Abstract
Objective:
Favorable effects of walking levels on glycemic control have been hypothesized to be mediated through reductions in abdominal adiposity, but this has not been well studied. We addressed this issue in patients treated for type 2 diabetes.
Design:
Cross-sectional analysis.
Subjects:
A total of 201 subjects with type 2 diabetes underwent assessments of pedometer-measured daily step counts, blood pressure, A1C and anthropometric measures (2006–2010). Associations of anthropometric indicators of abdominal adiposity (that is, waist circumference; waist-to-hip ratio (WHR)) with A1C were evaluated through linear regression models adjusting for age, ethnicity, sex and the use of insulin and oral hypoglycemic agents. Models including waist circumference were additionally adjusted for body mass index (BMI). A similar approach was used to examine A1C and daily step associations.
Results:
Among the 190 subjects (mean age 60 years; mean BMI 30.4 kg m−2), mean values (s.d.) for waist circumference and WHR were respectively, 99.1 cm (13.3) and 0.88 (0.07) in women, and 104.5 cm (13.1) and 0.97 (0.06) in men. Mean A1C and daily step count were respectively, 7.6% (1.4) and 5 338 steps per day (2609), and were similar for both sexes.
There was a 0.51% (95% confidence interval (CI): 0.10, 0.93) A1C increment per s.d. increase in waist circumference and a 0.32% (95% CI: 0.08, 0.56) A1C increment per s.d. increase in WHR in fully adjusted models. Each s.d. increase in daily step count was associated with clinically significant reductions in waist circumference and BMI. Each s.d. increase in daily steps was associated with a 0.21% (95% CI: 0.02, 0.41) A1C decrement that declined to 0.16% (95% CI: −0.35, 0.04) with further adjustment for anthropometric indicators of abdominal adiposity.
Conclusion:
Higher daily steps may be associated with lower A1C values both directly and via changes in abdominal adiposity.
Keywords: waist circumference, waist-to-hip ratio, pedometer, A1C, epidemiology
Introduction
The development of diabetes has been shown to be associated with an increase in visceral or intra-abdominal adipose tissue (abdominal adiposity), a decrease in s.c. adipose tissue and a loss of muscle mass.1 Abdominal adiposity, specifically visceral adiposity, not only increases the risk of developing type 2 diabetes2 but may also affect the degree of glycemic control even after the development of diabetes.3, 4 The latter is thought to be because of changes in insulin resistance occurring in the liver and peripheral muscle, mediated in part by higher levels of free fatty acids and the liberation of inflammatory mediators. However, studies of the associations between abdominal adiposity and A1C have not accounted for objective measures of physical activity. Physical activity has been shown to improve glycemic control among patients with type 2 diabetes.5 In interventional studies, walking has also been shown to be associated with reductions in fat mass.6 This would suggest that the favorable effects of walking are at least partly mediated through reductions in abdominal adiposity.
Quantification of the relative contributions of physical activity and abdominal adiposity with A1C may be clinically useful to physicians and patients with type 2 diabetes. This information would help to facilitate the development of anthropometric and/or daily step count targets for achieving metabolic control. In a clinical setting, anthropometric parameters provide an approximation of visceral and s.c. abdominal fat and are inexpensive, noninvasive techniques that can be easily applied in clinical practice. The primary objective of this study is to better delineate the interrelationships of abdominal adiposity and daily step counts with A1C in adults treated for type 2 diabetes.
Patients and methods
Study design and population
Adults with established type 2 diabetes were recruited from McGill University-affiliated outpatient clinics and presented for a baseline visit. Data collection procedures have been detailed previously,7, 8 but are summarized here. Participants were required to have a normal gait9 and a body mass index (BMI) ⩽40 kg m−2.10 Persons with stable cardiovascular disease were eligible to participate, but those with other chronic medical illnesses were excluded. Women who were pregnant or contemplating pregnancy were also excluded.
Demographic information and medical histories were obtained for each participant and anthropometric parameters, blood pressure and A1C were measured. Dietary information was obtained by means of the Food Frequency Questionnaire,11 and pedometer-based estimates of physical activity were calculated as outlined below.
Ethics statement
Procedures were approved by the Institutional Review Boards of McGill University and participating institutions (McGill University Health Centre, Sir Mortimer Davis Jewish General Hospital, and Centre de Santé et de Services Sociaux de la Montagne). All study participants provided written informed consent before clinical assessments.
