Abstract
Background:
Alterations in the composition of gut microbiota —known as dysbiosis— have been proposed to contribute to the development of obesity, thereby supporting the potential interest of nutrients acting on the gut microbes to produce beneficial effect on host energetic metabolism. Non-digestible fermentable carbohydrates present in cereals may be interesting nutrients able to influence the gut microbiota composition.
Objective and design:
The aim of the present study was to test the prebiotic potency of arabinoxylan oligosaccharides (AXOS) prepared from wheat bran in a nutritional model of obesity, associated with a low-grade chronic systemic inflammation. Mice were fed either a control diet or a high fat (HF) diet, or a HF diet supplemented with AXOS during 8 weeks.
Results:
AXOS supplementation induced caecal and colon enlargement associated with an important bifidogenic effect. It increased the level of circulating satietogenic peptides produced by the colon (peptide YY and glucagon-like peptide-1), and coherently counteracted HF-induced body weight gain and fat mass development. HF-induced hyperinsulinemia and the Homeostasis Model Assessment of insulin resistance were decreased upon AXOS feeding. In addition, AXOS reduced HF-induced metabolic endotoxemia, macrophage infiltration (mRNA of F4/80) in the adipose tissue and interleukin 6 (IL6) in the plasma. The tight junction proteins (zonula occludens 1 and claudin 3) altered upon HF feeding were upregulated by AXOS treatment suggesting that the lower inflammatory tone was associated with the improvement of gut barrier function.
Conclusion:
Together, these findings suggest that specific non-digestible carbohydrates produced from cereals such as AXOS constitute a promising prebiotic nutrient in the control of obesity and related metabolic disorders.
Keywords: prebiotic, arabinoxylan oligosaccharides, high fat diet, endotoxemia, gastrointestinal peptides, obesity
Introduction
In most Western countries, obesity and related diseases, such as diabetes and cardiovascular diseases, have emerged as major health problems. In parallel, the consumption of cereals —representing a major source of dietary fibres— has decreased dramatically in Western countries over the last century.1 It has been suggested that the decrease in fibre intake contributes to the development of these health disorders.2, 3, 4 Studies performed in rodents and humans suggest that obesity is associated with an altered composition of gut microbiota.5, 6, 7, 8, 9, 10 Several mechanisms are proposed, linking events occurring in the gut upon carbohydrate fermentation and the control of metabolic disorders.7, 8, 11, 12, 13, 14 Changes in the synthesis of gastrointestinal peptides controlling food intake (glucagon like peptide-1 (GLP-1), peptide YY (PYY), ghrelin, amylin, pancreatic polypeptide) occur. The regulation of fat storage takes place namely through modification of intestinal expression of the lipoprotein lipase inhibitor, fasting-induced adipose factor. Energy sparing is partly due to the fermentation of non-digestible carbohydrates into short chain fatty acids that are used by host tissues. The release of bacterial metabolites (short chain fatty acids, conjugated linoleic acid) allow them to act as regulators of adiposity (via G-protein-coupled receptors (GPR43) and/or peroxisome proliferator-activated receptor-γ (PPARγ)-dependent mechanisms). Finally, the changes in the activity of peptides/systems involved in the control of gut permeability and metabolic endotoxemia (glucagon like peptide-2 (GLP-2), tight junction proteins such as zonula occludens-1 (ZO1), endocannabinoid system,) also contribute to modulate host metabolic alterations, such as inflammation and endotoxemia. Inulin and fructooligosaccharides are non-digestible carbohydrates obtained from chicory root. We have previously shown that those inulin-type fructans restored the drop of bifidobacteria occurring in the caeco-colon of high-fat (HF) diet-fed mice. They improve metabolic alterations induced by HF feeding including dyslipidemia, impaired gut permeability, metabolic endotoxemia and diabetes.7, 8, 14, 15, 16 These effects are largely dependent on the production and action of gut-derived peptides, such as GLP-1 and GLP-2 actions.14, 16 Inulin-type fructans are typically studied as they were the first compounds to respond to the prebiotic concept. Prebiotic is defined as the selective stimulation of growth and/or activity of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host.17 Other non-digestible/fermented carbohydrates gradually fermented throughout the colon can be prepared from cereals. They may be valuable nutrients that could improve health thanks to their influence on gut microbiota composition.
The most important non-digestible carbohydrates present in wheat are the non-starch polysaccharides arabinoxylans. Arabinoxylans represent 50% of dietary fibres present in wheat bran.18, 19 Of particular interest, the highly polymerised arabinoxylan from bran may be hydrolysed through wheat-associated endoxylanases or specific intestinal bacteria possessing arabinoxylan-degrading enzymes. This hydrolysis leads to the formation of arabinoxylan oligosaccharides (AXOS). AXOS represent a new class of candidate prebiotics depending on their structure (degree of polymerisation and degree of arabinose substitution).18, 20 Little information is available on the prebiotic potency of AXOS in vivo. One study compared the prebiotic effects of AXOS and inulin using simulator of the human intestinal microbial ecosystem.21 The authors demonstrated that AXOS fermentation and short chain fatty acid production took place in the more distal colon region as compared with the place where inulin fermented. AXOS fermentation results also in a strong propionate production suggesting that this prebiotic might be a good prebiotic candidate to impact fat metabolism in the host.21 The aim of the present study was to assess the effect of AXOS prepared from wheat bran on diet-induced obesity and inflammation.
