SUMMARY
The KEAP1/NRF2/ARE pathway and the heat shock response are inducible cytoprotective systems regulated by transcription factors NRF2 and HSF1, respectively. We report that structurally distinct small molecule NRF2 activators, all of which react with sulfhydryl groups but differ in potency by 15,000-fold, upregulate Hsp70, a prototypic HSF1-dependent gene. Hsp70 upregulation requires HSF1 but is NRF2 independent. We further demonstrate that a sulfoxythiocarbamate inducer conjugates to the negative regulator of HSF1, Hsp90. The differential concentration dependence of the two responses suggests that activation of NRF2 precedes that of HSF1: the KEAP1/NRF2/ARE pathway is at the fore-front of cellular defense, protecting against instant danger; the heat shock response closely follows to resolve subsequent potentially devastating damage, saving the proteome. This uncovered duality undoubtedly contributes to the cytoprotective effects of such molecules in models of carcinogenesis, cardiovascular disease, and neurodegeneration.
INTRODUCTION
Eukaryotic organisms have evolved highly efficient protective mechanisms that enable their survival under conditions of stress. Most prominent are the heat shock response and the KEAP1/NRF2/ARE pathway, known as “the electrophile counterattack response.” There is a growing interest in the discovery and development of small molecule inducers of these systems for prevention and treatment of chronic degenerative disease (Talalay, 2000; Westerheide and Morimoto, 2005). Induction leads to upregulation of large networks of cytoprotective proteins that detect, prevent, and counteract the consequences of thermal, oxidative, and electrophilic stress, and promote survival. Furthermore, both responses are involved in multiple interactions that link homeostatic metabolism with stress biology, and they ultimately play crucial roles in determining health and life span (Akerfelt et al., 2010; Wakabayashi et al., 2010).
The heat shock response and the KEAP1/NRF2/ARE pathway are controlled by two central regulators, heat shock factor 1 (HSF1) and nuclear factor-erythroid 2-related factor 2 (NRF2), respectively. Under homeostatic conditions, HSF1 is an inactive monomer bound to heat shock protein 90 (Hsp90). Following stimulation, HSF1 dissociates from the Hsp90 complex, trimerizes, and binds to heat shock elements (HSEs) of its target genes, thereby driving their expression (Westerheide and Morimoto, 2005). In addition a number of posttranslational modifications, such as phosphorylation, acetylation, and sumoylation, are involved in regulating the transcriptional activity of HSF1, and there is also negative feedback regulation by heat shock proteins (Akerfelt et al., 2010). NRF2, under basal conditions, is targeted for ubiquitination and proteasomal degradation by its repressor KEAP1 (Kelch-like ECH-associated protein 1). Inducers, all of which possess a single common chemical property, i.e., reactivity with sulfhydryl groups (Talalay et al., 1988), chemically react with KEAP1, rendering it unable to repress NRF2, permitting the nuclear translocation of the transcription factor that binds to antioxidant response elements (AREs) as a heterodimer with a small Maf protein, driving expression of target genes (Motohashi and Yamamoto, 2004).
Activation of NRF2 counteracts oxidative and electrophilic stress; activation of HSF1 prevents protein misfolding. Due to the versatility of their target genes, the combined action of these transcription factors allows for maintenance of normal cellular functions under various stress conditions. Conversely, their suppression results in accelerated pathogenesis in numerous models of disease. Therefore, it is rather curious that in addition to thermal stress, environmental redox changes and electrophiles also induce heat shock genes. Thus, murine HSF1 is activated by H2O2 in a manner dependent on C35 and C105 (Ahn and Thiele, 2003). Similarly, an intermolecular disulfide bond between C36 and C103 within human HSF1 causes trimerization and DNA binding, whereas an intramolecular disulfide bridge (in which C153, C373, and C378 participate) is inhibitory (Lu et al., 2008). The electrophilic nitro-oleic acid (but not the nonelectrophilic oleic acid), the cyclopentenone (but not the cyclopentanone) prostaglandins, the triterpenoid celastrol, the isothiocyanate sulforaphane, and dithiolethione, all of which possess cysteine reactivity, are activators of both HSF1- and NRF2-dependent genes (Kwak et al., 2004; Westerheide and Morimoto, 2005; Trott et al., 2008; Kansanen et al., 2009; Gan et al., 2010). Therefore, we hypothesized that a common signal that is sensed through cysteine modification(s) within KEAP1 and HSF1, or a negative regulator of HSF1, serves as a trigger for both cytoprotective pathways. To test this hypothesis, we asked: (1) Do NRF2 activators that differ in their chemical structures, but share reactivity with sulfhydryl groups, also induce Hsp70, a prototypic HSF1-target gene?; and (2) Is the ability to upregulate Hsp70 mediated by transcription factors HSF1, NRF2, or both?
