Abstract
Neuregulin 1 (NRG-1) and its receptor ErbB4 have emerged as biologically plausible schizophrenia risk factors, modulators of GABAergic and dopaminergic neurotransmission, and as potent regulators of glutamatergic synaptic plasticity. NRG-1 acutely depotentiates LTP in hippocampal slices, and blocking ErbB kinase activity inhibits LTP reversal by theta-pulse stimuli (TPS), an activity-dependent reversal paradigm. NRG-1/ErbB4 signaling in parvalbumin (PV) interneurons has been implicated in inhibitory transmission onto pyramidal neurons. However, the role of ErbB4, in particular in PV interneurons, for LTP reversal has not been investigated. Here we show that ErbB4-null (ErbB4−/−) and PV interneuron-restricted mutant (PV-Cre;ErbB4) mice, as well as NRG-1 hypomorphic mice, exhibit increased hippocampal LTP. Moreover, both ErbB4−/− and PV-Cre;ErbB4 mice lack TPS-mediated LTP reversal. A comparative behavioral analysis of full and conditional ErbB4 mutant mice revealed that both exhibit hyperactivity in a novel environment and deficits in prepulse inhibition of the startle response. Strikingly, however, only ErbB4−/− mice exhibit reduced anxiety-like behaviors in the elevated plus maze task and deficits in cued and contextual fear conditioning. These results suggest that aberrant NRG-1/ErbB4 signaling in PV interneurons accounts for some but not all behavioral abnormalities observed in ErbB4−/− mice. Consistent with the observation that PV-Cre;ErbB4 mice exhibit normal fear conditioning, we find that ErbB4 is broadly expressed in the amygdala, largely by cells negative for PV. These findings are important to better understand ErbB4's role in complex behaviors and warrant further analysis of ErbB4 mutant mice lacking the receptor in distinct neuron types.
Introduction
Maintaining the balance between excitation and inhibition is important for the homeostatic control of normal brain function and behavior, and imbalances are believed to contribute to the pathology of multiple psychiatric disorders, including schizophrenia (Benes and Berretta, 2001; Coyle, 2006; Kehrer et al., 2008; Lisman et al., 2008). Recent rodent work from several laboratories suggests that the schizophrenia risk genes Neuregulin 1 (NRG-1) and ERBB4 may play an important role in regulating hippocampal and frontal cortical pyramidal neurons (for review, see Mei and Xiong, 2008; Buonanno, 2010), while disrupted NRG-1/ErbB4 signaling perturbs neuronal network activity (Fisahn et al., 2009; Nason et al., 2011), consistent with the notion of schizophrenia representing a disorder of functional connectivity and synaptic plasticity (Stephan et al., 2006).
Acutely administered NRG-1 peptide blocks or reverses LTP at Schaeffer collateral-to-CA1 (SC→CA1) glutamatergic synapses in the hippocampus (Huang et al., 2000; Kwon et al., 2005; Bjarnadottir et al., 2007). Conversely, pharmacological blockade of ErbB receptor activity prevents LTP reversal by theta-pulse stimuli (TPS), which model a naturally occurring neural activity pattern in the hippocampus (Stäubli and Chun, 1996), suggesting that neural activity recruits the NRG/ErbB pathway to antagonize synapse strengthening (Kwon et al., 2005). While ErbB4, the main neuronal NRG receptor, is expressed in several interneuron subtypes in the cerebral cortex, it is not detected in pyramidal neurons (Vullhorst et al., 2009; Neddens and Buonanno, 2010; Neddens et al., 2011), indicating that NRG/ErbB effects on synaptic plasticity are mediated via local circuit mechanisms.
Parvalbumin (PV)-expressing fast-spiking basket cells are especially relevant for the possible involvement of NRG-1/ErbB4 signaling in psychiatric disorders because (1) most PV interneurons in the prefrontal cortex (PFC) and many in the hippocampus coexpress ErbB4 (Fisahn et al., 2009; Fazzari et al., 2010; Neddens and Buonanno, 2010); (2) they are involved in gamma oscillations that are regulated by NRG-1/ErbB4 signaling and abnormal in schizophrenia (Kwon et al., 1999; Wilson et al., 2008; Fisahn et al., 2009); and (3) their number is reduced in the hippocampus of ErbB4 mutant mice (Neddens and Buonanno, 2010), consistent with the reduced number of PV-expressing neurons in the PFC of individuals with schizophrenia (for review, see Lewis et al., 2005). However, while ErbB4 expression in the neocortex and hippocampus appears tightly correlated with interneurons, outside the cerebral cortex, ErbB4 is expressed in other neuron types as well (Steiner et al., 1999; Gerecke et al., 2001; Fox and Kornblum, 2005).