Materials and methods
Animals and diet intervention
Twenty-four male C57bl6/J mice (9-week-old at the beginning of the experiment, Charles River Laboratories, Lyon, France) were housed in groups of four per cage in a controlled environment (12-h daylight cycle) with free access to food and water. After 1 week of acclimatisation, the mice were divided into three groups (n=8 per group): a control group (CT), fed with a control diet (A04, SAFE, Villemoison-sur-Orge, France), a group fed a HF diet and a group fed the same HF diet, supplemented with AXOS (92.5% HF (w/w)+7.5%AXOS extract; HF-AXOS group). The composition of both the HF diet (D12492, Research Diets, New Brunswick, NJ, USA) and the A04 standard diet is presented in Supplementary Information 1. The caloric value of the diets were 3.1 Kcal g−1, 5.2 Kcal g−1 and 5.0 Kcal g−1 for CT, HF and HF-AXOS diets, respectively. Cosucra groupe (Warcoing, Belgium) supplied an extract of AXOS with a purity of 75% and a degree of substitution arabinose/xylose of 0.2–0.3. HPSEC analysis shows that 75% of the AXOS preparation have a molecular weight below 5900 Da. Food intake was recorded twice a week, taking into account the amount of diet eaten by four mice kept in the same cage, and by deducing food spillage. The results are presented for the whole study and represent the sum of the diet consumed during 8 weeks for four mice. The total caloric intake was obtained by multiplying total food intake (g) for four mice per cage (n=2) by the caloric value of the diets. After 8 weeks of dietary treatment and after a period of fasting (6 h), mice were anesthetised (intra peritoneal administration of 100 mg kg−1 ketamine and 10 mg kg−1 xylazine) and blood samples were harvested for further analysis. Portal blood was taken in <30 sec and directly flushed within tubes containing dipeptidyl peptidase IV (DPPIV) inhibitor (Millipore, St Charles, MO, USA) and a cocktail of general protease inhibitors containing EDTA, bestatin, leupeptin, aprotinin, E-64 and 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (P2714-Sigma protease cocktail, Sigma, Saint Louis, MO, USA and Roche Pefabloc SC, Roche Diagnostics, Vilvoorde, Belgium). Plasma was immediately removed and stored within tubes containing the same protease inhibitors at −80 °C for gut peptide determination. Liver, adipose tissues (visceral, epididymal and subcutaneous), proximal colon and caecum were carefully dissected, weighed and immersed in liquid nitrogen before storage at −80 °C. Mouse experiment was approved by and performed in accordance with the guidelines of the local ethics committee. Housing conditions were specified by the Belgian Law of 6 April 2010, regarding the protection of laboratory animals (Agreement LA1230314).
Blood parameters
Plasma insulin concentrations were determined using an ELISA kit (Mercodia, Uppsala, Sweden). The Homeostasis Model Assessment of insulin resistance (HOMA-IR) was calculated by the following formula: (HOMA-IR)=[fasting glycemia (mM)] × [fasting insulinemia (pM)]/22.5.22 Plasma triglycerides, cholesterol and non-esterified fatty acid concentrations were measured using kits coupling enzymatic reaction and spectrophotometric detection of reaction end products (Diasys Diagnostic and Systems, Holzheim, Germany). High-density lipoprotein cholesterol concentration was measured enzymatically after precipitation of other lipoproteins, such as very-low-density lipoprotein, chylomicrons and low-density lipoprotein cholesterol (Diasys Diagnostic and Systems). Low-density lipoprotein was estimated by the Friedewald formula.23
Systemic concentrations of interleukins (IL6, IL1α, IL1β, IL10, IL13), tumour necrosis factor alpha and monocyte chemotactic protein 1 were determined in 15 μl of plasma using a multiplex immunoassay kit (Bio-Plex Cytokine Assay, Bio-Rad, Nazareth, Belgium) and measured using Luminex technology (Bioplex, Bio-Rad). Portal concentrations of glucagon-like peptide 1 (GLP-1), PYY, amylin, pancreatic polypeptide, ghrelin and leptin were determined in 15 μl of plasma using a multiplex immunoassay kit (Bio-Plex Pro Assay, Bio-Rad) and measured using Luminex technology (Bioplex, Bio-Rad). Portal lipopolysaccharides (LPS) concentration was measured by using Endosafe-MCS (Charles River Laboratories) based on the limulus amaebocyte lysate kinetic chromogenic methodology that measures color intensity directly related to the endotoxin concentration in a sample. Serum were diluted 1/10 with endotoxin-free buffer to minimise interferences in the reaction (inhibition or enhancement) and heated for 15 min at 70 °C. Each sample was diluted with endotoxin-free limulus amaebocyte lysate reagent water (Charles River Laboratories) and treated in duplicate and two spikes for each sample were included in the determination. All samples have been validated for the recovery and the Coefficient Variation. The lower limit of detection was 0.01EU ml−1.24
Lipid analysis in the liver
Triglycerides and cholesterol were measured in the liver tissue after extraction with chloroform–methanol, as described by Neyrinck et al.25 Briefly, 100 mg of liver tissue was homogenised in 1 ml of phosphate buffer (pH 7.4). Lipids were extracted by mixing 125 μl of homogenate with 1 ml of chloroform:methanol (2:1). The chloroform phase was evaporated under nitrogen flux and the dried residue was solubilised in 100 μl of isopropanol. Triglycerides and total cholesterol were measured as previously described for plasma samples.
Microbial analysis of the caecal contents and expression of selected genes in tissues
At the end of the experiment, the total caecum content was collected and weighed before storage at −80 °C. Quantitative PCR (Q-PCR) for Bifidobacterium spp., Lactobacillus spp., Bacteroides-Prevotella spp. were performed as reported by Neyrinck et al.26 using Mesa Fast qPCR (Eurogentec, Seraing, Belgium). Primers are detailed in Supplementary Information 2. We have applied the amplification programme recommended by the manufacturers; the annealing temperature being 58 °C. Real-time PCRs were performed with the StepOnePlus real-time PCR system and software (Applied Biosystems, Den Ijssel, The Netherlands).
Total RNA was isolated from tissues (colon and visceral adipose tissue) using the TriPure isolation reagent kit (Roche Diagnostics). Complementary DNA (cDNA) was prepared by reverse transcription of 1 μg total RNA using the Kit Reverse transcription System (Promega, Leiden, The Netherlands). Real-time PCRs were performed with the StepOnePlus real-time PCR system and software (Applied Biosystems, The Netherlands) using Mesa Fast qPCR (Eurogentec) for detection according to the manufacturer's instructions. RPL19 RNA was chosen as housekeeping gene. Primer sequences for the targeted mouse genes are detailed in Supplementary Information 2. All samples were run in duplicate in a single 96-well reaction plate and data were analysed according to the 2-ΔCT method. The identity and purity of the amplified product was checked through analysis of the melting curve carried out at the end of amplification.
