Abstract
Objective:
Subcutaneous (SC) adipose tissue stearic acid (18:0) content and stearoyl-CoA desaturase-1 (SCD1)-mediated production of oleic acid (18:1) have been suggested to be altered in obesity. The objective of our study was to examine abdominal adipose tissue fatty acid content and SCD1 mRNA/protein level in women.
Subjects and methods:
Fatty acid content was determined by capillary gas chromatography in SC and omental (OM) fat tissues from two subgroups of 10 women with either small or large OM adipocytes. Samples from 10 additional women were used to measure SCD1 mRNA and protein expression, total extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphorylated ERK1/2 protein as well as insulin receptor (IR) expression levels.
Results:
OM fat 18:0 content was significantly lower in women with large OM adipocytes compared with women who had similar adiposity, but small OM adipocytes (2.37±0.45 vs 2.75±0.30 mg per 100 g adipose tissue, respectively, P⩽0.05). OM fat 18:0 content was negatively related to the visceral adipose tissue area (r=−0.44, P=0.05) and serum triglyceride levels (r=−0.56, P<0.05), while SC fat 18:0 content was negatively correlated with total body fat mass (BFM) (r=−0.48, P<0.05) and fasting insulin concentration (r=−0.73, P<0.005). SC adipose tissue desaturation index (18:1/18:0), SCD1 expression and protein levels were positively correlated with BFM. Moreover, obese women were characterized by a reduced OM/SC ratio of SCD1 mRNA and protein levels. A similar pattern was observed for ERK1/2 and IR expression.
Conclusion:
The presence of large adipocytes and increased adipose mass in a given fat compartment is related to reduced 18:0 content and increased desaturation index in women, independently of dietary fat intake. The depot-specific difference in ERK1/2 expression and activation, as well as in SCD1 and IR expression in obese women is consistent with the hypothesis that they may predominantly develop SC fat, which could in turn help protect from metabolic disorders.
Keywords: visceral adipose tissue, omental adipose tissue, subcutaneous adipose tissue, computed tomography
Introduction
In both men and women, excess accumulation of adipose tissue and hypertrophic adipocytes on anatomical structures such as the mesentery and greater omentum, also termed intra-abdominal or visceral obesity, has emerged as a critical determinant of obesity-related metabolic disorders eventually leading to insulin resistance, type 2 diabetes and cardiovascular disease.1, 2 Specific features of each adipose tissue compartment may help explain the preferential association of visceral fat with cardiometabolic risk markers, including adipocyte metabolism, adipokine secretion and tissue expandability.1 However, a relatively small number of studies focused on fatty acid content.3, 4
Adipose tissue fatty acid composition is thought to reflect dietary fatty acid intake over a relatively long period. On the basis of the half-life of adipose tissue fatty acids, a temporal relationship of approximately 1–3 years has been proposed.5, 6, 7 Correlations were reported between dietary polyunsaturated fatty acids intake and their content in subcutaneous (SC) adipose tissue.4, 8, 9, 10, 11, 12, 13 However, discrepancies have been noted for the association between dietary intake and the fat tissue content of saturated fatty acids (SFA), in particular for 14:0, palmitate (16:0) or stearate (18:0) and monounsaturated fatty acids (MUFA).4, 5
Regarding regional differences, fatty acid composition of femoral, abdominal and upper arm fat appears to be similar.5 A few studies reported small (2–10%) but significant differences in the proportions of SFA and MUFA between those sites.5, 14 Furthermore, studies comparing SC and visceral adipose tissue have demonstrated a higher proportion of MUFA in the former compared with the latter, and the increase in MUFA occurred at the expense of SFA content, suggesting a desaturation process. However, available information is incomplete as most studies examined SC adipose tissue obtained from only one site irrespective of other compartments.5
18:0 is one of the most abundant dietary SFA in the western diet.15, 16 This fatty acid can also be synthesized by the elongation of 16:0.16 The 18:0 content of adipose tissue and the 18:0/16:0 ratio (elongation index) were recently shown to be inversely correlated with body mass index (BMI) and SC adipocyte cell size in humans,4 although this finding is not unanimous.17 A positive relationship between adipose tissue 18:0 content and insulin sensitivity measured by the homeostatic model assessment of insulin sensitivity was also reported.4 Although the 18:0 fatty acid has multiple fates such as elongation, oxidation or esterification, it is the major substrate for stearoyl-CoA desaturase-1 (SCD1).15
SCD1 catalyzes the insertion of a double bond between carbons 9 and 10 of 16:0 and 18:0 converting them to palmitoleate and oleate, respectively.15 A recent study in human adipocytes showed that upregulation of de novo lipogenesis was accompanied by increased elongation and desaturation, which channels newly synthesized SFA to oleic acid.18 Studies in SCD1-null mice showed that animals are lean and protected from diet-induced obesity as well as insulin resistance.15 In humans, Roberts et al.4 showed that subjects with a lower 18:1/18:0 ratio (desaturation index) had a lower BMI and reduced SC adipocyte cell size.
Adipocyte differentiation is orchestrated by numerous transcription factors such as CCAAT-enhancer-binding protein (C/EBP) family members and SREBP-1c (sterol regulatory element-binding protein-1c).19, 20 Mitogen-activated protein kinase (MAPK) and ERK1/2 constitute key factors in this process. ERK1-null mice have reduced adipose tissue mass and adipocyte number.21 The MAPK ERK1/2 pathway was shown to be involved in the activation of the C/EBP family members22, 23 and represents an early component of adipogenesis.22, 23 Hence, high ERK1/2 phosphorylation rates in adipose tissue may reflect a high potential of this tissue to initiate adipocyte differentiation. Activation of the adipogenic program coordinates the expression of lipogenic genes, including Scd1, to initiate and maintain adipocyte functions such as glucose uptake, lipid synthesis and storage.20 Recent studies have shown that SCD1 knockdown impairs insulin-stimulated glucose uptake in human adipocytes.18
Despite the prevalent idea that fatty acid composition of adipose tissue reflects dietary fat intake, no clear answer has been provided on regional differences in 18:0 content and how it relates to obesity or visceral obesity. The present study was designed to examine the fatty acid content of SC and omental (OM) adipose tissue in women. We tested the hypothesis that adipose tissue 18:0 content would be reduced in expanded fat compartments with large adipocytes. This would occur with an increase of the desaturation index and SCD1 expression.