Here we analyzed LTP and LTP reversal at hippocampal SC→CA1 synapses in ErbB4−/−, PV-Cre;ErbB4, and NRG-1 hypomorphic mice. We also compared ErbB4−/− and PV-Cre;ErbB4 mice in a battery of behavioral tests pertinent to schizophrenia and anxiety/fear. Our results unequivocally establish the requirement of ErbB4 signaling in PV interneurons for mediating NRG's effects on synaptic plasticity, and suggest that while ErbB4 activity in PV interneurons is important for behaviors associated with exploratory activity and sensorimotor gating, NRG/ErbB4 effects on other neural populations contribute to the regulation of anxiety and fear-related behaviors.
Materials and Methods
Animals
Full and conditional Erbb4 knock-out alleles have been described previously (Gassmann et al., 1995; Golub et al., 2004). Both ErbB4 alleles target the second coding exon, removal of which destabilizes RNA and leads to the complete loss of detectable ErbB4 protein by Western blotting and immunofluorescence histology (Vullhorst et al., 2009). Full ErbB4 mutant mice were rescued from embryonic lethality by transgenic ErbB4 overexpression in the heart (ErbB4MHC-ErbB4) (Tidcombe et al., 2003). ErbB4MHC-ErbB4 mice were backcrossed to C57BL/6J mice for >15 generations. In our facility, ErbB4lox mice were backcrossed for three generations to C57BL/6J and then maintained by inbreeding. NRG-1 hypomorphic mice harbored a targeted deletion in the EGF domain (NRG1ΔEGF+/−), and were backcrossed to C57BL/6J mice for >15 generations (Erickson et al., 1997). The PV-Cre mouse line Pva1btm1(cre)Arbr, expressing Cre recombinase from the Pva1b locus by insertion of the gene into its 3′ untranslated region, was obtained from The Jackson Laboratory (Hippenmeyer et al., 2005). PV-Cre mice were on a mixed 129×C57BL/6J background, backcrossed in our facility for two generations to C57BL/6J and maintained as inbreds from then on. ErbB4-2A-Cre-ERT2 mice were provided by Dr. Hongkui Zeng (Allen Institute for Brain Science, Seattle, WA), and mice expressing tdTomato from the Rosa26 locus (Ai14) were obtained from The Jackson Laboratory (Madisen et al., 2010). For more details on the generation and expression properties of ErbB4-Cre-ERT2 mice, please go to http://transgenicmouse.alleninstitute.org. Adult ErbB4-2A-CreERT2 × Ai14 mice were injected intraperitoneally for 5 consecutive days with 1 mg of tamoxifen (Sigma). All mice were maintained under a 12/12 h light/dark cycle and received food ad libitum. Animal procedures were reviewed and approved by the NIH Animal Care and Use Committee.
Reagents
The NRG-1β1 peptide (R&D Systems) encompasses the EGF-like domain between amino acids 176–246, which is necessary and sufficient to activate the ErbB signaling pathway (Buonanno and Fischbach, 2001). The NRG-1β1 peptide was used because, in contrast to the entire extracellular domain, it penetrates the tissue and effectively activates ErbB receptors, as previously demonstrated (Kwon et al., 2005). PD158780 was from Calbiochem and was reconstituted in DMSO at a stock concentration of 10 mm. Mouse monoclonal anti-parvalbumin antibody (clone PARV-19) was from Sigma. Rabbit monoclonal antibody mAB10 against ErbB4 has been described previously (Vullhorst et al., 2009). A mouse monoclonal antibody against clathrin heavy chain (clone TD.1) was from Santa Cruz Biotechnology.