TaqMan low-density array performed on reverse-transcribed RNA samples extracted from the colon
Low-density DNA array on-demand was used to detect 48 different transcript species and configured into eight identical 48-transcript sets (Applied Biosystems, Foster City, CA, USA). Three reliable housekeeping transcripts -RPL19, GAPDH and 18S- were chosen in this model as reference transcripts for normalisation. Forty-five other transcripts of interest were chosen as their translation products are involved in inflammation, lipid metabolism, short chain fatty acid response, gut permeability, gut peptide production and response, cell differentiation and endocannabinoid system. Total RNA was isolated using the TriPure isolation reagent kit (Roche Diagnostics). Total RNA (2 μg) was reverse-transcribed using the high-capacity cDNA Archive Kit (Applied Biosystems, USA) following manufacturer's instructions. A total of 49 μl RNase/DNase-free water with 1 μl cDNA (100 ng of retrotranscribed total RNA) and 50 μl TaqMan Universal PCR Master Mix (Applied Biosystems, USA) was added to each line of LDA. LDA was sealed before centrifugation in a Heraus Multifuge 3S centrifuge (Cardinal Health, Dublin, OH, USA) for 1 min 4 × at 1200 r.p.m. The PCR amplifications were performed in a 7900HT Fast Real-Time PCR system: 10 min at 94.5 °C, followed by 40 cycles of denaturation at 97 °C for 30 s and annealing/extension at 59.7 °C for 1 min. mRNA abundances values were normalised to the expression value of RPL19 and calculations based on the comparative Ct (cycle threshold) method (2−ΔΔCt). Results were presented as the fold change relative to the control mice and we have also presented the ratio between the HF-AXOS group versus the HF group.
Statistical analysis
Results are presented as mean±s.e.m. Statistical analysis was performed by analysis of variance (ANOVA) followed by post hoc Tuckey's multiple comparison test (GraphPad Software, San Diego, CA, USA). Correlations between parameters were assessed by Pearson's correlation test. P<0.05 was considered as statistically significant. Principal component analysis and hierarchical clustering heat map profile were performed using and JMP 8.0.1 (SAS Campus Drive, Cary, NC, USA).
Results
Supplementation with AXOS decreases body weight gain, caloric intake and fat mass development
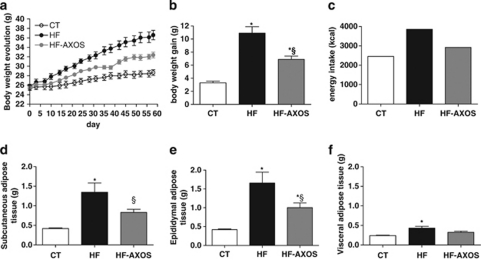
The final body weight of mice was 28.7±0.6, 36.7±1.0* and 32.4±0.6 g*§ for CT, HF and HF-AXOS group, respectively (*P<0.05 versus CT and §P<0.05 versus HF, ANOVA).
The HF diet, due to its higher energy density (5.2 versus 3.1 Kcal g−1 for the control diet) significantly increased body weight gain and the development of epididymal, visceral and subcutaneous fat as compared with CT group (Figure 1). AXOS supplementation in the HF diet decreased energy density of the diet (5.0 Kcal g−1) and decreased body weight gain by about 37% compared with HF group (Figure 1b). AXOS treatment also decreased fat mass in a significant way in the epididymal and subcutaneous adipose tissues compared with HF treatment alone (Figures 1d and e). The weight of subcutaneous adipose tissue came back to the control value in HF-AXOS mice. The total caloric intake, obtained by multiplying total food intake (measured during 8 weeks) by the caloric value of the diets, was higher in HF group than in controls (n=2) (Figure 1c). This effect was blunted when AXOS was added in the HF diet. The loss of body weight/fat gain taking into account the caloric intake of mice revealed that AXOS treatment did not significantly decrease the HF-induced feed efficiency (weight gain divided by calories consumed during the whole treatment), which was 5.4±0.5, 11.1±0.9* and 9.5±0.7* for CT, HF and HF-AXOS, respectively; *P<0.05 HF group versus CT group (ANOVA).
Figure 1.
Body weight evolution (a), body weight gain (b), caloric intake (c) and weight of epididymal (d), subcutaneous (e) and visceral (f) adipose tissues of mice fed a control diet (CT), a high fat diet (HF) or a HF diet supplemented with arabinoxylan oligosaccharides (HF-AXOS) for 8 weeks. *P<0.05 versus CT and §P<0.05 versus HF (ANOVA).
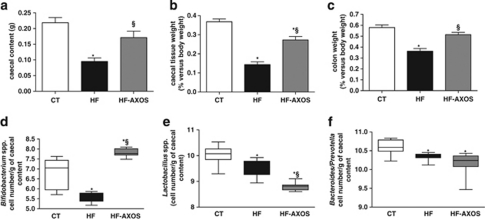
Supplementation with AXOS induces colon and caecal enlargement and promotes the growth of bifidobacteria
HF feeding decreased the weight of caecal content, caecal tissue and colon tissue as compared with the control condition (Figure 2a,b,c). Quantitative analysis in caecal content showed that HF diet decreased the gram-positive bifidobacteria and lactobacilli as well as the gram-negative bacteria such as Bacteroides–Prevotella spp. (Figures 2d–f). AXOS supplementation induced caecal and colon enlargement together and produced a 2-log increase of the bifidobacteria number in the caecal content as compared with HF-fed mice. The weight of the colon and the weight of the caecal content returned to the control values. The bifidogenic effect was also significant as compared with control mice. The number of lactobacilli decreased upon HF+AXOS diet as compared with HF diet alone or to the CT diet whereas the number of Bacteroides–Prevotella spp. was not significantly modified by AXOS treatment. If we exclude the weight of the colon and the caecum, the final body weight of mice was 28.2±0.6, 36.4±1.0* and 31.9±0.6 g*§ for CT, HF and HF-AXOS group, respectively (*P<0.05 versus CT and §P<0.05 versus HF, ANOVA).