Anthropometric measurements
Weight and height were assessed to the nearest 0.1 kg (SECA 882 electronic scale) and 0.1 cm (SECA 214 stadiometer), respectively, with the subject wearing light clothing and with shoes removed. Waist circumference was measured midway between the iliac crest and the lower rib margin. Hip circumference was measured at the point of greatest posterior extension of the buttocks. The waist-to-hip ratio (WHR) was calculated by dividing the waist circumference by the hip circumference. BMI was calculated by dividing the weight in kilograms by the square of the height in meters.
A1C measurement
A1C was measured from blood samples collected at each visit using the high-pressure liquid chromatography method (Bio-Rad Variant II, High Performance Liquid Chromatography System, Bio-Rad Laboratories, Hercules, CA, USA).
Daily step and physical activity measurement
Daily step counts were estimated using Yamax SW-200 pedometers (YAMASA TOKEI KEIKI CO., LTD, Tokyo, Japan), the accuracy and reliability of which had been previously demonstrated.12, 13 Each participant was provided with three pedometers labeled A, B and C. Participants were instructed to wear pedometer A during waking hours for 7 consecutive days, after which it was removed and replaced by pedometer B for another 7 consecutive days. Pedometers A, B and C were then mailed to our study center in a prepaid, pre-addressed and padded courier envelope. Pedometer C served to measure (false) steps registered during the mailing process (postman steps). These postman steps were subtracted from the step count of each of pedometers A and B, and the sum of the remaining steps were averaged over the total time period worn (that is, 14 days). The short, last 7 days self-administered format of the International Physical Activity questionnaire14 was used to assess self-reported levels of overall physical activity and to calculate metabolic equivalents per week.
Statistical methods
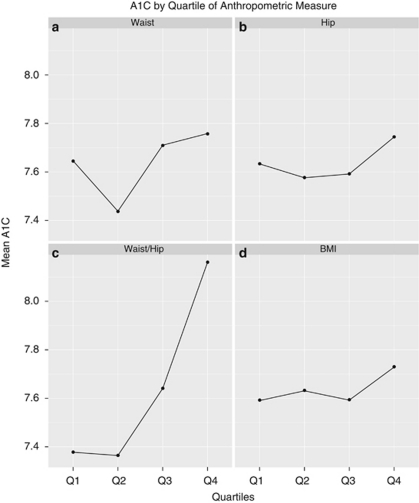
Preliminary trends across the data were evaluated by generating plots of A1C against quartiles of each anthropometric measure (Figure 1). Participant characteristics were examined by quartiles of A1C (Table 1). Characteristics were computed within each quartile as appropriate (that is, mean and s.d. for continuous variables; proportions for categorical variables).
Figure 1.
(a–d) Mean A1C by quartiles of waist circumference (a), hip circumference (b), WHR (c) and BMI (d).
Table 1. Participant characteristics (n=190) by quartiles of HbA1c.
| Characteristic, mean (s.d.) |
Quartiles of HbA1c |
|||
|---|---|---|---|---|
| 1st n=51 | 2nd n=46 | 3rd n=46 | 4th n=47 | |
| HbA1c, (%) | ⩽6.8 | 6.9–7.6 | 7.7–8.2 | >8.2 |
| Women, no. (%) | 24 (47) | 19 (41) | 22 (48) | 22 (47) |
| Age (years) | 60.4 (10.6) | 58.7 (9.2) | 61.5 (10.7) | 58.5 (11.5) |
| Diabetes duration (years) | 5.9 (6.0) | 9.6 (7.7) | 12.9 (9.9) | 10.6 (6.9) |
| Europid, no. (%) | 67 (34) | 74 (34) | 72 (33) | 64 (30) |
| Completed high school, no. (%) | 90 (46) | 89 (41) | 78 (36) | 83 (39) |
| Cardiovascular disease, no. (%) | 10 (20)a | 8 (17)b | 9 (20)b | 7 (15)a |
| Current tobacco use, no. (%) | 4 (8)c | 5 (11)a | 4 (9)a | 5 (11)c |
| Medications | ||||
| Statin use, no. (%) | 38 (75) | 35 (76) | 32 (70) | 33 (70) |
| Insulin use, no. (%) | 4 (8) | 12 (26) | 22 (48) | 26 (55) |
| Metformin, no. (%) | 39 (76) | 42 (91) | 33 (72) | 34 (72) |
| Sulfonylurea, no. (%) | 17 (33) | 20 (43) | 19 (41) | 19 (40) |
| Other OHAd, no. (%) | 1 (2) | 1 (2) | 1 (2) | 0 (0) |
| One OHA, no. (%) | 33 (65) | 18 (39) | 20 (43) | 17 (36) |
| Two OHA, no. (%) | 17 (33) | 25 (54) | 20 (43) | 24 (51) |
| Three OHA, no. (%) | 1 (2) | 3 (7) | 6 (13) | 6 (13) |
| Daily step counte | 5400 (2700) | 6000 (2200) | 4900 (2200) | 5000 (3100) |
| Anthropometric parameters | ||||
| Waist circumference (cm) | ||||