RESULTS AND DISCUSSION
Celastrol, an Electrophilic Quinone Methide, Induces NRF2- and HSF1-Dependent Genes
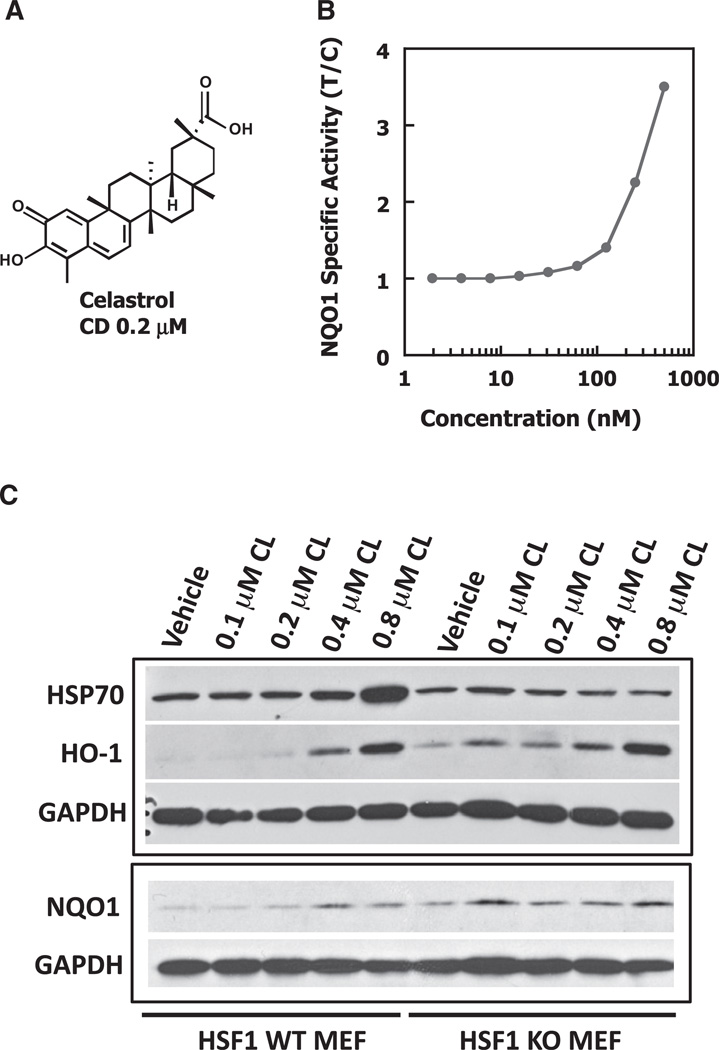
Using a quantitative bioassay (Prochaska and Santamaria, 1988), we identified among a large series of synthetic pentacyclic triterpenoids and tricyclic bis(cyanoenone)s the most potent (active at sub- to low-nanomolar concentrations) NRF2 activators known to date (Dinkova-Kostova et al., 2005, 2010). Structure-activity studies established the essential requirement for electrophilic Michael acceptor group(s) for inducer activity. Based on this finding, we predicted that celastrol, a naturally occurring triterpenoid that has an electrophilic quinone methide functionality (Figure 1A), will be an inducer. Indeed, we found a potent dose-dependent induction of NAD(P)H:quinone oxidoreductase 1 (NQO1), a prototypic NRF2-dependent enzyme, by celastrol with a CD (concentration that doubles the specific activity of the enzyme) of 0.2 µM (Figure 1B). Notably, celastrol is a classical HSF1 activator and inducer of the heat shock response (Westerheide and Morimoto, 2005; Trott et al., 2008). In close agreement we found a coordinate dose-dependent upregulation of Hsp70 and heme oxygenase 1 (HO-1), also known as Hsp32, when mouse embryonic fibroblasts (MEFs) were exposed to celastrol (Figure 1C).