Figure 2.
Weight of caecal content (a), caecal tissue (b) and colon tissue (c). Caecal bacterial content of Bifidobacterium spp. (d), Lactobacillus spp. (e) and Bacteroides-Prevotella spp. (f). Mice were fed a control diet (CT), a high fat diet (HF) or a HF diet supplemented with arabinoxylan oligosaccharides (HF-AXOS) for 8 weeks. *P<0.05 versus CT and §P<0.05 versus HF (ANOVA).
Supplementation with AXOS does not modify lipid contents in the serum and in the liver but decreased hyperinsulinemia and the HOMA-IR
Lipids (triglycerides and cholesterol) in the serum and in the liver were not significantly affected by HF feeding and/or AXOS supplementation (Supplementary Information 3). HF feeding increased significantly insulinemia and the HOMA-IR (Supplementary Information 3). AXOS treatment was able to blunt both hyperinsulinemia and HF-increased HOMA-IR as values returned to the control values.
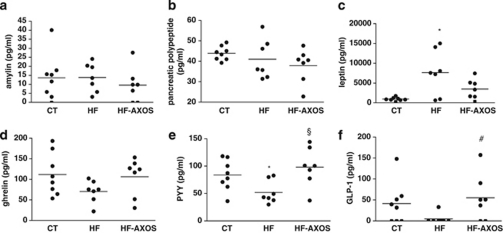
Supplementation with AXOS modifies gut peptides regulating food intake
We have measured a panel of hormones regulating appetite that were secreted by the adipose tissue (leptin), by the pancreas (amylin and pancreatic polypeptide), by the stomach (ghrelin) or by the intestinal L-cells (PYY and GLP-1) (Figure 3). The levels of gut hormones, such as PYY, GLP-1, pancreatic polypeptide and ghrelin, were decreased in the plasma 8 weeks after HF diet; this effect being significant for PYY. In contrast, the adipokine leptin significantly increased upon HF feeding. When AXOS was included in the HF diet, we observed higher concentration of PYY with a mean value near from the control value (P<0.05 HF-AXOS versus HF group, ANOVA). We detected levels of GLP-1 in five HF-AXOS mice of seven with a mean value of 55±21 pg ml−1, whereas only one HF mouse of seven had detectable GLP-1 concentration of 33 pg ml−1 (P<0.05, Chi-square test, HF-AXOS versus HF group). The effects of AXOS on other gut peptides were not significant. These results suggest that AXOS promote gut peptides (PYY and GLP-1) coming from the colon, which are able to regulate food intake by increasing satiety.
Figure 3.
Portal amylin (a), pancreatic polypeptide (b), leptin (c), ghrelin (d), peptide YY (PYY) (e) and glucagon-like peptide 1 (GLP-1) (f) concentrations in mice fed a control diet (CT), a high fat diet (HF) or a HF diet supplemented with arabinoxylan oligosaccharides (HF-AXOS) for 8 weeks. Data show individual values with a bar representing the mean value. *P<0.05 versus CT and §P<0.05 versus HF (ANOVA). #P<0.05, Chi-square test, HF-AXOS versus HF group.
Supplementation with AXOS decreases inflammatory markers induced upon HF feeding
We observed higher levels of proinflammatory mediators such as IL6 in the plasma upon HF feeding (Table 1). AXOS supplementation significantly decreased IL6 concentrations in the plasma as compared with HF-fed mice, at a level near the control value. Plasma IL6 negatively correlates with Bifidobacterium spp. (r=−0.48; P<0.05 Pearson's r correlation). In accordance with inflammatory parameters measured in the plasma, AXOS supplementation counteracted the HF-induced F4/80 expression in visceral adipose tissue, suggesting a lower macrophage infiltration in this tissue (F4/80 mRNA were 1.00±0.25, 2.47±0.45* and 1.18±0.32§ for CT, HF and HF-AXOS groups respectively; *P<0.05 versus CT and §P<0.05 versus HF, ANOVA).
Table 1. Inflammatory markers in the plasma.
| CT | HF | HF-AXOS | Δ(HF-AXOS-HF) | |
|---|---|---|---|---|
| MCP-1 | 41±10 | 189±97 | 39±11 | −151 |
| IL6 | 4.4±0.5 | 11.4±3.5 * | 3.9±0.5§ | −8 |
| IL1β | 21±4 | 14±4 | 11±2 | −3 |
| IL13 | 249±13 | 242±48 | 216±42 | −26 |
| TNFα | 89±9 | 67±14 | 63±12 | −4 |
| IL1α | 128±19 | 183±54 | 176±35 | −6 |
| IL10 | 151±19 | 113±40 | 164±34 | 51 |
Abbreviations: CT, control diet; HF, high fat diet; HF-AXOS, HF diet supplemented with arabinoxylan oligosaccharides; IL, interleukin; MCP, monocyte chemoattractant protein; TNF, tumour necrosis factor.
Mice were fed a CT diet, a HF diet or a HF-AXOS diet for 8 weeks. *P<0.05 versus CT and §P<0.05 versus HF (ANOVA).
All values are expressed in pg ml−1.
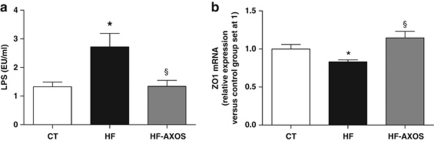
Supplementation with AXOS reduces endotoxemia and improves gut barrier function
Given that inflammatory disorders occurring upon HF feeding are related to alterations in gut permeability leading to metabolic endotoxemia,14 we have quantified LPS in the portal blood and we have measured the expression of a tight junction protein (ZO1) in the colon. HF feeding decreased mRNA content of ZO1 in the colon, whereas it increased the level of LPS in the portal blood (Figure 4). AXOS supplementation counteracted the HF-induced metabolic endotoxemia (Figure 4a). AXOS treatment significantly increased the level of ZO1 expression in the colon as compared with HF group (Figure 4b). This suggests an improvement of gut barrier function through AXOS treatment.
Figure 4.