| Women | 98.4 (14.9) | 96.0 (13.4) | 102.3 (10.3) | 99.7 (13.7) |
| Men | 105.3 (12.2) | 100.6 (14.2) | 104.0 (10.6) | 107.9 (14.6) |
| Hip circumference (cm) | ||||
| Women | 114.4 (14.9) | 110.9 (10.6) | 115.5 (10.7) | 110.7 (11.6) |
| Men | 109.6 (9.8) | 106.1 (13.4) | 107.1 (9.5) | 109.9 (11.6) |
| WHR | ||||
| Women | 0.86 (0.06) | 0.86 (0.07) | 0.89 (0.06) | 0.90 (0.07) |
| Men | 0.96 (0.06) | 0.95 (0.06) | 0.98 (0.07) | 0.98 (0.06) |
| BMI (kg m−2) | ||||
| Women | 31.4 (6.9) | 29.8 (6.0) | 33.1 (5.3) | 30.0 (4.7) |
| Men | 30.0 (5.0) | 28.8 (6.0) | 30.2 (4.3) | 30.5 (6.5) |
Abbreviations: BMI, body mass index; OHA, oral hypoglycemic agents; WHR, waist-to-hip ratio.
Data for two participants missing.
Data for four participant missing.
Data for one participants missing.
Other OHA: meglitinides, thiazolidinediones and incretin agents.
Rounded to the nearest hundred steps.
Multivariate linear regression was applied to these data to evaluate the associations of each anthropometric parameter with A1C, after adjustment for potential confounders. We identified potential confounders by comparing the beta-coefficients of the anthropometric parameter of interest across a variety of models containing various combinations of covariates. The covariates having the strongest impact on this beta-coefficient were identified as statistically important confounders and retained in the final model. Clinically important confounders such as daily step count, age, ethnicity, sex as well as the use of insulin and oral hypoglycemic medications were also included. Previous studies in this population have adjusted for BMI when evaluating models containing anthropometric parameters such as waist circumference.3 We, therefore, compared models with and without BMI to determine whether it was an important confounder of the associations of interest in this study. Variables meeting the above criteria were retained in the final models.
Multivariate regression was also employed to evaluate the associations between daily step counts and anthropometric parameters, as well as A1C. Sex-specific analyses were additionally conducted.
Residual plots of the most representative models were examined to verify that the assumptions of linear regression were met. All analyses were conducted using the R statistical package.15
Results
A total of 201 individuals (106 men and 95 women) completed the assessment. In all, 11 were excluded from the present analyses because of missing A1C or pedometer data. The remaining 190 participants (87 women and 103 men) were 60 years of age on average, with mean diabetes duration of 10 years. The cohort was predominantly Europid (69%), with 18% reporting prevalent cardiovascular disease (27% in men; 7% in women). The mean BMI was 30.4 kg m−2, and the mean daily step count rounded to the nearest hundred steps was 5300, placing participants, on average, in the low-active category, according to the classification scheme developed by Tudor-Locke and Bassett.16 The average A1C and systolic/diastolic blood pressure were 7.6% and 137/80 mm Hg, respectively. There was a greater prevalence of insulin use in the higher quartiles of A1C (Table 1), and the mean WHR and BMI were higher in the fourth compared with the first quartile of A1C, particularly among the women. The mean waist circumference was higher in the fourth compared with the first quartile of A1C, particularly among the men.
Identification of confounders
Using the approach described previously, age, ethnicity and sex were determined to be statistically important confounders and as such they were included in all models. Dietary covariates were evaluated in all models but were not found to be statistically important confounders. Though they did not significantly impact the associations of interest, medication use was retained in the final models because of their clinical importance.