Figure 1. Induction of NQO1, HO-1, and Hsp70 by Celastrol.
(A) Chemical structure of celastrol.
(B) Hepa 1c1c7 cells (104 per well) in 96-well plates were exposed to serial dilutions of the quinone methide triterpenoid celastrol. NQO1 activity was measured 48 hr later in cell lysates. Results are mean values (n = 8). The SD in each case was <10% of the value. CD, Concentration that doubles the specific activity of NQO1.
(C) Wild-type (WT) or HSF1-knockout (KO) MEFs (2 × 105 per well) in 6-well plates were exposed to vehicle (0.1% DMSO) or increasing concentrations of celastrol (CL) for 4 hr. Cells were lysed in RIPA buffer 20 hr later, aliquots from cell lysates were resolved by SDS/PAGE, transferred to immobilon-P, and probed with antibodies against Hsp70 (StressMarq Biosciences Inc.; 1:1000 dilution), HO-1 (Enzo Life Sciences; 1:1000 dilution), and NQO1 (1:3000 dilution; a kind gift from John D. Hayes, University of Dundee). The antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Sigma-Aldrich; 1:5000 dilution) was used as a loading control. The data are representative of two independent experiments.
To investigate the role of HSF1 in the mechanism of induction of Hsp70 and HO-1, we used MEFs isolated from HSF1-knockout mice (Xiao et al., 1999). In contrast to their wild-type counterparts, no induction of Hsp70 was observed in HSF1-deficient cells, demonstrating the essential requirement for HSF1 for upregulation of this heat shock protein (Figure 1C). Curiously, the basal levels of HO-1 and NQO1 were higher in HSF1-knockout MEFs. However, unlike Hsp70, HO-1 and NQO1 were upregulated independently of HSF1, implying alternative complementary mechanism(s) in their induction by celastrol.
HSF1-Mediated Upregulation of Hsp70 Is a Common Property of Inducers of the KEAP1/NRF2/ARE Pathway
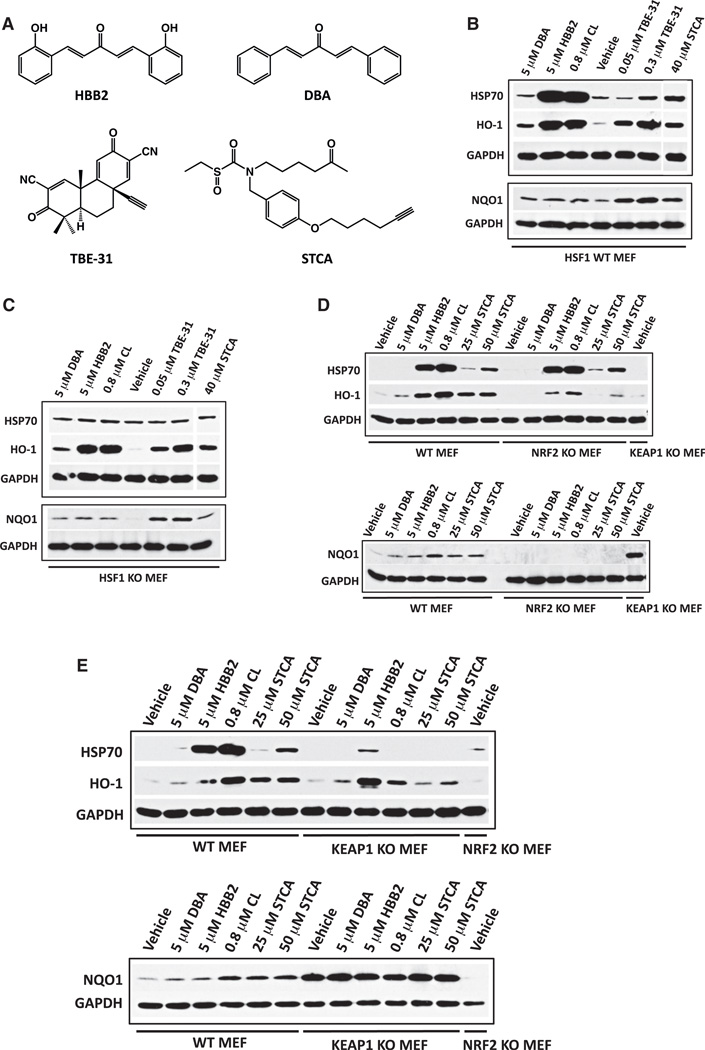
Because NQO1 gene expression is mediated by NRF2, whereas expression of Hsp70 is controlled by HSF1, it appears that celastrol activates both transcription factors. To test whether upregulation of the heat shock response might be a common property of activators of the KEAP1/NRF2/ARE pathway, we exposed MEFs to three structurally distinct compounds, i.e., the double Michael acceptor bis(2-hydroxybenzylidene)acetone (HBB2), the tricyclic bis(cyanoenone)-31 (TBE-31), and the sulfoxythiocarbamate-alkyne STCA (Figure 2A). In addition we examined the effects of the nonhydroxylated analog of HBB2, bis(benzylidene)acetone (DBA), which, although structurally similar, is 100-fold less potent as an NQO1 inducer than HBB2 in Hepa1c1c7 cells (Dinkova-Kostova et al., 2001). Of note, because of the lower sensitivity