Protein extraction and Western blotting
Whole tissue lysates were generated from PV-Cre;ErbB4f/f and ErbB4f/f mice by sonication in 8 m urea/1% SDS. Proteins were reduced with 5 mm tributylphosphine (Sigma) and alkylated with 15 mm iodoacetamide (Sigma). Sixty micrograms of protein were size-fractionated on 4–12% acrylamide/Tris-glycine gels (Invitrogen) and electrophoretically transferred onto nitrocellulose membranes. Excess binding capacities were blocked with 5% nonfat dry milk in Tris-buffered saline (137 mm NaCl, 3 mm KCl, 25 mm Tris-HCl, pH 7.4) containing 0.1% Tween 20 (TBS-T). Membranes were probed sequentially with anti-ErbB4 (mAB10; 1 μg/ml) and mouse monoclonal antibody against clathrin heavy chain (0.03 μg/ml) in 3% bovine serum albumin (BSA) in TBS-T. Antibody binding was visualized with secondary antibodies conjugated to horseradish peroxidase using enhanced chemiluminescence (GE Healthcare).
Electrophysiology
Transverse slices (300–350 μm) from the dorsal hippocampus were transferred to a submerged recording chamber continuously perfused at 2 ml/min (30°C) with ACSF containing the following (in mm): 125 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2, and 11 glucose. Whole-cell voltage-clamp recordings were performed with glass microelectrodes (6–7 MΩ) filled with internal solution (in mm: 130 Cs-methanesulfonate, 10 HEPES, 0.5 EGTA, 4 Mg-ATP, 0.3 Na-GTP, 5 QX-314, 8 NaCl, 10 phosphocreatine, pH 7.2 adjusted with CsOH). Schaffer collateral/commissural fibers were stimulated at 0.05 Hz (0.1 ms, 20–40 μA) using a borosilicate two barrel filled with oxygenated ACSF or concentric bipolar stimulating electrode. Baseline EPSCs were set to 40–50% of maximum responses and recorded for at least 10 min after obtaining a stable baseline. Of note, stimulus intensities were not significantly different between genotypes. For the induction of LTP, a general pairing protocol was used, composed of 100 pulses at 2 Hz and a holding potential of −10–0 mV, as previously described (Kim and Lisman, 1999; Kwon et al., 2008). Slices showing <130% LTP were excluded from analyses. Depotentiation was elicited by TPS (5 Hz, 1 min), as described by Stäubli and Chun (1996). Picrotoxin (100 μm) was included in some experiments. Recorded data were filtered at 3 kHz, sampled at 10 kHz using pClamp, and analyzed with Clampfit (Molecular Devices). Results are presented as mean ± SEM and analyzed for statistical significance using the Wilcoxon signed-rank test.
Immunofluorescence histology
Mice were transcardially perfused with 4% PFA in 0.1 m PBS, pH 7.4. Brains were postfixed overnight in the same fixative and 50 μm sections were cut on a vibratome and saved for up to 3 weeks in 0.1 m PBS with sodium azide at +4°C. Sections were blocked in 20% normal goat serum (NGS), 1% BSA, 0.25% Triton X-100 in 0.1 m PBS for 1 h at room temperature (RT) and incubated with anti-PV primary antibody (1:6000) and/or rabbit monoclonal antibody mAB10 against ErbB4 (1 μg/ml) in 0.1 m PBS with 2% NGS and 0.25% Triton X-100 (dilution buffer) for 24 h at +4°C with gentle rocking. Slices were washed in 0.1 m PBS with 0.25% Triton X-100 for at least 30 min before incubation with goat-anti-mouse Alexa 647 and/or anti-rabbit Alexa 594 secondary antibodies (Invitrogen) for 90 min at RT in dilution buffer. After extensive washes in PBS with 0.25% Triton X-100, sections were mounted on gelatin-coated slides, dried, and mounted in Mowiol-DABCO. Fluorescence was analyzed on a Zeiss 510 Meta confocal microscope (Zeiss Microimaging) at 20× and 40× magnification. Images were adjusted for overall brightness and contrast in Adobe Photoshop (Adobe Systems).
Behavioral testing battery
Cohorts.
