it difficult to tell whether both hormones are necessary or whether progesterone alone could account for the blunted response to 2DG. Further, recent research has shown that although E2 replacement in castrated females did not restore insulin sensitivity in adipose tissue, testosterone replacement in castrated males did restore insulin sensitivity in the same adipose tissue pads.9 Thus, it is possible that other gonadal hormones contribute to the sexual dimorphism observed with nutrient sensing.
In conclusion, the results of this study suggest that males respond to a more mild glucoprivic stimulation whereas females respond to a more mild lipoprivic stimulus and suggest that this effect is not dependent upon E2 alone. We hypothesize that females may have evolved increased sensitivity to fat availability in order to spare glucose for longer periods of food scarcity or stress. These data add to our knowledge regarding the influence of sex on regulation of energy homeostasis by suggesting that males and females respond differently to not only circulating hormones (insulin and leptin) as previously published, but also to nutrient status to control feeding behavior.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK75365 to D.S., DK54080, DK54890 and DK056863 to RJS and DK 017844 to SCW). We would like to thank Priya Makadia for her technical assistance in the studies completed within this manuscript.
RJS has received grant support from Zafgen and Ethicon Endo-Surgery. RJS also currently has stock/stock options with Zafgen and consults for Zafgen, Eli Lilly and Ethicon Endo-Surgeries. DAS has received grant support from Ethicon Endo-Surgery, Roche, Mannkind, and Pfizer. The other authors declare no conflict of interest.
References
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeters MR, Sauerwein HP, Groener JE, Aerts JM, Ackermans MT, Glatz JF, et al. Gender-related differences in the metabolic response to fasting. J Clin Endocrinol Metab. 2007;92:3646–3652. doi: 10.1210/jc.2007-0552. [DOI] [PubMed] [Google Scholar]
- Davis SN, Fowler S, Costa F. Hypoglycemic counterregulatory responses differ between men and women with type 1 diabetes. Diabetes. 2000;49:65–72. doi: 10.2337/diabetes.49.1.65. [DOI] [PubMed] [Google Scholar]
- Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Ertl AC, Richardson MA, Tate DB, Davis SN. Estrogen blunts neuroendocrine and metabolic responses to hypoglycemia. Diabetes. 2003;52:1749–1755. doi: 10.2337/diabetes.52.7.1749. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Gong B, Davis SN. Antecedent short-term central nervous system administration of estrogen and progesterone alters counterregulatory responses to hypoglycemia in conscious male rats. Am J Physiol Endocrinol Metab. 2007;293:E1511–E1516. doi: 10.1152/ajpendo.00340.2007. [DOI] [PubMed] [Google Scholar]
- D′Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Davidson TL. Interoceptive sensory signals produced by 24-hr food deprivation, pharmacological glucoprivation, and lipoprivation. Behav Neurosci. 1996;110:168–180. [PubMed] [Google Scholar]
- Sanders NM, Ritter S. Repeated 2-deoxy-D-glucose-induced glucoprivation attenuates Fos expression and glucoregulatory responses during subsequent glucoprivation. Diabetes. 2000;49:1865–1874. doi: 10.2337/diabetes.49.11.1865. [DOI] [PubMed] [Google Scholar]
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- Dibattista D. Conditioned taste aversion produced by 2-deoxy-D-glucose in rats and hamsters. Physiol Behav. 1988;44:189–192. doi: 10.1016/0031-9384(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Koss MD, Brandt K, Geary N, Langhans W, Leonhardt M. Dissociation of mercaptoacetate′s effects on feeding and fat metabolism by dietary medium- and long-chain triacylglycerols in rats. Nutrition. 2008;24:360. doi: 10.1016/j.nut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond (Biol) 1953;140:579–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Mayer J. Genetic, traumatic and environmental factors in the etiology of obesity. Physiol Rev. 1953;33:472–508. doi: 10.1152/physrev.1953.33.4.472. [DOI] [PubMed] [Google Scholar]
- Langhans W. Fatty acid oxidation in the energostatic control of eating--a new idea. Appetite. 2008;51:446. doi: 10.1016/j.appet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Li A-J. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Langhans W. Fatty acid oxidation and control of food intake. Physiol Behav. 2004;83:645. doi: 10.1016/j.physbeh.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab. 2007;293:E96–E101. doi: 10.1152/ajpendo.00415.2006. [DOI] [PubMed] [Google Scholar]
- Adams JM, Legan SJ, Ott CE, Jackson BA. Modulation of hypoglycemia-induced increases in plasma epinephrine by estrogen in the female rat. J Neurosci Res. 2005;79:360–367. doi: 10.1002/jnr.20369. [DOI] [PubMed] [Google Scholar]
- Davis SN, Galassetti P, Wasserman DH, Tate D. Effect of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J Clin Endocrinol Metab. 2000;85:224–230. doi: 10.1210/jcem.85.1.6328. [DOI] [PubMed] [Google Scholar]
- Davis SN, Shavers C, Costa F. Differential gender responses to hypoglycemia are due to alterations in CNS drive and not glycemic thresholds. Am J Physiol Endocrinol Metab. 2000;279:E1054–E1063. doi: 10.1152/ajpendo.2000.279.5.E1054. [DOI] [PubMed] [Google Scholar]
- Bauche F, Sabourault D, Giudicelli Y, Nordmann J, Nordmann R. 2-Mercaptoacetate administration depresses the beta-oxidation pathway through an inhibition of long-chain acyl-CoA dehydrogenase activity. Biochem J. 1981;196:803–809. doi: 10.1042/bj1960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer E. Control of food intake by fatty acid oxidation and ketogenesis. Nutrition. 1999;15:704–714. doi: 10.1016/s0899-9007(99)00125-2. [DOI] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54:471–477. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Sajapitak S, Tsukamura H, Kinoshita M, Matsuyama S, Ohkura S, et al. Acute lipoprivation suppresses pulsatile luteinizing hormone secretion without affecting food intake in female rats. J Reprod Dev. 2006;52:763–772. doi: 10.1262/jrd.18066. [DOI] [PubMed] [Google Scholar]
- Kaneko KJ, Gelinas C, Gorski J. Activation of the silent progesterone receptor gene by ectopic expression of estrogen receptors in a rat fibroblast cell line. Biochemistry. 1993;32:8348–8359. doi: 10.1021/bi00083a039. [DOI] [PubMed] [Google Scholar]
- McDermott LJ, Jorgensen DE, Byers DJ. Estradiol and progesterone suppress feeding induced by 2-deoxy-D-glucose. Physiol Behav. 1984;32:731–736. doi: 10.1016/0031-9384(84)90186-0. [DOI] [PubMed] [Google Scholar]