Subjects and methods
Women of this study were recruited through the elective surgery schedule of the Gynaecology Unit of the Laval University Medical Center. The study was approved by the Medical Ethics Committees of Laval University Medical Center. All subjects provided written informed consent before their inclusion in the study. We selected a subsample of 20 women to be included in two subgroups of 10 women with either small (n=10) or large (n=10) OM adipocytes, but individually matched for age, total body fat mass (BFM) and SC adipocyte size. Women of the study elected for total abdominal hysterectomies, some with bilateral salpingo ovariectomy (n=9). Reasons for surgery included one or more of the following: myoma/fibroids (n=14), menorrhagia/menometrorrhagia (n=9), benign cyst (n=6), incapacitating dysmenorrhea (n=4), endometriosis (n=4), chronic pelvic pain (n=3), endometrial polyp (n=2), incapacitating premenstrual syndrome (n=1), hydatid of Morgani (n=1) or corpus luteum (n=1). SCD1 mRNA expression as well as SCD1 and ERK1/2 protein levels were measured in an additional subsample of 10 women. These women were aged 40.1–58.9 years (mean age 48.70±5.96 years), had an average BMI of 27.6±6.1 kg m−2 (range 19.3–36.6 kg m−2). They also elected for total abdominal hysterectomies, some with bilateral salpingo ovariectomy (n=9). Reasons for surgery included myoma/fibroids (n=7), menorrhagia/menometrorrhagia (n=5), ovarian cyst (n=1), endometriosis (n=1) and endometrial polyp (n=1).
Dietary data
Dietary intakes were assessed using a validated food frequency questionnaire24 containing 91 food items available in the Quebec City area and reflecting Canadian eating habits. The participants were questioned about the amount and frequency of intake of items among the following food groups: vegetables; fruits; legumes; nuts and seeds; cereals and grain products; milk and dairy products; meat/processed meat; poultry; fish; eggs; sweets; oil and fats; fast foods and drinks.
Body fatness and body fat-distribution measurements
Dual-energy X-ray absorptionmetry was used to measure total BFM and lean body mass. We used a Hologic QDR-2000 densitometer and the enhanced array whole-body software V5.73A (Hologic, Bedford, MA, USA). Computed tomography was used to measure the SC and visceral adipose tissue cross-sectional areas using a GE Light Speed 1.1 CT scanner (General Electric Medical Systems, Milwaukee, WI, USA) as described.25
Plasma lipid–lipoprotein measurements
After 12 h of fasting on the morning of the surgery, blood samples were obtained. A Technicon RA-analyzer (Bayer, Etobicoke, ON, Canada) and enzymatic methods were used to measure cholesterol and triglyceride levels in plasma. Plasma very low-density lipoproteins were isolated by ultracentrifugation, and the high-density lipoprotein fraction was obtained by precipitation of the low-density lipoproteins from the infranatant with heparin and MnCl2.26 The cholesterol content of the infranatant was measured before and after precipitation, and low-density lipoprotein cholesterol concentration was calculated by difference.
Adipose tissue sampling
SC and OM adipose tissue samples were collected at the site of the surgical incision (lower abdomen) and at the distal portion of the greater omentum, respectively, and immediately carried to the laboratory. Adipocyte isolation was performed by collagenase digestion of a portion of the fresh adipose tissue sample. Average cell population diameters were measured by image analysis.27 Remaining tissue was frozen in liquid nitrogen and stored at −80 °C for subsequent analyses.
Fatty acid profile
Adipose tissue total lipids were extracted, fatty acids were methylated and the fatty acid profile of each sample was determined by capillary gas chromatography. Fatty acids were identified according to their retention time in the column, using a standard mixture of 43 fatty acids as a basis for comparison (FAME 37 mix, Supelco, Bellefonte, PA, USA), the C15:0 fatty acids, as well as the following fatty acids: C22:5 w6 (Larodan AB, Malmö, Sweden), C22:5 w3 (Supelco), C22:5 w3 cis-12, and the mixture of 31 fatty acids GLC-411 (NuChek Prep, Elysian, MN, USA), C14:1 trans-9, C16:1 trans-9 and isoforms of C18:1 (cis-6, cis-11, cis-12, cis-13-trans-6, trans-11) (Supelco). Results were expressed as grams of fatty acids per 100 g of adipose tissue.28
Messenger RNA expression
Total RNA was isolated from SC and OM adipose tissues, tested for quality and concentration, and used in cDNA synthesis as described.29 Real-time cDNA amplification was performed in triplicate using the LightCycler 480 (Roche Diagnostics, Indianapolis, IN, USA) and the SYBR Green I Master (Applied Biosystems, Hayward, CA, USA) as described.29 Target gene amplifications were normalized using expression levels of the following housekeeping genes: hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1), ATP synthase O subunit (ATP5O) and glucose-6-phosphate dehydrogenase (G6PD). Associations observed in the present analyses were not attributable to housekeeping gene variation. Primer sequences were (sense/anti-sense):
for SCD1: 5′-CTCCACTGCTGGACATGAGA-3′/5′-AATGAGTGAAGGGGCACAAC-3′
for ATPase5o: 5′-ATTGAAGGTCGCTATGCCACAG-3′/5′-AACGACTCCTTGGGTATTGCTTAA-3′
for HPRT1: 5′-AGTTCTGTGGCCATCTGCTTAGTA G-3′/5′-AAAAAACAATCCGCCCAAAGG-3′
and for G6PD: 5′-GATGTCCCCTGTCCCACCAACTCTG-3′/5′-GCAGGGCATTGAGGTTGGGAG-3′.