Only male mice were used for behavioral testing. Heterozygous ErbB4MHC-ErbB4 breeders were used to establish the full ErbB4 knock-out (ErbB4−/−) cohort and its respective wild-type littermate control group. For the conditionally ablated ErbB4 mutant mice, the PV-Cre driver line Pva1btm1(cre)Arbr was crossed with mice harboring a floxed Erbb4 allele to obtain both PV-Cre;ErbB4f/f and PV-Cre;ErbB4f/− mutant mice. The respective control groups were ErbB4f/f and ErbB4f/− without PV-Cre. Data were not different between ErbB4f/f and ErbB4f/− mice, indicating that knocking out one allele of ErbB4 does not affect any behavioral parameters investigated in this work. Since there was no difference in the behavioral outcomes between the two control groups, the PV-Cre; ErbB4f/f, and PV-Cre;ErbB4f/− cohorts were pooled and referred to as PV-Cre;ErbB4. Cohort size range was between 7 and 17, depending on test and genotype. Age range was between 3 and 6 months at the time of testing. Before testing, groups were assessed for general health, reflexes, and sensory and neurological functions. Cohorts were subjected to multiple behavioral tests in the following order: open field, elevated plus maze, prepulse inhibition (PPI) of acoustic startle, and fear conditioning. A second cohort of ErbB4−/− mice and control littermates was used for the resident intruder and saccharin preference tests.
Open field test.
Locomotor activity was measured in a 35 × 35 cm open field arena. Mice were placed in one corner of the arena and movements were recorded for 30 min with TopScan Suite of CleverSystems (CleverSys). The open field chamber was wiped between trials with a 70% alcohol solution.
Prepulse inhibition of the startle response.
The SR-Lab Startle Response System (San Diego Instruments) was used to measure PPI. Mice were placed in a clear Plexiglas holding cylinder and, after 5 min acclimation, presented with random trials of startle (40 ms at an intensity of 120 dB), prepulse + startle (20 ms of 3, 6, 12, and 17 dB above background noise followed, 100 ms later, by 40 ms at an intensity of 120 dB) and non-startling-stimulus (NSS). A 65 dB background sound was presented throughout the session. Intertrial intervals averaged 15 s (range: 5–20 s). Percentage PPI was calculated as [(startle response − NSS) − (prepulse + startle response − NSS)/(startle response − NSS)] * 100.
Elevated plus maze.
A Plexiglas plus-shaped maze containing two dark enclosed arms and two open lit arms elevated 50 cm above ground was used to examine anxiety-related behaviors. The arms were 30 × 5 cm with a 5 × 5 cm center area, and the walls of the closed arms were 20 cm high. Trials were started by placing mice in the center of the maze. Mice were tracked for 5 min with a video camera, and then returned to their home cage. The plus maze was wiped clean between trials with a 70% alcohol solution. Time spent in the maze and frequency of visits to different zones of the maze was scored using Any Maze program (Stoelting).
Contextual and cued fear conditioning.
Mice were trained and tested for fear conditioning using the San Diego Instruments Freeze Monitor. Freezing, defined as the lack of all movement other than respiration, was used as the measure of conditioned fear. Freezing was scored using the Freeze Detector System (San Diego Instruments), an automatic freezing scoring program. During training, mice were placed in the chamber and exposed to two conditioned (CS; 85 dB white noise, 30 s) and unconditioned (US; 0.5 mA foot shock, 2.0 s) stimulus. The CS–US stimuli were paired and separated by a 120 s intertrial interval. Following the last CS–US stimulus, the training session ended with a 3 min period during which freezing behavior was recorded. Contextual fear conditioning testing was performed 24 h after training. Mice were returned to the training chambers and freezing was scored for 5 min. Twenty-four hours later, mice were placed in an altered context to test for generalized freezing and for cued fear conditioning. For generalized freezing, freezing behavior was scored for 2 min before the CS and for cued fear conditioning 3 min after the CS.
Resident intruder test.
ErbB4 mutants and their WT control cohorts were single-housed 1 week before testing. A C57BL/6J male mouse was introduced into the home cage of the tested mouse. Their activities were videotaped for 10 min and scored for time spent on olfactory investigations (sniffing), and for the number of side approaches, tail rattlings, and attack bitings.
Saccharin preference test.