and semiquantitative nature of the western blot in comparison with the highly sensitive and quantitative enzyme assay, in the experiments described in this paper, we used saturating concentrations of these inducers with respect to upregulation of NQO1. Our choice of compounds was determined by the following considerations: (1) they have distinct chemical structures; (2) they react with sulfhydryl groups; and (3) they differ enormously (by 15,000-fold) in inducer potency: TBE-31 (CD = 0.9 nM) being the most potent, and DBA (CD = 15 µM) being the least potent. HBB2, TBE-31, and STCA induced Hsp70, HO-1, and NQO1 (Figure 2B). Importantly, as was found for celastrol, induction of Hsp70, but not of HO-1 or NQO1, by all compounds examined was completely abolished in HSF1-knockout cells, supporting the essential role played by HSF1 in upregulation of Hsp70 (Figure 2C).
Figure 2. Induction of Hsp70 by Inducers of the KEAP1/NRF2/ARE Pathway Depends on HSF1 but Is Independent of NRF2.
(A) Chemical structures of DBA, HBB2, TBE-31, and STCA. Wild-type (B, D, and E), HSF1-knockout (C), NRF2-knockout (D), or KEAP1-knockout (E) MEFs (2 × 105 per well) in 6-well plates were exposed to vehicle (0.1% acetonitrile), DBA, HBB2, celastrol (CL), TBE-31, or STCA for 4 hr. The levels of Hsp70, HO-1, and NQO1 were evaluated by western blot 20 hr later. Of note, the STCA-treated sample in (B) and (C) was run on the same gel, but the lane between “TBE-31” and “STCA” was pulled out. The data are representative of three independent experiments.
HBB2, TBE-31, and STCA react readily with cysteine residues of KEAP1, leading to activation of NRF2 and induction of its downstream target genes, including NQO1 (Liby et al., 2008; Dinkova-Kostova et al., 2002; 2010; Ahn et al., 2010). Furthermore, there is a correlation between reactivity with sulfhydryl groups and NQO1-inducer potency (Talalay et al., 1988; Dinkova-Kostova et al., 2001). In the experiments described in this report, we were guided by our prior knowledge of the reactivity of each compound with sulfhydryl groups when selecting the dose to use. Thus, despite their structural similarity, HBB2 and DBA differ greatly in their reactivity with sulfhydryl groups: the second-order rate constant for the reaction with glutathione is ~30-fold higher for HBB2 than for DBA, correlating with the 100-fold greater NQO1-inducer potency of HBB2 (Dinkova-Kostova et al., 2001). The profound difference in the ability of this pair of compounds to induce Hsp70 and the essential requirement for HSF1 strongly suggest that, similar to activation of NRF2, cysteine reactivity is also critical for activation of HSF1.