Western blot analyses
Tissues were homogenized in Tris buffer (pH 7.4) with sucrose, EDTA, proteases and phosphatase inhibitors. Excess fat was removed by centrifugation at 1000 g. Homogenates were centrifuged at 150 000 g for 90 min, and the supernatant was recovered and stored at −80 °C until analyzed. Protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Mississauga, ON, Canada). A total of 40 μg protein were mixed with 6 × Laemmli sample buffer (2% SDS, 2% β-mercaptoethanol, 10% v/v glycerol and 50 mg l−1 bromophenol blue in 0.1 M Tris-HCl buffer, pH 6.8), heated at 100 °C for 5 min, subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred to Immobilon-P membranes for immunoblotting. The membranes were incubated for 1 h in blocking buffer (1 × Tris-buffered saline (TBS), 0.1% Tween-20) containing 5% milk and then overnight in a buffer containing 5% bovine serum albumin (BSA) and various antibodies raised against SCD1 (1/5000) (generous gift from Dr J Ozols, Farmington, CT, USA), phospho-ERK1/2 (Thr-202; Tyr-204) (1/1000), total ERK1/2 (1/1000), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1/2500) (Cell Signalling Technology, Danvers, MA, USA) or the anti-insulin receptor (anti-IR α-960) (generous gift of Dr BI Posner, McGill University, QC, Canada). After three washes in tris-buffered saline and tween 20, the membranes were incubated at room temperature in tris-buffered saline and tween 20 with an anti-Rabbit IgG binding to horseradish peroxidase (1/10 000) (Bio-Rad). Signals were revealed using ECL-plus detection reagent (Roche Diagnostics, Laval, PQ, Canada). The appropriate bands were quantified using the phospho-imager system (Molecular imager FX, Bio-Rad).30
Statistical analyses
Student's t-tests were used to compare the characteristics of the subjects, dietary intakes and adipose tissue fatty acid profile between the two subgroups of women matched for adiposity, but showing small or large OM adipocytes. Pearson correlations were performed to quantify the associations of adipose tissue fatty acids with anthropometric, adiposity or blood lipid values. Pearson correlations were computed to quantify the associations between SCD1 mRNA expression or protein level in adipose tissue and other variables of interest. Statistical analyses were performed using JMP 7.0 software (SAS Institute, Cary, NC, USA).
Results
Characteristics of the subjects
Characteristics of women with small (n=10) or large OM adipocytes (n=10) are shown in Table 1. By design, the subgroups had similar total BFM, lean body mass and waist circumference. However, OM adipocyte size was higher in women with large OM adipocytes (P<0.0001). Although total adipose tissue areas were similar for both groups of women, those with large OM adipocytes had higher visceral adipose tissue area and triglyceride levels.
Table 1. Characteristics of the subjects.
| Characteristics | Small omental adipocytes (n=10) | Large omental adipocytes (n=10) | P |
|---|---|---|---|
| Age (years) | 47.3±3.9 | 47.7±3.3 | 0.81 |
| Weight (kg) | 71.8±12.0 | 71.0±14.9 | 0.65 |
| BMI (kg m−2) | 26.4±4.7 | 27.4±5.2 | 0.65 |
| Waist circumference (cm) | 91.5±11.2 | 96.0±12.7 | 0.38 |
| Total body fat mass (kg) | 27.3±7.6 | 26.7±7.8 | 0.74 |
| Lean body mass (kg) | 43.7±6.0 | 42.1±8.6 | 0.65 |
| Adipose tissue area (cm2) | |||
| Total | 398±121 | 394±141 | 0.93 |
| Visceral | 61±15 | 98±50 | 0.02 |
| Subcutaneous | 336±122 | 312±117 | 0.63 |
| Lipid profile | |||
| Cholesterol (mmol l−1) | 4.55±0.98 | 5.17±0.87 | 0.15 |
| HDL-cholesterol (mmol l−1) | 1.50±0.37 | 1.59±0.27 | 0.50 |
| LDL-cholesterol (mmol l−1) | 2.58±0.74 | 2.84±0.67 | 0.42 |
| Triglycerides (mmol l−1) | 1.05±0.30 | 1.62±0.61 | 0.01 |
| Cholesterol/HDL-C ratio | 3.14±0.73 | 3.34±0.80 | 0.54 |
| Apolipoprotein B (mg dl−1) | 0.84±0.19 | 0.96±0.21 | 0.19 |
| Insulin (μU ml−1) | 8.43±2.67 | 12.24±7.28 | 0.23 |
| Glycemia (mmol l−1) | 5.56±0.64 | 5.99±0.44 | 0.09 |
| Abdominal adipocyte diameters (μm) | |||
| Omental adipocyte size | 74.4±7.6 | 97.6±10.9 | <0.0001 |
| Subcutaneous adipocyte size | 98.3±11.7 | 99.5±12.1 | 0.83 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Data are mean±s.d.
Table 2 shows the dietary data of the subjects. Women were eating on average 2084 kcal per day. Women in both groups had a similar dietary intake in terms of energy, macronutrients and fibers. Both groups consumed the same amount of 16:0, 16:1, 18:0 and oleic (18:1) fatty acids.