Plasma lipopolysaccharides (LPS) (a) and zonula occludens 1 (ZO1) expression in the colon (b) in mice fed a control diet (CT), a high fat diet (HF) or a HF diet supplemented with arabinoxylan oligosaccharides (HF-AXOS) for 8 weeks. *P<0.05 versus CT and §P<0.05 versus HF (ANOVA).
The role of the colon in the metabolic effect of AXOS
In this study, we highlighted several effects of AXOS suggesting that the colon may be a key metabolic crossroad between the gut microbiota and the management of metabolic parameters (colon enlargement, higher circulating satietogenic peptides produced by the L cells in the colon, higher expression of ZO1 in the colon). We decided to explore several genes involved in inflammation, lipid metabolism, short chain fatty acid response, gut permeability, gut peptide production and response, cell differentiation and endocannabinoid system using a TaqMan low-density array. This technique allows for high throughput screening in functional genomics by simultaneously measuring mRNA relative abundances of multiple genes of interest.27 Among 48 cDNA species analysed, 42 cDNA species were successfully amplified by LDA; 6 cDNA species were not detected or detected in few samples (that is, interferon-gamma (IFNγ), IL12, IL1α, IL4, macrophage inflammatory protein alpha (MIP1α), glucose-dependent insulinotropic polypeptide (GIP)). Among transcript species in which relative abundance could be analysed, eight transcript species were significantly and differentially abundant in proximal colon upon HF feeding versus control diet. Six transcript species were less abundant, in particular four genes encoding proteins involved in gut barrier function (Table 2). AXOS treatment restored the presence of three transcript species encoding tight junction protein, with significant abundance for the claudin 3 gene. Although the abundance of transcript species encoding proinflammatory cytokines was not significantly affected by dietary treatments (HF alone or HF+AXOS), we observed a significant upregulation of the anti-inflammatory cytokine IL10 as compared with control mice. Of note, AXOS supplementation downregulated fasting-induced adipose factor expression as compared with either the control group or the HF group.
Table 2. Gene expression profile identified by TLDA analysis in the proximal colon.
| Gene | NM | HF | HF-AXOS | Ratio |
|---|---|---|---|---|
| Inflammation | ||||
| IL10 | NM_010548.1 | 1.76±0.49 | 3.07±0.55* | 1.75 |
| TNFa | NM_013693.2 | 0.58±0.14 | 0.89±0.18 | 1.53 |
| MCP-1 | NM_011333.3 | 0.91±0.06 | 1.22±0.47 | 1.34 |
| TLR4 | NM_021297.2 | 0.77±0.05* | 1.00±0.10 | 1.31 |
| IL6 | NM_031168.1 | 0.79±0.09 | 0.98±0.23 | 1.24 |
| COX2 | NM_011198.3 | 0.78±0.12 | 0.94±0.13 | 1.20 |
| IL7 | NM_008371.4 | 0.96±0.05 | 1.09±0.11 | 1.14 |
| PPARg | NM_011146.2 | 0.76±0.06* | 0.73±0.08* | 0.95 |
| CD14 | NM_009841.3 | 1.27±0.13 | 1.08±0.12 | 0.85 |
| MIP-1a | NM_011337.2 | ND | ND | ND |
| IL12a | NM_008351.1 | ND | ND | ND |
| IFNg | NM_008337.3 | ND | ND | ND |
| IL1a | NM_010554.4 | ND | ND | ND |
| IL4 | NM_021283.1 | ND | ND | ND |
| Permeability | ||||
| Claudin 3 | NM_009902.3 | 0.81±0.02* | 1.03±0.08§ | 1.27 |
| ZO2 | NM_011597.3 | 0.77±0.04* | 0.94±0.12 | 1.22 |
| MUC-2 | NM_023566.2 | 0.56±0.05* | 0.67±0.11* | 1.18 |
| ZO1 | NM_009386.1 | 0.79±0.06* | 0.91±0.10 | 1.16 |
| ITF | NM_011575.1 | 1.03±0.04 | 1.07±0.08 | 1.03 |
| Occludin | NM_008756.2 | 0.86±0.06 | 0.82±0.07 | 0.95 |
| Claudin 2 | NM_016675.3 | 0.75±0.07 | 0.63±0.14 | 0.84 |
| Differentiation | ||||
| Neurog3 | NM_009719.6 | 0.91±0.10 | 1.08±0.14 | 1.19 |
| Atoh1 | NM_007500.4 | 0.72±0.06 | 0.80±0.11 | 1.10 |
| Neurod1 | NM_010894.2 | 0.89±0.11 | 0.79±0.09 | 0.89 |
| Gut peptides | ||||
| GLP-2r | NM_175681.2 | 0.74±0.14 | 0.97±0.07 | 1.31 |
| GLP-1r | NM_021332.2 | 0.78±0.15 | 0.79±0.10 | 1.02 |
| Preproglucagon | NM_008100.3 | 0.91±0.10 | 0.86±0.14 | 0.95 |
| Lepr | NM_146146.1 | 0.83±0.06 | 0.68±0.08* | 0.82 |
| PYY | NM_145435.1 | 1.47±0.18* | 1.16±0.10 | 0.79 |
| DPPIV | NM_010074.2 | 1.22±0.16 | 0.95±0.09 | 0.78 |
| GIPr | NM_001080815.1 | 1.30±0.20 | 0.92±0.15 | 0.70 |
| GIP | NM_008119.2 | ND | ND | ND |
| Lipid metabolism | ||||
| I-FABP | NM_007980.2 | 0.97±0.15 | 1.06±0.13 | 1.10 |
| PPARa | NM_011144.5 | 1.02±0.20 | 0.71±0.09 | 0.70 |
| FIAF | NM_020581.1 | 0.92±0.14 | 0.52±0.10*§ | 0.57 |
| Short chain fatty acid response | ||||
| Cathelicidin | NM_009921.1 | 0.49±0.12 | 0.96±0.30 | 1.95 |
| GPR41 | NM_001033316.2 | 0.88±0.13 | 1.27±0.14 | 1.45 |