Upregulation of Hsp70 by Inducers of the KEAP1/NRF2/ARE Pathway Is Independent of NRF2
The Keap1/Nrf2/ARE pathway is a validated target for HBB2, DBA, and STCA. Induction of NQO1 by these compounds is dependent on NRF2 and does not occur in cells that are deficient for this transcription factor (Liu et al., 2008; Ahn et al., 2010). Therefore, we investigated its potential contribution to induction of Hsp70 and HO-1 by comparing MEFs isolated from NRF2-knockout mice or their wild-type counterparts. Induction of Hsp70 was identical in both cell types upon exposure to HBB2, celastrol, or STCA, establishing that NRF2 is dispensable for upregulation of Hsp70 (Figure 2D). Importantly, no Hsp70 induction was observed when cells of either genotype were treated with DBA. In comparison to wild-type MEFs, HO-1, but not NQO1, induction by HBB2, celastrol, and STCA still occurred in NRF2-knockout cells, although to a lower degree (Figure 2D), and was not evident in NRF2-knockout cells exposed to DBA. The results show that: (1) induction of NQO1 by these compounds is fully dependent on NRF2, (2) NRF2 is partially responsible for the upregulation of HO-1, and (3) induction of Hsp70 is clearly independent of this transcription factor.
MEFs that are deficient in KEAP1, the negative regulator of NRF2, constitutively express high levels of NRF2 target genes and are resistant to the toxicities of xenobiotics (Kwak et al., 2004). We next compared the expression levels of Hsp70 and HO-1 in KEAP1-knockout MEFs to those in their wild-type counterparts following exposure to DBA, HBB2, celastrol, or STCA. In sharp contrast with wild-type cells, induction of Hsp70 in KEAP1-knockout cells was observed only by HBB2, although even in this case it was quite diminished (Figure 2E). In the absence of KEAP1, the levels of NQO1 were constitutively upregulated and not affected by inducers. In contrast the basal levels of HO-1 were only slightly higher in KEAP1-knockout cells, supporting the conclusion that, in this cell type, transcription factor NRF2 is not the major contributor to the gene expression of this cytoprotective protein. Induction of HO-1 was also reduced under conditions of constitutive NRF2 upregulation, with the same exception, induction by HBB2. The results suggest that the bioavailability of these compounds for their protein targets is lower in KEAP1-knockout MEFs, most likely due to more efficient metabolism and excretion. Indeed, cells and animals that lack KEAP1 overexpress a plethora of drug-metabolizing enzymes and efflux pumps (Kwak et al., 2004; Yates et al., 2009).
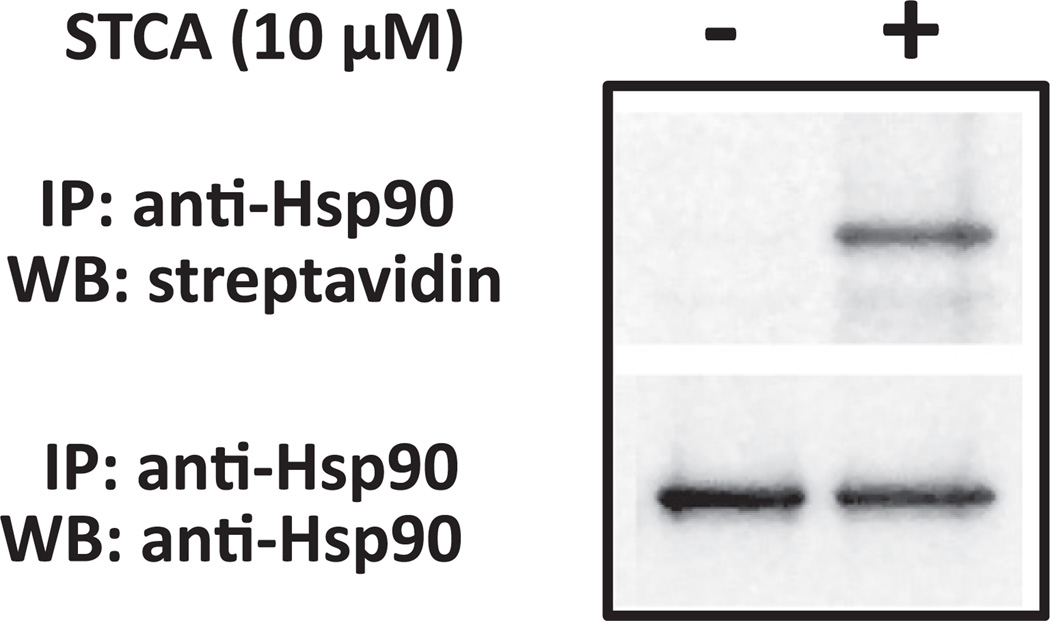
STCA Covalently Binds to Hsp90