Table 2. Dietary intakes of energy, macronutrients, fatty acids and fibers in women with small or large omental adipocytes, but similar total adiposity or subcutaneous adipocyte sizes.
| Small omental adipocytes (n=10) | Large omental adipocytes (n=10) | P | |
|---|---|---|---|
| Energy (kcal/d) | 2084±396 | 1912±347 | 0.3 |
| Total protein (g/d) | 89.4±15.2 | 74.6±21.1 | 0.09 |
| Total CHO (g/d) | 246±58 | 238±55 | 0.8 |
| Total fat (g/d) | 81.8±21.1 | 73.2±21.2 | 0.3 |
| Total SFA (g/d) | 24.1±7.5 | 22.1±6.5 | 0.5 |
| Total MUFA (g/d) | 36.2±10.7 | 32.1±10.8 | 0.4 |
| Total PUFA (g/d) | 15.4±4.4 | 13.1±5.1 | 0.3 |
| Total 16:0 (g/d) | 13.2±3.4 | 12.0±3.4 | 0.4 |
| Total 16:1 (g/d) | 1.2±0.3 | 1.1±0.4 | 0.7 |
| Total 18:0 (g/d) | 5.5±1.8 | 5.3±1.7 | 0.6 |
| Total 18:1 (g/d) | 34.2±10.4 | 30.3±10.4 | 0.4 |
| Total fiber (g/d) | 23.3±5.9 | 21.2±9.4 | 0.6 |
| Soluble fiber (g/d) | 7.4±1.9 | 7.1±2.8 | 0.8 |
| Insoluble fiber (g/d) | 15.8±4.1 | 14.1±6.6 | 0.5 |
Abbrevations: CHO, carbohydrates; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Data are mean±s.d.
Fatty acid composition of adipose tissues and the diet appear similar on a relative basis
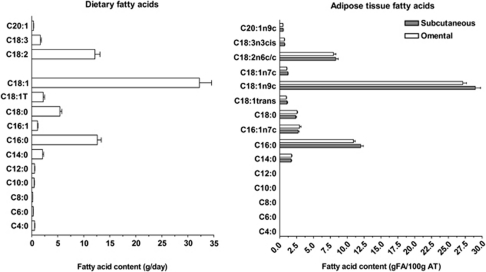
Figure 1 shows a comparison of fatty acid content in the diet (left panel) and in SC or OM adipose tissue (right panel). On a relative basis, adipose tissue fatty acid composition of both the SC and OM compartment was strikingly similar to dietary fatty acid intakes. The relative content of the main fatty acids, namely 18:1, 16:0, 18:2 and 18:0, were also similar. Positive associations were found between 14:0 and 20:1 fatty acid intakes and their content in SC (r=0.49, P⩽0.03) or OM (r=0.47, P⩽0.04) adipose tissue, respectively. Positive trends were also observed between 18:2 fatty acid intake and their content in SC and OM adipose tissues (r=0.42, P=0.06; r=0.40, P=0.08, respectively). Other dietary fatty acids were not correlated with their counterparts in the adipose tissue.
Figure 1.
Comparison of dietary fatty acid content (left panel) and SC or OM adipose tissue fatty acid composition (right panel). Only fatty acids that were detected at a value higher than 0.5 g fatty acid per 100 g adipose tissue are represented.
Lower 18:0 content and higher desaturation index is associated with the presence of large OM adipocytes
Adipose tissue fatty acid composition of the subgroups with small vs large OM adipocytes is shown in Table 3. Women with large OM adipocytes had significantly lower 18:0 content in OM adipose tissue. Similarly, women with large OM adipocytes had a lower 18:0/16:0 ratio. The desaturation index (18:1/18:0 ratio) was higher in women with larger OM adipocytes.
Table 3. Subcutaneous and omental adipose tissue fatty acid composition in women with either small or large omental adipocytes, but similar total adiposity or subcutaneous adipocyte sizes.
| Small omental adipocytes (n=10) | Large omental adipocytes (n=10) | P | |
|---|---|---|---|
| SC | |||
| Total SFA | 15.8±2.5 | 16.4±2.4 | 0.6 |
| Total MUFA | 35.2±5.4 | 35.1±2.7 | 1.0 |
| Total PUFA | 10.1±1.5 | 10.0±2.4 | 0.9 |
| 16:0 | 11.7±1.9 | 12.3±1.6 | 0.4 |
| 16:1 | 2.6±0.7 | 2.8±0.8 | 0.5 |
| 18:0 | 2.4±0.4 | 2.3±0.6 | 0.8 |
| 18:1 | 29.0±4.1 | 29.0±2.7 | 0.9 |
| 18:0/16:0 | 0.21±0.03 | 0.19±0.03 | 0.2 |
| 18:1/18:0 | 12.3±1.9 | 13.0±2.8 | 0.5 |
| OM | |||
| Total SFA | 15.4±1.2 | 15.2±1.7 | 0.7 |
| Total MUFA | 32.9±2.8 | 33.5±2.9 | 0.6 |
| Total PUFA | 9.6±1.3 | 9.3±2.2 | 0.7 |
| 16:0 | 10.8±0.9 | 11.0±1.3 | 0.7 |
| 16:1 | 2.8±0.5 | 3.1±1.3 | 0.6 |
| 18:0 | 2.7±0.3 | 2.4±0.4 | 0.04 |
| 18:1 | 27.1±2.7 | 27.2±2.4 | 0.9 |
| 18:0/16:0 | 0.25±0.03 | 0.22±0.05 | 0.05 |
| 18:1/18:0 | 9.9±0.7 | 12.0±0.7 | 0.05 |
Abbreviations: MUFA, monounsaturated fatty acids; OM, omental; PUFA, polyunsaturated fatty acids; SC, subcutaneous; SFA, saturated fatty acids.
Data are mean±s.d. (g per 100 g of fatty acids).