Anthropometric parameters as predictors of A1C
Plots suggested a positive curvilinear association between mean A1C and WHR quartiles (Figure 1c). This was not apparent for waist, hip circumference or BMI (Figures 1a, b and d).
In age, ethnicity and sex-adjusted models, each s.d. increase in waist circumference (13.4 cm) was associated with a 0.26% (95% confidence interval (CI): 0.04, 0.47) higher A1C (Table 2). Further adjustment for BMI markedly increased these associations, whereas additional adjustment for insulin use and the number of hypoglycemic agents being used marginally decreased the magnitude of association. In the fully adjusted model, each s.d. increase in waist circumference was associated with a statistically and clinically important 0.51% (0.10, 0.93) increment in A1C.
Table 2. Change in A1C (95% CI) per s.d. increase in each anthropometric measure.
| Anthropometric measure (s.d.) | All | Women | Men |
|---|---|---|---|
| Waist circumference (13.4 cm) | |||
| Adjusted for age, ethnicity, sex | 0.26 (0.04, 0.47) | 0.20 (−0.09, 0.48) | 0.29 (−0.03, 0.61) |
| Adjusted for age, ethnicity, sex, BMI | 0.81 (0.37, 1.24) | 0.81 (0.31, 1.31) | 0.84 (0.04, 1.63) |
| Adjusted for age, ethnicity, sex, BMI, steps | 0.72 (0.27, 1.17) | 0.70 (0.17, 1.24) | 0.78 (−0.01, 1.58) |
| Adjusted for age, ethnicity, sex, BMI, steps, insulin use | 0.54 (0.12, 0.97) | 0.54 (0.03, 1.05) | 0.62 (−0.13, 1.36) |
| Adjusted for age, ethnicity, BMI, steps, insulin use, number of OHA | 0.51 (0.10, 0.93) | 0.55 (0.04, 1.07) | 0.62 (−0.09, 1.33) |
| WHR (0.08) | |||
| Adjusted for age, ethnicity, sex | 0.46 (0.21, 0.71) | 0.52 (0.20, 0.84) | 0.41 (0.03, 0.80) |
| Adjusted for age, ethnicity, sex, BMI | 0.48 (0.21, 0.74) | 0.56 (0.23, 0.89) | 0.39 (−0.03, 0.82) |
| Adjusted for age, ethnicity, sex, steps | 0.41 (0.15, 0.67) | 0.46 (0.11, 0.81) | 0.39 (0.01, 0.77) |
| Adjusted for age, ethnicity, sex, steps, insulin use | 0.35 (0.11, 0.59) | 0.40 (0.07, 0.72) | 0.33 (−0.02, 0.69) |
| Adjusted for age, ethnicity, sex, steps, insulin use, number of OHA | 0.32 (0.08, 0.56) | 0.41 (0.08, 0.75) | 0.27 (−0.08, 0.61) |
Abbreviations: BMI, body mass index; CI, confidence interval; OHA, oral hypoglycemic agents; WHR, waist-to-hip ratio.
In age-, ethnicity- and sex-adjusted models, each s.d. increase in WHR (0.08) was associated with a 0.46% (95% CI: 0.21, 0.71) increment in A1C (Table 2). This estimate did not change importantly after further adjustment for BMI or daily steps. In the fully adjusted model, the association between each s.d. increase in WHR and A1C was 0.32 % (0.08, 0.56).
Associations of daily step count with anthropometric measures
In fully adjusted models, each s.d. increment in daily step count (2609) was associated with clinically important decreases in BMI and waist circumference, among both women and men (Table 3). The decrease in WHR was statistically significant among women but not men.
Table 3. Change in each anthropometric measure (95% CI) per s.d. increase in daily stepsa, in models adjusted for age, ethnicity, sex, insulin use and number of OHA.
| Measure | All | Women | Men |
|---|---|---|---|
| Waist, (cm) | −4.6 (−6.4, −2.8) | −5.3 (−8.2, −2.4) | −4.1 (−6.4, −1.7) |
| WHR | −0.01 (−0.02, −0.00) | −0.03 (−0.04, −0.01) | 0 (−0.02, 0.03) |
| BMI, (kg m−2) | −1.6 (−2.4, −0.8) | −1.4 (−2.8, −0.1) | −1.7 (−2.7, −0.7) |
Abbreviations: BMI, body mass index; CI, confidence interval; OHA, oral hypoglycemic agents; WHR, waist-to-hip ratio.
s.d. for daily step count: 2609 (combined); 2438 (women); 2796 (men).