Our results indicate that reactivity with cysteine(s) plays a critical role in the mechanism of HSF1 activation. Therefore, it is tempting to speculate that, similar to the KEAP1/NRF2/ARE pathway, a common signal that is sensed through cysteine modification(s) within HSF1, or a negative regulator of HSF1, triggers the heat shock response. Notably, induction of NRF2-dependent genes occurs at lower inducer concentrations than those that cause upregulation of Hsp70. Thus, the concentration that doubles (CD value) the activity of NQO1 for STCA, celastrol, HBB2, and TBE-31 is 5.5, 0.2, 0.15, and 0.0009 µM, respectively (Ahn et al., 2010; Dinkova-Kostova et al., 2001, 2010). Although it is not possible to compare directly a highly quantitative enzymatic assay with semiquantitative western blot analysis, induction of HO-1, as judged by our western blots, requires concentrations that are similar to those that induce NQO1. In contrast, upregulation of Hsp70 becomes apparent when cells are exposed to 25 µM STCA, 0.8 µM celastrol, 1 µM HBB2, and 0.05 µM TBE-31. One reason could be a difference in the abundance of the protein targets that lead to activation of NRF2 and HSF1, respectively. KEAP1, the negative regulator of NRF2, is a very low abundant protein. In contrast, Hsp90, a negative regulator of HSF1, is one of the most abundant cellular proteins. Curiously, by use of a proteomics approach, we previously identified Hsp90 among the proteins that were modified when HEK293 cells were exposed to STCA (Ahn et al., 2010). In this study we validated this finding by immunoprecipitation-western blot analysis, taking advantage of the stability of the STCA-cysteine conjugate and its alkyne moiety that allows use of click chemistry. Thus, Hsp90 was immunoprecipitated from lysates of HEK293 cells treated with STCA or vehicle, and subjected to click reaction with biotin azide. Immunoblotting with streptavidin or Hsp90 antibody revealed a clear band migrating identically to Hsp90 that was present only in lysates from STCA-treated cells (Figure 3), implying that Hsp90 was covalently modified by STCA.
Figure 3. STCA Conjugates on Hsp90.
HEK293 cells were grown and exposed to 10 µM STCA or vehicle for 2.5 hr as described (Ahn et al., 2010). Hsp90 was immunoprecipitated from cell lysates and subjected to click reaction with biotin azide on beads. Eluted samples were immunoblotted with streptavidin or Hsp90 antibody (BD Biosciences; 1:1000 dilution). The data are representative of two independent experiments.
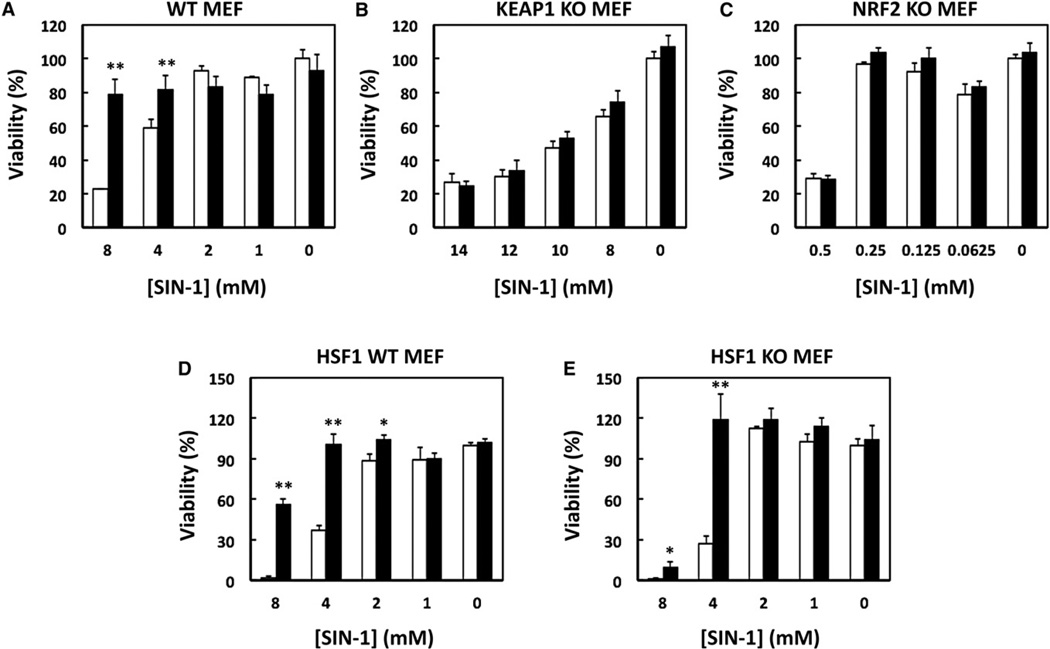
Exposure to TBE-31, a Dual Inducer of the KEAP1/NRF2/ARE Pathway and Hsp70, Protects against Peroxynitrite-Induced Cytotoxicity