Table 4 shows the correlation coefficients between measures of 16:0, 18:0, elongation or desaturation, and adiposity or blood lipid values. Measures of overall adiposity, such as the BMI and total BFM, were inversely correlated with 18:0 content in SC adipose tissue. SC adipose tissue area tended to be negatively associated with 18:0 content in the same depot. Furthermore, SC adipocyte size was also inversely correlated with the elongation index, again in SC adipose tissue. BMI and SC adipose tissue area were positively associated with the desaturation index in the SC compartment, while a positive trend was observed with total BFM. When examining visceral adiposity, a higher visceral adipose tissue area was associated with a lower 18:0 content and a higher desaturation index in the OM depot. Plasma cholesterol concentration tended to be associated with the 18:0 content of OM adipose tissue. Furthermore, low-density lipoprotein-cholesterol levels were found to be inversely correlated with the elongation index in the SC depot. Triglyceride concentrations were negatively associated with the 18:0 content and elongation index, and positively associated with desaturation in OM fat. Finally, fasting insulin was negatively correlated with the 18:0 content, elongation and desaturation in SC fat. This correlation was absent in the OM depot.
Table 4. Pearson correlation coefficients between measures of 16:0, 18:0, elongation index or desaturation index and adiposity or blood lipid profile measures.
|
16:0 g FA per 100g AT |
18:0 g FA per 100g AT |
Elongation index (18:0/16:0) |
Desaturation index (18:1/18:0) |
|||||
|---|---|---|---|---|---|---|---|---|
| SC | OM | SC | OM | SC | OM | SC | OM | |
| BMI | −0.04 | −0.08 | −0.53** | −0.36 | −0.60** | −0.28 | 0.52** | 0.30 |
| Waist circumference | 0.03 | 0.03 | −0.50* | −0.51* | −0.64** | −0.46* | 0.41 | 0.37 |
| Total body fat mass | 0.01 | −0.12 | −0.48* | −0.24 | −0.57** | −0.16 | 0.42† | 0.10 |
| SAT area | 0.03 | −0.12 | −0.42† | −0.31 | −0.54** | −0.23 | 0.43* | 0.19 |
| VAT area | −0.10 | 0.09 | −0.33 | −0.44* | −0.33 | −0.42† | 0.27 | 0.45* |
| SC adipocyte diameter | 0.08 | 0.13 | −0.40 | −0.40 | −0.56** | −0.42† | 0.40 | 0.28 |
| OM adipocyte diameter | 0.21 | 0.05 | 0.04 | −0.33 | −0.20 | −0.32 | 0.06 | 0.29 |
| Cholesterol | 0.24 | 0.07 | −0.14 | −0.43† | −0.40 | −0.41 | 0.28 | 0.32 |
| Triglycerides | −0.18 | 0.08 | −0.33 | −0.56** | −0.27 | −0.50* | 0.33 | 0.59** |
| HDL-Cholesterol | 0.20 | −0.22 | 0.14 | −0.22 | −0.05 | −0.1 | −0.02 | 0.1 |
| LDL-Cholesterol | 0.29 | 0.17 | −0.14 | −0.28 | −0.43* | −0.33 | 0.26 | 0.17 |
| Cholesterol/HDL ratio | −0.04 | 0.30 | −0.35 | −0.21 | −0.36 | −0.32 | 0.28 | 0.23 |
| Fasting insulin | −0.33 | −0.16 | −0.73** | −0.35 | −0.56** | −0.20 | 0.61** | 0.31 |
Abbreiations: AT, adipose tissue; BMI, body mass index; FA, fatty acid; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OM, omental; SAT, subcutaneous adipose tissue; SC, subcutaneous; VAT, visceral adipose tissue.
*P⩽0.05, **P⩽0.01, †P=0.06.
Different expression pattern of SCD1, ERK1/2 and IR in OM vs SC adipose tissue
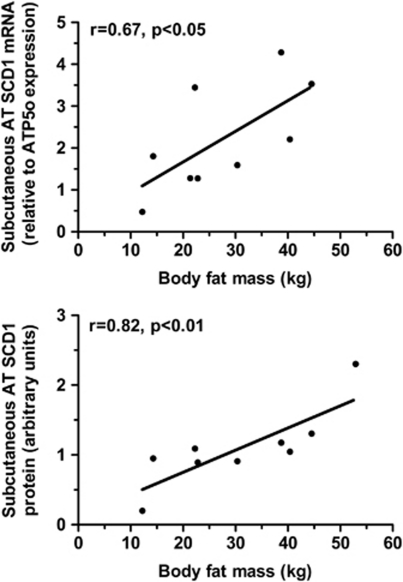
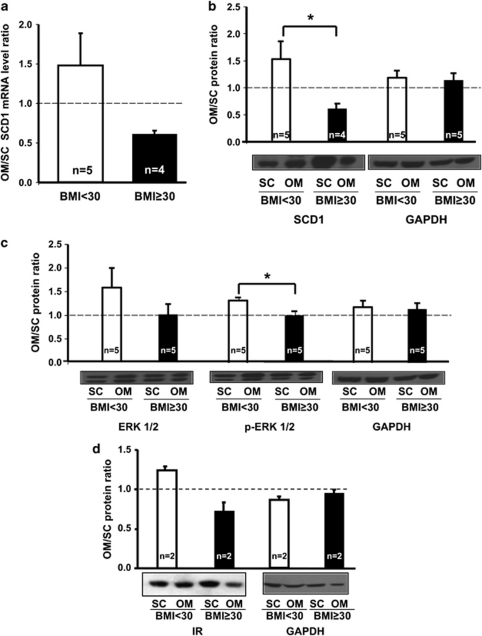
Significant positive associations between SCD1 mRNA and protein expression levels in SC adipose tissue and BFM were observed (Figure 2 and data not shown). However, this association was not observed in OM. Figure 3 shows the OM/SC ratio in normal or overweight women (BMI<30 kg m−2; n=5) vs obese women (BMI⩾30 kg m−2; n=4) of SCD1 mRNA (panel A) and protein (panel B) levels. Panel C represents the OM/SC ratio of total and phosphorylated ERK1/2 (p-ERK1/2), while panel D presents the ratio of IR expression. Obese subjects were characterized by a reduced OM/SC ratio of SCD1 mRNA and protein levels as well as a lower OM/SC ratio of total ERK1/2 protein level and its phosphorylated form (p-ERK1/2). Similar results were observed when examining the ratio of the IR expression.