| GPR43 | NM_146187.3 | 0.87±0.14 | 0.76±0.14 | 0.87 |
| Endocannabinoid system | ||||
| CB1 | NM_007726.3 | 0.65±0.27 | 1.16±0.20 | 1.80 |
| GRP119 | NM_181751.2 | 0.99±0.14 | 1.20±0.22 | 1.22 |
| GRP55 | NM_001033290.2 | 0.84±0.19 | 1.03±0.14 | 1.22 |
| CB2 | NM_009924.3 | 1.87±0.23* | 1.17±0.23 | 0.63 |
| Other | ||||
| RXRa | NM_011305.3 | 0.83±0.10 | 0.93±0.07 | 1.12 |
| GLUT-2 | NM_031197.1 | 2.09±0.58 | 1.89±1.18 | 0.90 |
| CRBPII | NM_009034.3 | 0.91±0.16 | 0.81±0.13 | 0.89 |
Abbreviations: Atoh, atonal homolog; CB, cannabinoid receptor; COX, cyclooxygenase; CRBP, retinol binding protein; CT, control diet; DPPIV, dipeptidyl peptidase IV; FIAF, fasting-induced adipose factor; GIP(r), gastric inhibitory polypeptide (receptor); GLP, glucagon-like peptide; GPR or GRP, G protein-coupled receptor; HF, high fat diet; HF-AXOS, HF diet supplemented with arabinoxylan oligosaccharides; I-FABP, intestinal fatty acid binding protein; IFN, interferon; IL, interleukin; ITF, trefoil factor 3; Lepr, leptin receptor; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; MUC, mucin; ND, not detected or detected in few samples; Neurog, Neurogenin; Neurod, Neurogenic differentiation; NM, GenBank accession number; PPAR, peroxisome proliferator-activated receptor; PYY, peptide YY; RXR, retinoid X receptor; TLR, toll-like receptor; TNF, tumour necrosis factor; ZO, zonula occludens.
Results obtained from TLDA analysis representing the ratio of mRNA abundance as compared with control mice (using the 2−ΔCt method where ΔCt=Ct−Ct RPL19). mRNAs were classified in function categories. Ratio represents mean fold change in abundance of HF-AXOS group relative to the HF group. Mice were fed a HF diet or a HF diet supplemented with arabinoxylan oligosaccharides (HF-AXOS) for 8 weeks. *P<0.05 versus CT and §P<0.05 versus HF (analysis of variance).
PCA
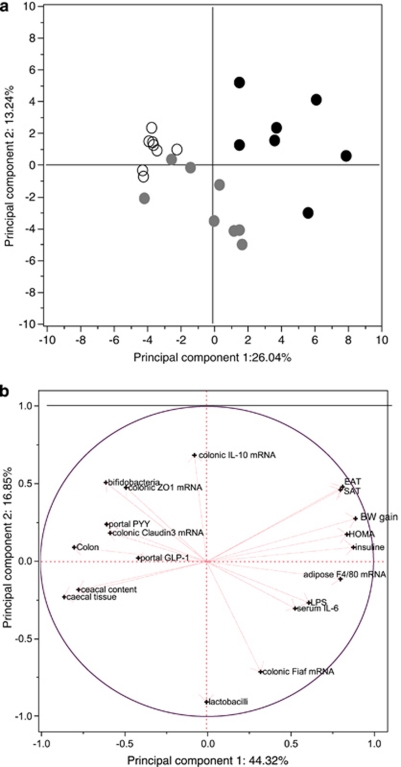
The results of PCA of all parameters —related to gut bacteria, gut peptide, colonic gene expression, gut barrier function, endotoxemia, inflammatory markers, body weight, fat mass, lipid profiles and glucose homeostasis— analysed in this study are illustrated in Figure 5a. The first and the second principal components (PCs) were responsible for only 39% of the total variance. The projection of the parameters in the plane by these PC supported the presence of a separate cluster for mice fed with a HF diet. PCA revealed regrouping between control mice and AXOS-treated mice. We performed a second PCA taking into account only parameters significantly modified by AXOS supplementation (Figure 5b). The first and the second PC explained, in this case, 61% of the total variance. Overall, PC1 was mainly characterised by body weight gain, fat mass development, HOMA-IR and fasted insulinemia with values up to 0.8 (right side), whereas PC2 was mainly characterised by IL-10 expression in the colon and lactobacilli content in the caecum with absolute values up to 0.5. In contrast, portal GLP-1 and PYY were situated on the left side. Both portal LPS and circulating IL6 were in opposition to bifidobacteria content in the caecum and expression of proteins involved in tight junctions (ZO1 and claudin 3) in the colon. These loadings were in accordance with Pearson's correlation test (data not shown). PC2 clearly discriminated between lactobacilli content in the caecum and the expression of IL10 in the colon in accordance with Pearson's correlation test (r=−0.61; P<0.01).
Figure 5.
PCA. (a) Score plot of all parameters investigated in this study in mice fed a standard diet (○), a high fat (HF) diet (•) or a HF diet supplemented with arabinoxylan oligosaccharides (HF-AXOS) ( ) for 8 weeks. (b) Projection of the parameters significantly modified by AXOS treatment in the plane defined by the two first PCs (loading plot). BW, body weight; IL, interleukin; PYY, peptide YY; ZO, zonula occludens; SAT subcutaneous adipose tissue weight; EAT, epididymal adipose tissue weight; LPS, endotoxemia; FIAF, fasting-induced adipose factor; GLP-1, glucagon-like peptide-1; HOMA, Homeostasis Model Assessment of insulin resistance.
) for 8 weeks. (b) Projection of the parameters significantly modified by AXOS treatment in the plane defined by the two first PCs (loading plot). BW, body weight; IL, interleukin; PYY, peptide YY; ZO, zonula occludens; SAT subcutaneous adipose tissue weight; EAT, epididymal adipose tissue weight; LPS, endotoxemia; FIAF, fasting-induced adipose factor; GLP-1, glucagon-like peptide-1; HOMA, Homeostasis Model Assessment of insulin resistance.