Hedonic-like behavior was measured using the saccharin preference test. Mice were single-housed for 1 week and then tested for their preference for saccharin in their home cage. Mice were given access for 2 d to two 2 drinking bottles containing tap water. On day 3, one of the water bottles was replaced with a bottle containing 0.01% saccharin solution, and the consumption of water and saccharin was measured for 2 consecutive days. The position of the bottles was changed daily to prevent side preference.
Results
NRG-1β inhibits LTP induction elicited by a pairing protocol
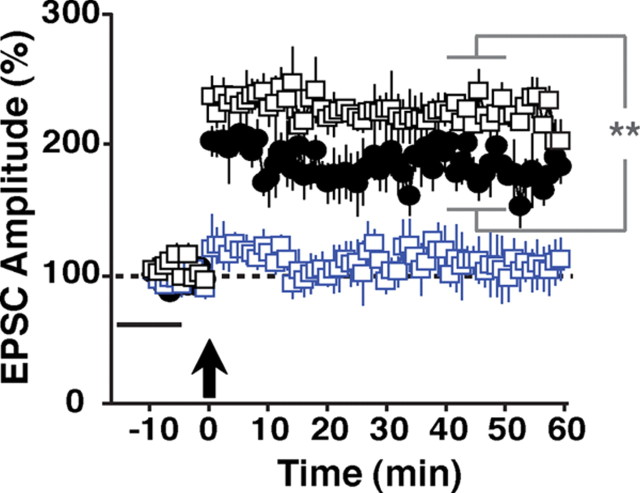
LTP can be broadly separated into induction and expression phases. At SC→CA1 glutamatergic synapses, LTP induction depends on NMDA receptor activation and expression manifests as an increase in synaptic AMPA receptor currents. We previously demonstrated that a recombinant protein encompassing the EGF domain of NRG-1β1 (hereafter referred to as NRG-1β), added 20 min after LTP induction, reverses expression of LTP elicited by theta-burst stimulation or pairing by reducing AMPAR currents to preinduction levels (Kwon et al., 2005, 2008). To analyze the effects of NRG-1β on LTP induction using a pairing protocol, we perfused hippocampal slices with 1 nm NRG-1β 15 min before pairing for a total of 10 min. Of note, NRG-1β had no effect on baseline EPSCs in the absence of LTP-inducing stimuli, as shown previously (Kwon et al., 2005). As illustrated in Figure 1, pairing failed to induce LTP in NRG-1β pretreated slices (−10–0 min: 95 ± 14%; 40–50 min: 105 ± 19%), while untreated control slices exhibited robust LTP (188 ± 15%; p < 0.01 for the comparison between controls and NRG-1β at 40–50 min after pairing). NRG-1β did not block LTP induction when slices were pretreated with the pan-ErbB receptor kinase inhibitor PD158780 (10 μm). Moreover, compared with untreated controls, a small but significant enhancement of LTP was observed, suggesting that inhibition of endogenous NRG/ErbB signaling by PD158780 augments LTP (229 ± 9% vs 188 ± 15%; p < 0.01).
Figure 1.
Effects of acute ErbB receptor activation on LTP induction at SC→CA1 synapses in WT mice. Synaptic responses were measured in whole-cell voltage-clamp mode beginning 10 min before and continuing for 60 min following LTP induction by pairing (arrow). EPSC amplitudes are relative to baseline (set as 100%; dotted line). Perfusion with 1 nm NRG-1β for 10 min before LTP induction (−15 min to −5 min relative to pairing; black bar) effectively inhibited LTP induction (blue squares). Following pretreatment with 10 μm PD158780, NRG-1β no longer inhibited LTP induction (black squares). Rather, EPSC amplitudes exhibited a small but significant increase compared with normal controls (filled circles). N = 5 (normal control), 6 (NRG-1β), 5 (NRG-1β + PD158780). **p < 0.01.