The generation of peroxynitrite from the reaction of nitric oxide with superoxide is critically involved in the pathogenesis of cardiovascular disease, diabetes, chronic inflammatory diseases, cancer, and neurodegenerative disorders, and the development of pharmacological agents that reduce the toxicity of peroxynitrite is actively being pursued (Pacher et al., 2007). Therefore, we examined the ability of TBE-31, the most potent inducer in this series, to protect against the cytotoxicity of the peroxynitrite donor, 3-morpholinosydnonimine (SIN-1). Exposure to SIN-1 caused a dose-dependent loss of cell viability. Thus, in wild-type MEFs, treatment with 8 mM SIN-1 resulted in ~80% cell death (Figure 4A, white bars). Pretreatment with 50 nM TBE-31 24 hr prior to SIN-1 exposure was highly protective, and ~80% of the cells were viable (Figure 4A, black bars). Compared to wild-type, KEAP1-knockout MEFs were much more resistant to SIN-1 cytotoxicity, and 65% of the cells were still viable at 8 mM SIN-1 (Figure 4B, white bars), whereas NRF2-knockout MEFs were very sensitive with 70% cell death occurring at 0.5 mM SIN-1 (Figure 4C, white bars). Importantly, the protective effect of TBE-31 was essentially abolished in the absence of either KEAP1 (Figure 4B, black bars) or NRF2 (Figure 4C, black bars), suggesting that protection was primarily due to NRF2 upregulation. In close agreement, although HSF1-knockout MEFs (Figure 4E, white bars) were more sensitive to SIN-1 than their wild-type counterparts (Figure 4D, white bars), TBE-31 was generally protective in both cell types (Figures 4D and 4E, black bars). However, at 8 mM SIN-1, protection by TBE-31 was compromised in the HSF1-knockout cells, implying that at high concentrations (at which cell damage is greatest), the HSF1 pathway also plays a role in the protective mechanisms.
Figure 4. TBE-31 Protects against Peroxynitrite-Induced Cytotoxicity.
Wild-type (A) (1.3 × 104 per well), KEAP1-knockout (B) (0.5 × 104 per well), NRF2-knockout (C) (1.5 × 104 per well), HSF1-wild-type (D) (0.5 × 104 per well), or HSF1-knockout (E) (1.3 × 104 per well) MEFs were grown on 96-well plates overnight. Cells were then treated with either vehicle (0.1% acetonitrile, white bars) or 50 nM TBE-31 (black bars) in complete medium for 4 hr, incubated for a further 20 hr in the absence of TBE-31, and finally exposed to serial dilutions of SIN-1 in Hank’s buffered saline solution for 2 hr. Cell viability was assessed by the MTT assay following incubation in complete medium 22 hr later. Results are mean ± SD (n = 4) and representative of two to three independent experiments. The asterisks indicate statistically significant differences (Student’s t test) from vehicle-treated control; *p < 0.01; **p < 0.001.