Discussion
The objective of the work performed in this paper, was to assess the potential relevance for host health of the modulation of the gut microbial composition occurring in obese mice upon supplementation with fermentable carbohydrates produced from cereals: the AXOS. We describe specific changes in gut microbiota composition that occur upon AXOS feeding in vivo (increase in bifidobacteria and decrease in lactobacilli), those effects being associated with improvement of inflammation and of gut barrier integrity in obese mice.
Over the past 5 years, the gut microbiota is increasingly considered as a symbiotic partner for the maintenance of health.28 Several data suggest that the activity of the gut microbiota is a factor to take into account when assessing the risk factors related to obesity, and associated disorders, such as dyslipidemia, inflammation, insulin resistance and diabetes.6, 7, 8, 11, 29, 30, 31 The hypothesis that specific modulation of the bifidobacteria community in obesity is supported by several studies obtained in mice as well as in humans.14, 15, 32, 33, 34 We previously showed that HF/carbohydrate-free diet led to obesity and diabetes and changes bacterial populations in the intestinal microbiota. Indeed, Bifidobacterium spp. Bacteroides-related bacteria and Eubacterium rectale–Clostridium coccoides group were reduced in the caecum of mice fed a HF diet during 4 or 14 weeks.15, 35 It was described that among the different gut bacteria analysed, the metabolic endotoxemia negatively correlated with the bifidobacteria count.15 Here, we show that feeding mice during 8 weeks with a HF diet containing carbohydrates (20% in energy), decreased the dominant members of the mouse intestinal microbiota — Bacteroides-Prevotella spp. from the Bacteroidetes phylum. The specific populations of lactobacilli from the Firmicutes phylum and bifidobacteria from the Actinobacteria phylum were also reduced upon HF feeding.36 AXOS supplementation led to a huge increase of bifidobacteria (with a level even higher than control mice), whereas lactobacilli number was further reduced in HF-AXOS mice. This contrasting effect had never been described with other prebiotic (inulin-type fructan) in HF diet-induced obese mice and needs further investigations clearly focusing on specific changes inside the Lactobacillus genus.15, 37 The higher caecal content of bifidobacteria was associated with improvement of metabolic endotoxemia and inflammatory markers as described for inulin-type fructan.14, 15 We observed that an anti-inflammatory interleukin (IL10) was upregulated in the colon tissue after HF-AXOS treatment as compared with the control diet. The increase of both plasma IL6 and F4/80 mRNA in the adipose tissue because of the HF feeding were blunted due to AXOS supplementation.
Several mechanisms have been proposed in order to explain the positive effect of the prebiotic approach in obesity models. Among those hypotheses, the modulation of endocrine function that occurs in prebiotic-fed animals, is a phenomenon that contributes to the improvement of obesity and associated disorders.7, 8, 16 The ingestion of inulin-type fructans has been shown to increase the number of L cells in the proximal colon of rats or mice, leading to the secretion of different peptides, including GLP1, GLP2 and PYY. These gut peptides have a role in the control of gut barrier, appetite and glucose homeostasis.14, 16, 38, 39 The resulting increase in GLP-2 contributes to the improvement of gut barrier function, namely through the higher expression of tight junction protein.14 Consequently, metabolic endotoxemia was reduced in those obese mice fed with prebiotic.14 In the present study, we observed that the tight junction proteins ZO1 and claudin 3 were upregulated in the colon after AXOS supplementation. More importantly, AXOS treatment blunted the HF-induced LPS concentration determined in the portal serum, supporting this hypothesis.
GLP-1 is a gut peptide able to regulate food intake and glucose homeostasis.7, 8 We have shown more detectable level of GLP-1 in the portal serum of HF-AXOS group than in HF group. The level of PYY, another satietogenic peptide, was also significantly higher in the portal plasma upon AXOS supplementation. Both effects might be involved in the lower food intake observed in this study, leading probably to the lower body weight gain and fat mass development as already described for inulin-type fructan.15, 16 The HF diet-induced fasting hyperinsulinemia and the higher HOMA-IR were significantly decreased under AXOS supplementation. These results suggest improved insulin sensitivity in mice fed with this bifidogenic compound.
Due to its propionate-producing effect upon fermentation, AXOS is proposed as a prebiotic prone to lower cholesterol and to beneficially affect fat metabolism of the host.21 However, we did not observe any effect of AXOS on lipid profiles both in the liver and in the plasma.
Several parameters suggested that the colon may be relevant in the metabolic effects observed through the AXOS supplementation (the colon enlargement, the higher circulating satietogenic peptides produced by the L cells in the colon, the higher expression of a tight junction protein in the colon). Therefore, we decided to explore several genes in this organ using a technique for high throughput screening in functional genomics by simultaneously measuring mRNA expression of multiple genes (TaqMan low-density array). We have shown that a protein involved in tight junctions —claudin3— was significantly upregulated in the colonic tissue of AXOS-treated mice through this technique. Tight junctions are important for the permeability properties of epithelial and endothelial barriers as they restrict diffusion along the paracellular space.40 They consist of transmembrane proteins such as claudins, which mediate adhesion and barrier formation as well as selective paracellular diffusion. These membrane proteins interact with a cytoplasmic plaque consisting of junction adaptors, such as the zonula occludens proteins, that contain multiple protein–protein interaction domains. In our study, quantitative PCR analysis revealed that ZO1 was also induced through the AXOS treatment. Such a junction adaptor form a protein network that links the junction to the actin cytoskeleton and recruits different types of signalling proteins that regulate junction assembly and function as well as epithelial proliferation and differentiation.40 Those results suggest that AXOS improves gut barrier function and decreases gut permeability.
AXOS also induced the expression of IL10 as compared with control mice, which could therefore contribute to the higher level of this anti-inflammatory cytokine in the serum. Concomitantly with the lower endotoxemia, we postulate that the lower inflammatory tone observed upon AXOS supplementation is the consequence of the higher production of IL10 coming from the colon.