Association of daily step count with A1C
In models adjusting for age, ethnicity, sex, insulin use and the number of oral hypoglycemic agents being used, each s.d. increase in daily step counts was associated with a 0.21%. (95% CI: 0.41, 0.02) lower A1C (Table 4). Further adjustment for either waist circumference and BMI or WHR reduced the estimates of this association by approximately one-third, with CI that now cross zero.
Table 4. Change in A1C (with 95% CI) per s.d. increase in daily step counta.
| Variables adjusted in model | All | Women | Men |
|---|---|---|---|
| Age, ethnicity, sex, insulin, number of OHA | −0.21 (−0.41, −0.02) | −0.24 (−0.52, 0.04) | −0.26 (−0.52, 0.01) |
| Age, ethnicity, sex, insulin, number of OHA, waist, BMI | −0.16 (−0.37, 0.05) | −0.14 (−0.44, 0.16) | −0.24 (−0.52, 0.04) |
| Age, ethnicity, sex, insulin, number of OHA, WHR | −0.16 (−0.35, 0.04) | −0.09 (−0.39, 0.21) | −0.24 (−0.50, 0.02) |
Abbreviations: BMI, body mass index; CI, confidence interval; OHA, oral hypoglycemic agents; WHR, waist-to-hip ratio.
SD for daily step count: 2609 (combined); 2438 (women); 2796 (men).
Discussion
Our findings demonstrate that among adults treated for type 2 diabetes, anthropometric indicators of abdominal adiposity are positively associated with A1C levels. Each s.d. increase in waist circumference (13.4 cm) was associated with an approximately 0.5% higher A1C in models adjusted for age, sex, ethnicity, BMI and anti-hyperglycemic medication use. Each s.d. (0.08 unit) increase in WHR was associated with an approximately 0.35% higher A1C in models adjusted for age, sex and ethnicity. The associations of these anthropometric measures with A1C were only marginally decreased by adjustment for daily step counts. Increments of one s.d. increase in daily step counts were associated with reductions in A1C of approximately 0.2% in age-, ethnicity-, sex- and medication-adjusted models. Further adjustment for anthropometric measures of abdominal adiposity reduced the estimates of these associations by approximately one-third and resulted in a loss of statistical significance, though the associations were still potentially of clinical importance. Finally, we determined step counts to be associated with clinically important reductions in both waist circumference and BMI among both women and men, and with reductions in WHR only among women.
Our analyses indicate that abdominal adiposity, as captured by anthropometric parameters, is importantly related to A1C, even after accounting for daily step counts. In fact, adjustment for daily step counts resulted in only small attenuations of the associations between anthropometric parameters and A1C. In contrast, whereas higher step counts are associated with a lower A1C, roughly one-third of this association is accounted for by reductions in abdominal adiposity, according to our models.
Our findings are consistent with those of the Atherosclerosis Risk in Communities Study study, in which persons with type 2 diabetes in the highest quartile of WHR (1.00–1.27) had a 0.8% greater A1C compared with persons in the lowest quartile (WHR 0.64–0.93).4 In contrast to this study, however, we additionally examined effects of waist circumference adjusted for BMI, accounted for daily steps and also medication use. In a recent cross-sectional analysis of the Guangzhau Biobank Cohort Study, among anthropometric parameters, WHR was the strongest predictor of type 2 diabetes, higher levels of physical activity were associated with a lower risk of type 2 diabetes, and WHR was more strongly associated with diabetes risk than physical activity.17 Our study complements this work by assessing the associations with glycemic control in a diabetic population and accounting for physical activity through an objective measure, pedometer-based estimates of daily step counts.
Our study highlights the importance of abdominal adiposity as a possible determinant of A1C. Waist circumference and WHR are highly correlated with intra-abdominal adipose tissue, which is strongly linked to development of insulin resistance and type 2 diabetes itself.1 This is due in part to its association with ectopic fat and its greater lipotoxic effects through the liberation of free fatty acids and inflammatory cytokines.18, 19, 20, 21 It is well established that glycemic control deteriorates over time.22, 23 This is felt to be because of the decline in beta cell function.24 Our findings would suggest that abdominal adiposity is a strong determinant of A1C even in a population being treated for type 2 diabetes, and may in fact be an important factor that determines the degree to which the A1C deteriorates in this population.