Figure 2.
Associations between SCD1 mRNA expression or SCD1 protein levels in SC adipose tissue (AT) and total BFM. Messenger RNA expression normalized according to ATP5O mRNA expression (similar results were obtained using other housekeeping genes, see Subjects and Methods); protein levels normalized with GAPDH. Pearson correlation coefficients are shown.
Figure 3.
OM/SC ratios of SCD1 mRNA expression (a), SCD1 protein level (b), total ERK1/2 protein level, ERK 1/2 phosphorylation state (p-ERK 1/2) (c) and IR expression (d) in normal or overweight women (BMI<30 kg m−2) vs obese women (BMI⩾30 kg m−2). SCD1 mRNA expression values were normalized with G6PD whereas protein expressions were normalized by GAPDH (b–d). *P<0.05.
Discussion
Obesity-related alterations in adipose tissue fatty acid composition have attracted scientific interest recently, as 18:0 content has been specifically shown to be altered in obesity.4 The aim of the present study was to examine the fatty acid content of SC and OM adipose tissues in women with or without abdominal obesity. We tested the hypothesis that adipose tissue 18:0 content would be reduced in expanded fat compartments with large adipocytes and that this would be observed with concomitant increases of the desaturation index and SCD1 expression. We found that fatty acid content of each adipose tissue compartment was relatively similar to that of the diet. However, 18:0 content was reduced in OM fat of women with visceral obesity and large OM adipocytes, while overall obesity was related to increased SC desaturation, as well as SCD1 mRNA and protein levels. Adipose tissue 18:0 content has previously been studied in relation to obesity, but we are the first to address this question with respect to fat distribution.
Previous studies relating adipose tissue fatty acid composition and dietary fatty acids are equivocal. General consensus is that adipose tissue fatty acids reflect dietary composition.5 Accordingly, we found that the relative content of most fatty acids was similar in the diet and adipose tissue. However, when examining the correlations between specific dietary fatty acids and their content in adipose tissue, results were less consistent. Only dietary 14:0 and 20:1 were significantly correlated with their counterparts in a given compartment. We cannot exclude a type 2 error related to our sample size. The trend for a positive association that we found between dietary intake of 18:2 and its content in adipose tissue is consistent with previous data,5 showing a correlation between dietary polyunsaturated fatty acids and their adipose tissue concentration. On the other hand, we did not find correlations between trans fatty acid consumption and adipose tissue content. This may be due to the new legislation banning trans fat in Canadian foods, which could have lead to an overestimation of trans fat intake with our food frequency questionnaire. Two studies by Garaulet et al.10, 17 examined adipose tissue and dietary fatty acid content. In the first study, 18:1n-9, 16:0 and 18:2n-6 were the most abundant adipose tissue fatty acids, matching the results of our study. The same group also found that SFAs were more abundant and MUFAs less abundant in visceral adipose tissue. We observed opposite results for SFAs and a trend for MUFAs. In the second study,17 a higher 16:1 and 18:1 content in visceral and SC adipose tissue, respectively, was reported in overweight subjects compared with obese and morbidly obese patients. We did not reproduce these findings. Discrepancies could be related to population differences such as the degree of obesity, sex or dietary patterns.
We examined two subgroups of 10 women with either small or large OM adipocytes that were matched for age, total BFM and SC adipocyte size. No difference was observed in the dietary profile of these subgroups. Adipose tissue fatty acid composition was also similar, except for 18:0 content, elongation and desaturation index, which were, respectively, lower and higher in women with large OM adipocytes. Results obtained with desaturation (18:1/18:0) and elongation (18:0/16:0) reflected those obtained with 18:0 alone, as 18:1, 16:0 and 16:1 contents were not related to any measure performed. Studies performed in human adipocytes suggest that elongation and desaturation processes are coordinately regulated to produce oleate.18, 31 Moreover, the abundance of SFAs is harmful to normal cell function32 and higher desaturation rates, and SCD1 expression was shown to protect against such lipotoxicity.33 This could possibly be the role of fatty acid desaturation and elongation in adipose tissue of women with abdominal obesity and large OM adipocytes. The absence of appreciable variation of 18:1 content may come from the fact that oleate is by far the most abundant fatty acid present in adipose tissue (Figure 1). Recent studies in mice and humans indicate that the content of 16:0 and 16:1 is associated with insulin resistance.34, 35, 36 Subjects in our cohorts were healthy and did not suffer from pronounced metabolic disorders, including diabetes. However, the fact that adiposity is related to the substrate rather than the product of the SCD1 reaction needs to be further investigated.
The 18:0 content of SC fat has been related to total adiposity.4 When examining the measures of SC or overall obesity, such as BMI, fat mass, SC adipose tissue area and SC adipocyte size, we found an inverse correlation between these measures and 18:0 content in the SC depot. Therefore, variables representing the SC depot are correlated to the size of that same compartment. This is not in contradiction with our findings in the matched design, where SC differences were not apparent. When examining OM 18:0 content in a matched design so that differences in visceral adipose tissue are emphasized, lower OM 18:0 content is observed in women with visceral obesity and large OM adipocytes. However, when focusing on the SC 18:0 content in relation to overall obesity, a correlation is also observed between BFM and 18:0 content in SC adipose tissue.
SCD1 is the enzyme that introduces a single double bound between carbons 9 and 10 of the 18:0 fatty acid, converting it to oleate.37 In our study, we found positive correlations between SC desaturation (18:1/18:0), an indirect measure of SCD1 activity, and BMI as well as SC adipose tissue area. When focusing on the visceral depot, we found a positive correlation between the desaturation index and visceral adipose tissue area. In the SC depot, a positive correlation between the levels of SCD1 mRNA/protein expression and total BFM (Figure 2) was observed. A similar correlation was also found with BMI (data not shown). These results are consistent with a recent study from Garcia-Serrano et al.38 showing that the variable mainly associated with SCD1 protein level in SC adipose tissue was BMI.