LTP is enhanced in full and PV interneuron-restricted ErbB4 mutant as well as NRG-1 hypomorphic mice
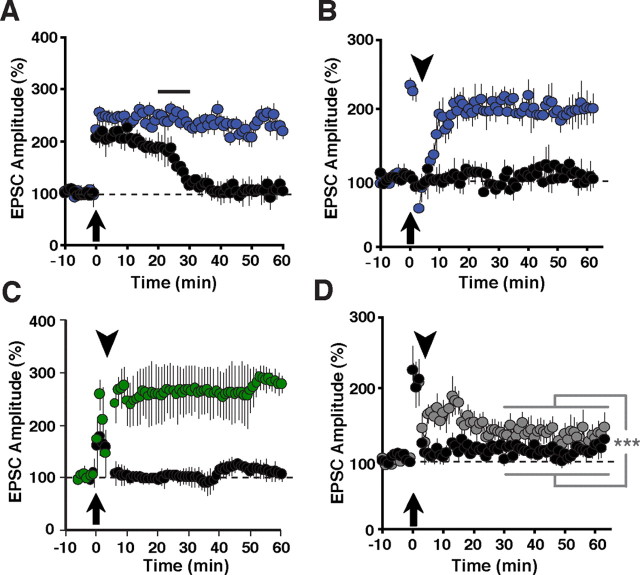
ErbB4 is the major neuregulin receptor in CNS neurons, and pharmacological ErbB receptor blockade by PD158780 blocks LTP reversal by NRG-1β and neural activity (Kwon et al., 2005). However, direct genetic evidence implicating ErbB4 in LTP reversal at SC→CA1 synapses has been lacking. We therefore began by comparing LTP in slices from wild-type and ErbB4MHC-ErbB4 mice harboring a mutant Erbb4 gene in all cells. These mice, hereafter referred to as ErbB4−/−, are rescued from embryonic lethality by transgenic expression of ErbB4 in the heart (Tidcombe et al., 2003). As shown in Figure 2A, LTP was significantly enhanced in mutants compared with control wild-types (ErbB4−/−: 260 ± 17% vs WT: 181 ± 12%, p < 0.001), consistent with the acute effects of ErbB receptor blockade by PD158780 on LTP in wild-type mice. These findings support the notion that NRG/ErbB4 signaling in the hippocampus serves as a counterbalance to the effects of potentiating stimuli. We also measured LTP in ErbB4 heterozygous mice and found that it was moderately enhanced as well (ErbB4−/+: 214 ± 27%; Fig. 2B). The magnitude of LTP was between those observed in wild-type and ErbB4−/− mice, and mean EPSC amplitudes between 40 and 50 min after pairing were significantly different from both (p < 0.001 for both comparisons), indicating that ErbB4 dose-dependently antagonizes LTP.
Figure 2.
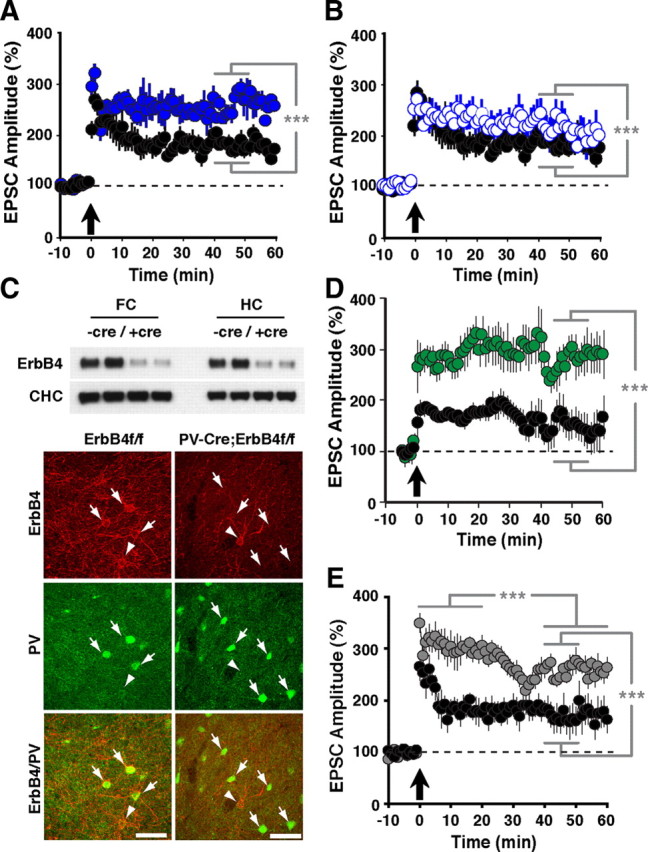
LTP in ErbB4 and NRG-1 mutant mice. EPSCs were measured as described in Figure 1. Compared with normal controls (A, B, D, E; black circles), hippocampal slices from ErbB4−/− (A; solid blue circles) and ErbB4+/− mice (B; open blue circles) exhibited enhanced LTP throughout the entire recording period following pairing. N = 6 (WT), 8 (ErbB4−/−), 4 (ErbB4−/+). C, Top, Western blot analysis of ErbB4 protein levels in whole tissue lysates from the frontal cortex (FC) and hippocampus (HC) of control ErbB4f/f (−cre) and PV-Cre;ErbB4 (+cre) mice. Representative results from two animals are shown for each genotype. Clathrin heavy chain (CHC) is included as a loading control. Bottom, Coimmunofluorescence of ErbB4 (red) and PV (green) in the frontal cortex of PV-Cre;ErbB4 mice. In ErbB4f/f controls, numerous PV(+) cells coexpress ErbB4 (arrows). In contrast, ErbB4 is not detected in PV(+) cells from PV-Cre;ErbB4 mice. Arrowheads mark ErbB4(+) cells that are negative for PV. Scale bars, 100 μm. D, Selective ablation of ErbB4 in PV interneurons (green) results in higher LTP, mimicking the effects of complete loss of ErbB4. N = 8 (ErbB4f/f controls) and 6 (PV-Cre;ErbB4; green). E, LTP was also increased in slices from NRG-1+/− mice (gray), although it runs down somewhat 25 min postinduction. N = 5 (WT), 8 (NRG-1+/−). ***p < 0.001.