We demonstrated an inverse relationship between daily step counts and A1C. Indeed, physical activity has been shown in several studies to improve A1C.25 However, few studies have concurrently examined physical activity, abdominal adiposity or its indicators, and glycemic control. Our findings indicate that approximately one-third of the inverse association between step counts and A1C may be explained by lower levels of abdominal adiposity. In a Cochrane review of exercise interventions for type 2 diabetes, 14 trials were evaluated but only two studies reported on changes in visceral adipose tissue. The latter was significantly reduced in the exercise intervention compared with the control group.26, 27 Consideration of these findings in combination with ours, supports the role of abdominal adiposity as a partial mediator of the reductions in A1C associated with increasing daily step counts.
In our study, adjustment for WHR or waist circumference decreased the magnitude of the association of daily step counts with A1C from −0.21% per s.d. increase in step count to −0.16%. Although the latter fell just below the threshold for statistical significance, these data suggest that higher daily step counts may be associated with a lower A1C independently of abdominal adiposity. Potential mechanisms for this might include an alleviation of the defect in insulin-stimulated glycogen metabolism in skeletal muscle, improvement in postprandial hyperglycemia by improving postprandial insulin secretion and lowering of hepatic glucose production.28, 29, 30
Our analyses offer a potential mechanistic explanation for the benefits of increasing daily step count levels in the management of type 2 diabetes. Specifically higher daily step counts are clinically associated with a smaller waist circumference, which in turn is associated with lower A1C levels. There was also a suggestion of a direct association between higher step counts and a lower A1C, independent of the associated changes in adiposity. These proposed mechanisms need to be evaluated further in intervention studies. The findings of this study, imply that there may be an opportunity for patients with type 2 diabetes to leverage the impact of higher daily step counts on waist circumference by walking more, reducing their waist circumference, and thereby improving their glycemic control. Further, they may also achieve direct (adiposity-independent) benefits of physical activity on A1C as well as important reductions in blood pressure, as we have previously demonstrated.8
The current study is limited by its cross-sectional nature, and potentially by its sample size. Attempts to minimize measurement error were taken by applying consistent and standardized methods for measuring blood pressure and HbA1c. We used anthropometric measures of body fat distribution specifically because they are cheaper, less invasive, more acceptable to patients and therefore more practical in a clinical setting. This might be considered a limitation as the latter are less accurate than radiological estimations of body fat distribution. Some studies suggest that the Yamax Digiwalker SW-200 might underestimate counted steps at higher BMIs.31, 32 However, these underestimates if they did occur are likely to have been similar across participants, and therefore unlikely to affect the demonstrated associations. Furthermore, our study participants were carefully trained on how to position the pedometer on their waistband or belt so as to ensure that it was placed in a vertical position. In addition, all pedometers registered a reading between nineteen and twenty-one when they were checked for accuracy by comparing their reading with twenty counted steps.
Our cohort was fairly sedentary with average daily step counts of approximately 5000. Because our associations were limited to these lower levels of physical activity, it is possible that the strength of the association between physical activity and A1C might be underestimated. Finally, while we attempted to adjust for all clinically and statistically important confounders, we may not have captured all such variables. Our findings need to be explored further in pedometer-based intervention trials. Despite these limitations, this is the only study, of which we are aware, that has employed objective measures of walking in examining the associations between anthropometric indicators of abdominal adiposity and A1C.
In summary, our study suggests that abdominal adiposity is an important determinant of A1C in adults treated for type 2 diabetes even after adjustment for daily step counts and medications. Daily step counts are inversely related to A1C and about one-third of this association may be explained by abdominal adiposity. Our findings provide quantification of the associations of decreasing waist size and increasing daily step counts on A1C levels among adults treated for type 2 diabetes.
Acknowledgments
This research was supported by a grant from the Canadian Institutes of Health Research to K Dasgupta (MOP-79275).
The authors declare no conflict of interest.