PCA is often used to reduce the dimensionality of the data profiles containing intercorrelated variables. Moreover, PCA aims to display the maximum amount of variation in a data profile within a few PCs. Hence, score plots derived from PCA are useful to find similarities and contrasts between samples, whereas correlations among variables can be identified in loading plots. In the present study, score plots revealed that control mice and AXOS-treated mice were closely associated considering all parameters analysed in the present study, whereas HF-fed mice were more distant. The loading plots of PCA indicate relationships between gut microbes and metabolic parameters involved in obesity-associated disorders such as caecal content of bifidobacteria and endotoxemia. The opposition between caecal content of lactobacilli and colonic expression of IL10 suggests that it would be interesting to evaluate lactobacilli with inflammatory effects in prebiotic-treated animals. At present, we cannot conclude that specific genera, classes or species of bacteria are positively or negatively associated with the obese phenotype.7 The aim of this study was to investigate the prebiotic effect of AXOS related to its bifidogenic effect in a context of obesity. We may not exclude the participation of other gut microbes in the interesting effects observed as evoked for examples with Roseburia.25, 26 Other fermentable carbohydrates can exert similar effect in HF diet-fed mice, than the one described with AXOS in our study. Resistant starch, when added at the level of 30%, is able to decrease body fat in HF (7%) diet-fed mice, and this effect seems to be rather related to caecal fermentation (decrease in pH), than to a decrease in energy intake.41, 42 In addition, resistant starch also promote gut peptides involved in appetite and metabolic regulation.43 The relation between resistant starch feeding and the gut microbial composition could be very interesting to analyse further, in order to extend the concept of prebiotic to other carbohydrates that are part of the current and healthy diet.
From this study it can be concluded that, AXOS derived from wheat bran has a huge bifidogenic effect as previously shown for inulin-type fructan.17 This prebiotic effect was accompanied by an improvement of the HF-induced body weight gain, fat mass development, hyperinsulinemia, insulin resistance, endotoxemia and inflammatory disorders in a model of HF diet-induced obesity. The higher levels of peptides coming from the colon seemed to be the key molecular targets. Indeed, the higher levels of GLP-1 and PYY measured in the portal serum of mice supplemented with AXOS were associated with the lower energy intake. On the other hand, the gut function dependent on GLP-2 was improved (higher tight junction proteins expression) upon AXOS treatment.
In view of correlation and PCA analyses, and consistent with our previous studies with the prebiotic inulin-type fructan, we postulate that all these beneficial effects can be related to modulation of gut microbiota such as higher content in bifidobacteria in the caecum. Altogether, these findings support a role for wheat-derived AXOS as interesting non-digestible nutrients with prebiotic properties related to prevention of obesity and related inflammatory disorders.
Acknowledgments
Financial support has been provided by a grant from the Walloon Region (WalNut 20 Project, convention 5459). PD Cani is research associate and O Toussaint is senior research associate from the FRS-FNRS (Fonds de la Recherche Scientifique) in Belgium.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Nutrition and Diabetes website (http://www.nature.com/nutd)
Supplementary Material
References
- Adam A, Levrat-Verny MA, Lopez HW, Leuillet M, Demigne C, Remesy C. Whole wheat and triticale flours with differing viscosities stimulate cecal fermentations and lower plasma and hepatic lipids in rats. J Nutr. 2001;131:1770–1776. doi: 10.1093/jn/131.6.1770. [DOI] [PubMed] [Google Scholar]
- Adam A, Lopez HW, Tressol JC, Leuillet M, Demigne C, Remesy C. Impact of whole wheat flour and its milling fractions on the cecal fermentations and the plasma and liver lipids in rats. J Agric Food Chem. 2002;50:6557–6562. doi: 10.1021/jf020418b. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Cani PD. A place for dietary fibre in the management of the metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2005;8:636–640. doi: 10.1097/01.mco.0000171124.06408.71. [DOI] [PubMed] [Google Scholar]
- Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21:411–418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact. 2011;10:S10. doi: 10.1186/1475-2859-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Crawford PA. Coordinated regulation of the metabolome and lipidome at the host-microbial interface. Biochim Biophys Acta. 2010;1801:240–245. doi: 10.1016/j.bbalip.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136:1476–1483. doi: 10.1053/j.gastro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Muccioli GG, Naslain D, Backhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de WT, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like Peptide 1 receptor. Diabetes. 2006;55:1484–1490. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Courtin CM, Verbeke K, Van de WT, Verstraete W, Delcour JA. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- Neyrinck AM, Delzenne NM. Potential interest of gut microbial changes induced by non-digestible carbohydrates of wheat in the management of obesity and related disorders. Curr Opin Clin Nutr Metab Care. 2010;13:722–728. doi: 10.1097/MCO.0b013e32833ec3fb. [DOI] [PubMed] [Google Scholar]
- Van CV, Swennen K, Dornez E, Van de WT, Marzorati M, Verstraete W, et al. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J Nutr. 2008;138:2348–2355. doi: 10.3945/jn.108.094367. [DOI] [PubMed] [Google Scholar]
- Grootaert C, Van den AP, Marzorati M, Broekaert WF, Courtin CM, Delcour JA, et al. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. 2009;69:231–242. doi: 10.1111/j.1574-6941.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25:2711–2721. doi: 10.1096/fj.10-176602. [DOI] [PubMed] [Google Scholar]
- Neyrinck AM, Possemiers S, Verstraete W, De BF, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51–59. doi: 10.1016/j.jnutbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Neyrinck AM, Possemiers S, Druart C, Van de WT, De BF, Cani PD, et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, roseburia and bacteroides/prevotella in diet-induced obese mice. PLoS One. 2011;6:e20944. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Xu J, Chen J, Yu J, Xu E, Lai M. TaqMan low density array is roughly right for gene expression quantification in colorectal cancer. Clin Chim Acta. 2008;389:146–151. doi: 10.1016/j.cca.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268:320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes. Obes Rev. 2010;12:272–281. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- Geurts L. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:1–17. doi: 10.3389/fmicb.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61:69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130:202–212. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Holle AV, Muccioli GG, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2010;22:712–722. doi: 10.1016/j.jnutbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–526. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity. 2006;14:1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- Zhou J, Martin RJ, Tulley RT, Raggio AM, Shen L, Lissy E, et al. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J Agric Food Chem. 2009;57:8844–8851. doi: 10.1021/jf901548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.