The activation of ERK1/2 is an early event of adipocyte differentiation.21, 39 Consistent with the study of Laviola et al.,40 we observed higher ERK1/2 expression and phosphorylation in OM vs SC adipose tissue of non-obese women. In obese patients, the ratio OM/SC was diminished, which suggests that the capacity for adipocyte differentiation may be impaired in OM adipose tissue of obese women. Accordingly, a depot-specific difference occurs in obese subjects showing lower SCD1 gene expression in OM vs SC adipose tissue. Our results are consistent with a recent study showing higher SCD1 expression in SC compared with OM fat in obese women. SCD1 expression was also associated with DGAT2 expression, the rate-limiting enzyme in TG synthesis.41 SCD1 expression is mainly regulated by SREBP-1c at the transcriptional level in response to insulin by a PI3-kinase-dependent signaling pathway.42, 43 A similar depot-specific difference in non-diabetic obese subjects was already observed for SREBP-1c.44 We and others also observed a depot-specific difference regarding the level of IR (Figure 3) as well as the phosphorylation state of insulin sensitive pathways, such as ERK1/2 and PI3-kinase (Figure 3).40, 45 Indeed, in obese subjects, insulin was shown to have a more pronounced effect in activating its associated signaling pathways, such as PI3-kinase in the SC adipose tissue.45 Additional analyses in our cohort revealed that SCD1 mRNA and protein levels are highly and significantly associated in the SC depot (r=0.85, P<0.01), whereas this correlation was absent in the OM depot. This result indirectly suggests the predominance of transcriptional regulation of SCD1 in the SC depot. In the OM depot, an alternative post-transcriptional mechanism might take place. To date, only polyunsaturated fatty acids have been shown to impair SCD1 mRNA stability in adipocytes.46 This may account for the depot-specific difference observed in our study.
Taken together, and in agreement with other studies,15, 16, 47 our observations suggest that enhanced fat storage in the insulin-sensitive SC depot is associated with increased SCD1 transcription and activity. We speculate that this may be associated with the insulin sensitivity levels in our patients, who have relatively minor metabolic alterations.
Limitations of the study should be taken into consideration. The cross-sectional nature of the design prevents us to establish cause-and-effect relationships. The use of a food frequency questionnaire can also be seen as a limitation. However, as the fatty acid intake was generally well represented in adipose tissue, we suggest that the questionnaire was representative, at least for lipid composition. Further studies are needed with a larger pool of subjects as well as male participants.
In conclusion, our study demonstrates that in women, the presence of large adipocytes and increased adipose mass in a given fat compartment is related to reduced 18:0 content and increased fatty acid desaturation. This is also associated with an altered fat depot distribution in SCD1 and IR expression, as well as ERK1/2 expression and activation. These alterations are independent of dietary fat intake and suggest that SCD1-mediated desaturation in SC adipose tissue may exert a protective effect from metabolic disorders.
Acknowledgments
We acknowledge the contribution of all surgeons, nurses and radiology technicians to the study. We thank Line Berthiaume for fatty acid-composition measurements. We also thank all the participants. The study was funded by the Canadian Institutes of Health research –Institute of Gender and Health to AT (MOP-64182) and a Quebec Heart and Stroke foundation grant to CM. AT is the recipient of a senior scholarship from Fonds de la recherche en santé du Québec. MCJ is the recipient of a FRSQ studentship.
The authors declare no conflict of interest.
References
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 2006;30:899–905. doi: 10.1038/sj.ijo.0803219. [DOI] [PubMed] [Google Scholar]
- Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, et al. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Plakke T, Berkel J, Beynen AC, Hermus RJ, Katan MB. Relationship between the fatty acid composition of the diet and that of the subcutaneous adipose tissue in individual human subjects. Hum Nutr Appl Nutr. 1983;37:365–372. [PubMed] [Google Scholar]
- van Staveren WA, Deurenberg P, Katan MB, Burema J, De Groot LC, Hoffmans MD. Validity of the fatty acid composition of subcutaneous fat tissue microbiopsies as an estimate of the long-term average fatty acid composition of the diet of separate individuals. Am J Epidemiol. 1986;123:455–463. doi: 10.1093/oxfordjournals.aje.a114260. [DOI] [PubMed] [Google Scholar]
- Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr. 2002;76:750–757. doi: 10.1093/ajcn/76.4.750. [DOI] [PubMed] [Google Scholar]
- Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Perez-Llamas F, Perez-Ayala M, Martinez P, de Medina FS, Tebar FJ, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74:585–591. doi: 10.1093/ajcn/74.5.585. [DOI] [PubMed] [Google Scholar]
- Knutsen SF, Fraser GE, Beeson WL, Lindsted KD, Shavlik DJ. Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24-hour recall and food frequency questionnaire in Black and White Adventists: the Adventist Health Study. Ann Epidemiol. 2003;13:119–127. doi: 10.1016/s1047-2797(02)00260-0. [DOI] [PubMed] [Google Scholar]
- Lopes C, Aro A, Azevedo A, Ramos E, Barros H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J Am Diet Assoc. 2007;107:276–286. doi: 10.1016/j.jada.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Popp-Snijders C, Blonk MC. Omega-3 fatty acids in adipose tissue of obese patients with non-insulin-dependent diabetes mellitus reflect long-term dietary intake of eicosapentaenoic and docosahexaenoic acid. Am J Clin Nutr. 1995;61:360–365. doi: 10.1093/ajcn/61.2.360. [DOI] [PubMed] [Google Scholar]