We next investigated the specific role of ErbB4 signaling in PV interneurons for hippocampal plasticity. PV-Cre;ErbB4 mice, obtained by crossing mice harboring a conditional ErbB4 allele with mice expressing Cre recombinase from the PV locus, show strongly reduced ErbB4 protein levels in extracts from the frontal cortex and hippocampus (Fig. 2C, top). This is consistent with the extensive coexpression of ErbB4 in PV interneurons in both areas (Neddens and Buonanno, 2010). Residual signals observed in extracts from PV-Cre;ErbB4 mice likely represent ErbB4 expression by other GABAergic interneurons that also coexpress ErbB4. To ascertain the faithfulness of PV-Cre-mediated ErbB4 ablation, we colabeled sections from the frontal cortex where virtually all PV interneurons coexpress ErbB4 (Neddens et al., 2011). As shown in Figure 2C (bottom), while we frequently observed ErbB4-IR in PV interneurons from control sections from ErbB4f/f mice, we were unable to find ErbB4-IR in PV interneurons from PV-Cre;ErbB4 mice. Occasional ErbB4-IR cells were immunonegative for PV and likely represented other types of GABAergic interneurons. Similar results were obtained in the hippocampus (data not shown). We then proceeded to measure LTP in PV-Cre;ErbB4 mice. Consistent with the notion that ErbB4 signaling in PV interneurons plays an important role in mediating the effects of NRG-1 on hippocampal plasticity, we found that normalized mean EPSC amplitudes were significantly increased in PV-Cre;ErbB4 mice compared with their control ErbB4f/f littermates (PV-Cre;ErbB4: 322 ± 43% vs ErbB4f/f: 157 ± 13%; p < 0.001).
The acute effects of NRG-1 on LTP have been studied by perfusing hippocampal slices with exogenous NRG-1β peptide (Fig. 1). To investigate the role of endogenous NRG-1, which is expressed in CA3 pyramidal neurons (Law et al., 2004; Longart et al., 2004), we compared LTP between wild-type mice and heterozygous NRG-1 mutant mice with targeted deletions of exons encoding the EGF-like domain; NRG-1-null mice die at E9.5 and could therefore not be used. As in the case of ErbB4 mutant and hypomorphic mice, NRG-1 heterozygous mice exhibited enhanced LTP (NRG-1+/−: 258 ± 14% vs WT: 166 ± 28%, p < 0.001; Fig. 2E). However, we noticed a somewhat different time course following LTP induction, with an apparent extended period of strongly enhanced LTP immediately after pairing, followed by a phase marked by decreasing LTP levels and eventual stabilization at intermediate levels that were remarkably similar to LTP in ErbB4-null mice (mean relative EPSC amplitudes: 306 ± 23% at 0–20 min vs 259 ± 16% at 40–60 min, p < 0.001). While these findings might suggest additional effects of NRG-1 hemizygosity on synaptic plasticity at SC→CA1 synapses, they are nevertheless consistent with the notion that genetic perturbations of both the ligand and the receptor result in similar outcomes and support the idea that endogenous NRG-1/ErbB4 signaling acts as a counterbalance to LTP-promoting processes.