Conclusions
Our results firmly establish that structurally diverse inducers of the KEAP1/NRF2/ARE pathway, all of which react with sulfhydryl groups, upregulate Hsp70 in an HSF1-dependent, but NRF2-independent, manner. Activation of HSF1 occurs at much higher inducer concentrations than those that activate NRF2. This observation suggests that activation of NRF2 precedes that of HSF1, and further supports the notion that the KEAP1/NRF2/ARE pathway is at the forefront of cellular defense and functions to remove and repair the consequences of instant danger. The heat shock response then follows to resolve a potentially devastating damage, save the proteome, and ensure survival.
SIGNIFICANCE
The heat shock response and the KEAP1/NRF2/ARE pathway provide the cell with carefully orchestrated broad defense mechanisms by regulating the expression of networks of several hundred genes that encode proteins with versatile cytoprotective functions. Deficiencies in these systems are associated with accelerated pathogenesis of many chronic degenerative diseases and aging. The identification of potent small molecule inducers of these pathways is being actively pursued as a strategy to prevent or delay disease onset and to extend healthy life span. Indeed, such compounds protect against the otherwise detrimental consequences of the toxic, neoplastic, and proinflammatory effects of a wide array of xenobiotics and endogenous substances in numerous experimental models of carcinogenesis, cardiovascular disease, and neurodegeneration. By use of a chemical approach, we found that small molecule inducers of the KEAP1/NRF2/ARE pathway that are structurally distinct, but commonly react with sulfhydryl groups, also share the ability to upregulate Hsp70 and, thus, have identified a previously unknown target for these molecules. Next, by taking a genetic approach and using cells that are deficient for transcription factors HSF1 or NRF2, we have obtained mechanistic understanding in that HSF1 is essential for induction of Hsp70, whereas NRF2 is dispensable. In addition our results provide a possible explanation for the cytoprotective effects that have been attributed to such compounds even in the absence of transcription factor NRF2. Taken together, these findings support the future development of potent “dual“ activators of this type as mechanism-based comprehensive cytoprotective agents.
ACKNOWLEDGMENTS
We acknowledge with gratitude the financial support of Research Councils UK, Cancer Research UK (C20953/A10270), and the NIH (U54 RR020839). We thank Masayuki Yamamoto (Tohoku University) for NRF2- and KEAP1-knockout MEFs and John D. Hayes (University of Dundee) for valuable comments.
REFERENCES
- Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YH, Hwang Y, Liu H, Wang XJ, Zhang Y, Stephenson KK, Boronina TN, Cole RN, Dinkova-Kostova AT, Talalay P, Cole PA. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc. Natl. Acad. Sci. USA. 2010;107:9590–9595. doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Talalay P, Sharkey J, Zhang Y, Holtzclaw WD, Wang XJ, David E, Schiavoni KH, Finlayson S, Mierke DF, Honda T. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J. Biol. Chem. 2010;285:33747–33755. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan N, Wu YC, Brunet M, Garrido C, Chung FL, Dai C, Mi L. Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J. Biol. Chem. 2010;285:35528–35536. doi: 10.1074/jbc.M110.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansanen E, Jyrkkänen HK, Volger OL, Leinonen H, Kivelä AM, Häkkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Ylä-Herttuala S, et al. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Ramos-Gomez M, Wakabayashi N, Kensler TW. Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes. Methods Enzymol. 2004;382:414–423. doi: 10.1016/S0076-6879(04)82022-6. [DOI] [PubMed] [Google Scholar]
- Liby K, Yore MM, Roebuck BD, Baumgartner KJ, Honda T, Sundararajan C, Yoshizawa H, Gribble GW, Williams CR, Risingsong R, et al. A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin. Cancer Res. 2008;68:6727–6733. doi: 10.1158/0008-5472.CAN-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Natl. Acad. Sci. USA. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Kim HE, Li CR, Kim S, Kwak IJ, Lee YJ, Kim SS, Moon JY, Kim CH, Kim DK, et al. Two distinct disulfide bonds formed in human heat shock transcription factor 1 act in opposition to regulate its DNA binding activity. Biochemistry. 2008;47:6007–6015. doi: 10.1021/bi702185u. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska HJ, Santamaria AB. Direct measurement of NAD(P) H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal. Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott A, West JD, Klaić L, Westerheide SD, Silverman RB, Morimoto RI, Morano KA. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol. Biol. Cell. 2008;19:1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J. Biol. Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]