References
- Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni M, Armellini F, Milani MP, De Marchi M, Todesco T, Robbi R, et al. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obes Relat Metab Disord. 1992;16:495–504. [PubMed] [Google Scholar]
- Sam S, Haffner S, Davidson MH, D'Agostino RB, Sr, Feinstein S, Kondos G, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care. 2009;32:1916–1920. doi: 10.2337/dc09-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- Uusitupa MI. Early lifestyle intervention in patients with non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Ann Med. 1996;28:445–449. doi: 10.3109/07853899608999106. [DOI] [PubMed] [Google Scholar]
- Walker KZ, Piers LS, Putt RS, Jones JA, O'Dea K. Effects of regular walking on cardiovascular risk factors and body composition in normoglycemic women and women with type 2 diabetes. Diabetes Care. 1999;22:555–561. doi: 10.2337/diacare.22.4.555. [DOI] [PubMed] [Google Scholar]
- Dasgupta K, Chan C, Da Costa D, Pilote L, De Civita M, Ross N, et al. Walking behaviour and glycemic control in type 2 diabetes: seasonal and gender differences—study design and methods. Cardiovasc Diabetol. 2007;6:1. doi: 10.1186/1475-2840-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjoo P, Joseph L, Pilote L, Dasgupta K. Sex differences in step count-blood pressure association: a preliminary study in type 2 diabetes. PLoS One. 2010;5:e14086. doi: 10.1371/journal.pone.0014086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyarto EV, Myers AM, Tudor-Locke C. Pedometer accuracy in nursing home and community-dwelling older adults. Med Sci Sports Exerc. 2004;36:205–209. doi: 10.1249/01.MSS.0000113476.62469.98. [DOI] [PubMed] [Google Scholar]
- Shepherd EF, Toloza E, McClung CD, Schmalzried TP. Step activity monitor: increased accuracy in quantifying ambulatory activity. J Orthop Res. 1999;17:703–708. doi: 10.1002/jor.1100170512. [DOI] [PubMed] [Google Scholar]
- Shatenstein B, Nadon S, Godin C, Ferland G. Development and validation of a food frequency questionnaire. Can J Diet Pract Res. 2005;66:67–75. doi: 10.3148/66.2.2005.67. [DOI] [PubMed] [Google Scholar]
- Schneider PL, Crouter SE, Bassett DR. Pedometer measures of free-living physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36:331–335. doi: 10.1249/01.MSS.0000113486.60548.E9. [DOI] [PubMed] [Google Scholar]
- Crouter SE, Schneider PL, Karabulut M, Bassett DR., Jr Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Team RDC. A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna; 2007. [Google Scholar]
- Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- Qin L, Corpeleijn E, Jiang C, Thomas GN, Schooling CM, Zhang W, et al. Physical activity, adiposity, and diabetes risk in middle-aged and older Chinese population: the Guangzhou Biobank Cohort Study. Diabetes Care. 2010;33:2342–2348. doi: 10.2337/dc10-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- Despres JP. The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patients' risk. Obes Res. 1998;6 (Suppl 1:8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- UK prospective diabetes study 16 Overview of 6 years' therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- Mourier A, Gautier JF, De Kerviler E, Bigard AX, Villette JM, Garnier JP, et al. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Effects of branched-chain amino acid supplements. Diabetes Care. 1997;20:385–391. doi: 10.2337/diacare.20.3.385. [DOI] [PubMed] [Google Scholar]
- Minuk HL, Vranic M, Marliss EB, Hanna AK, Albisser AM, Zinman B. Glucoregulatory and metabolic response to exercise in obese noninsulin-dependent diabetes. Am J Physiol. 1981;240:E458–E464. doi: 10.1152/ajpendo.1981.240.5.E458. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36:434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- Koivisto VA, Pelkonen R, Nikkila EA, Heding LG. Human and porcine insulins are equally effective in the regulation of glucose kinetics of diabetic patients during exercise. Acta Endocrinol (Copenh) 1984;107:500–505. doi: 10.1530/acta.0.1070500. [DOI] [PubMed] [Google Scholar]
- Crouter SE, Schneider PL, Bassett DR., Jr Spring-levered versus piezo-electric pedometer accuracy in overweight and obese adults. Med Sci Sports Exerc. 2005;37:1673–1679. doi: 10.1249/01.mss.0000181677.36658.a8. [DOI] [PubMed] [Google Scholar]
- Tyo BM, Fitzhugh EC, Bassett DR, Jr, John D, Feito Y, Thompson DL. Effects of body mass index and step rate on pedometer error in a free-living environment. Med Sci Sports Exerc. 2011;43:350–356. doi: 10.1249/MSS.0b013e3181e9b133. [DOI] [PubMed] [Google Scholar]