- Malcom GT, Bhattacharyya AK, Velez-Duran M, Guzman MA, Oalmann MC, Strong JP. Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. Am J Clin Nutr. 1989;50:288–291. doi: 10.1093/ajcn/50.2.288. [DOI] [PubMed] [Google Scholar]
- Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- Sampath H, Ntambi JM. The fate and intermediary metabolism of stearic acid. Lipids. 2005;40:1187–1191. doi: 10.1007/s11745-005-1484-z. [DOI] [PubMed] [Google Scholar]
- Garaulet M, Hernandez-Morante JJ, Tebar FJ, Zamora S. Relation between degree of obesity and site-specific adipose tissue fatty acid composition in a Mediterranean population. Nutrition. 2011;27:170–176. doi: 10.1016/j.nut.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Collins JM, Neville MJ, Hoppa MB, Frayn KN. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem. 2010;285:6044–6052. doi: 10.1074/jbc.M109.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- Mauvoisin D, Mounier C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie. 2011;93:78–86. doi: 10.1016/j.biochi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- Hu J, Roy SK, Shapiro PS, Rodig SR, Reddy SP, Platanias LC, et al. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J Biol Chem. 2001;276:287–297. doi: 10.1074/jbc.M004885200. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, et al. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet J, Nadeau G, Lapointe A, Lamarche B, Lemieux S. Validity and reproducibility of an interviewer-administered food frequency questionnaire for healthy French-Canadian men and women. Nutr J. 2004;3:13. doi: 10.1186/1475-2891-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes D, Couture P, Dupont P, Tchernof A. Subdivision of the subcutaneous adipose tissue compartment and lipid-lipoprotein levels in women. Obes Res. 2003;11:469–476. doi: 10.1038/oby.2003.64. [DOI] [PubMed] [Google Scholar]
- Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982;23:1206–1223. [PubMed] [Google Scholar]
- Tchernof A, Belanger C, Morisset AS, Richard C, Mailloux J, Laberge P, et al. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55:1353–1360. doi: 10.2337/db05-1439. [DOI] [PubMed] [Google Scholar]
- Counil E, Julien P, Lamarche B, Chateau-Degat ML, Ferland A, Dewailly E. Association between trans-fatty acids in erythrocytes and pro-atherogenic lipid profiles among Canadian Inuit of Nunavik: possible influences of sex and age. Br J Nutr. 2009;102:766–776. doi: 10.1017/S0007114509297182. [DOI] [PubMed] [Google Scholar]
- Blouin K, Nadeau M, Mailloux J, Daris M, Lebel S, Luu-The V, et al. Pathways of adipose tissue androgen metabolism in women: depot differences and modulation by adipogenesis. Am J Physiol Endocrinol Metab. 2009;296:E244–E255. doi: 10.1152/ajpendo.00039.2008. [DOI] [PubMed] [Google Scholar]
- Mauvoisin D, Prevost M, Ducheix S, Arnaud MP, Mounier C. Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the Stearoyl-CoA Desaturase (SCD1) gene expression in response to leptin. Mol Cell Endocrinol. 2010;319:116–128. doi: 10.1016/j.mce.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Collins JM, Neville MJ, Pinnick KE, Hodson L, Ruyter B, van Dijk TH, et al. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. J Lipid Res. 2011;52:1683–1692. doi: 10.1194/jlr.M012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, et al. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes. 2005;54:2917–2924. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- Iggman D, Arnlov J, Vessby B, Cederholm T, Sjogren P, Riserus U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia. 2010;53:850–857. doi: 10.1007/s00125-010-1669-0. [DOI] [PubMed] [Google Scholar]
- Stefan N, Kantartzis K, Celebi N, Staiger H, Machann J, Schick F, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. 2010;33:405–407. doi: 10.2337/dc09-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Dobrzyn A. Regulation of stearoyl-CoA desaturase expression. Lipids. 2004;39:1061–1065. doi: 10.1007/s11745-004-1331-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Serrano S, Moreno-Santos I, Garrido-Sanchez L, Gutierrez-Repiso C, Garcia-Almeida JM, Garcia-Arnes J, et al. Stearoyl-CoA desaturase-1 is associated with insulin resistance in morbidly obese subjects. Mol Med. 2011;17:273–280. doi: 10.2119/molmed.2010.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Laviola L, Perrini S, Cignarelli A, Natalicchio A, Leonardini A, De Stefano F, et al. Insulin signaling in human visceral and subcutaneous adipose tissue in vivo. Diabetes. 2006;55:952–961. doi: 10.2337/diabetes.55.04.06.db05-1414. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Cui W, Lu H, Simard S, Cianflone K. Association of adipocyte genes with ASP expression: a microarray analysis of subcutaneous and omental adipose tissue in morbidly obese subjects. BMC Med Genomics. 2010;3:3. doi: 10.1186/1755-8794-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor DE, Kim JB, Spiegelman BM, Edwards PA. Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J Biol Chem. 1999;274:20603–20610. doi: 10.1074/jbc.274.29.20603. [DOI] [PubMed] [Google Scholar]
- Mauvoisin D, Rocque G, Arfa O, Radenne A, Boissier P, Mounier C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J Cell Commun Signal. 2007;1:113–125. doi: 10.1007/s12079-007-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolehmainen M, Vidal H, Alhava E, Uusitupa MI. Sterol regulatory element binding protein 1c (SREBP-1c) expression in human obesity. Obes Res. 2001;9:706–712. doi: 10.1038/oby.2001.95. [DOI] [PubMed] [Google Scholar]
- Zierath JR, Livingston JN, Thorne A, Bolinder J, Reynisdottir S, Lonnqvist F, et al. Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-1 pathway. Diabetologia. 1998;41:1343–1354. doi: 10.1007/s001250051075. [DOI] [PubMed] [Google Scholar]
- Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J Lipid Res. 2006;47:1928–1939. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]