LTP reversal by NRG-1β or TPS is absent in ErbB4−/− mice
In wild-type controls, 1 nm NRG-1β, perfused 20 min after pairing for a total of 10 min, rapidly reverted LTP back to baseline levels (Fig. 3A). By contrast, slices from ErbB4-null mice were resistant to the acute effects of exogenously added NRG-1β (ErbB4−/−: 220 ± 16% vs WT: 104 ± 18%, p < 0.001), demonstrating the critical requirement of ErbB4 for NRG-1β-mediated LTP reversal. LTP can also be reversed by TPS (5 Hz, 1 min) if delivered shortly after LTP induction. TPS are modeled after normal hippocampal activity patterns observed during exploratory activity, and therefore represent a possible endogenous pathway for LTP reversal (Stäubli and Chun, 1996). We have previously shown that TPS-mediated LTP reversal is absent when slices are pretreated with the ErbB receptor inhibitor PD158780 (Kwon et al., 2005), implicating NRG/ErbB signaling in mediating the effects of TPS on early phase hippocampal LTP. As shown in Figure 3B, application of TPS 5 min after LTP induction by pairing is ineffective in slices from ErbB4−/− mice whereas they completely reverse LTP in slices from wild-type mice (ErbB4−/−: 199 ± 15% vs WT: 107 ± 15% of baseline, 40–50 min after LTP induction). Likewise, TPS were also without effect in PV-Cre;ErbB4 mice (PV-Cre;ErbB4: 261 ± 3% vs ErbB4f/f: 121 ± 3%; p < 0.002; Fig. 3C). Together, these data firmly establish ErbB4 as a critical mediator of the inhibitory effects of NRG-1 and TPS on early-phase LTP, and furthermore reveal PV interneurons as the primary locus of ErbB4 signaling underlying LTP reversal.
Figure 3.
LTP reversal in full and conditional ErbB4 knock-outs and hypomorphic NRG-1 mice. A, No LTP reversal by NRG-1β in SC→CA1 synapses from ErbB4−/− mice. EPSC amplitudes were recorded as described in Figure 1. Addition of 1 nm NRG-1β (black bar) triggers the rapid reversal of LTP in slices from wild-type control mice (black circles) but not from ErbB4 mutant mice (blue circles). N = 8 (WT controls and ErbB4−/−). B–D, Analysis of LTP reversal by theta-pulse stimuli. TPS (arrowheads), delivered 5 min after LTP induction (arrows), rapidly reverse LTP in hippocampal slices from normal controls (black circles) but not from ErbB4−/− (B, blue circles) or PV-Cre;ErbB4 (C, green) mice. In slices from NRG-1+/− mice (D, gray), TPS mostly, but not completely, reverse LTP. N = 5 (ErbB4−/− and WT controls), 7 (PV-Cre;ErbB4), 6 (ErbB4f/f controls), 6 (NRG-1+/− and WT controls). ***p < 0.002.
It is reasonable to assume that TPS might trigger LTP reversal by somehow promoting the release of endogenous NRG to activate ErbB4. We reasoned, therefore, that TPS in NRG-1 heterozygous mice might be less effective than in WT mice. Consistent with this idea, Figure 3C shows that unlike in WT slices, EPSC amplitudes in slices from NRG-1 heterozygous mice remained elevated after TPS and only slowly ran down (10–20 min: 164 ± 46% vs 30–40 min: 137 ± 41%; p < 0.01) to stabilize at levels that continued to be moderately potentiated (average EPSC amplitudes 30–60 min after pairing: NRG-1+/−: 134 ± 40% vs WT: 111 ± 21%; p < 0.001). These data are consistent with the idea that reduced levels of NRG-1 in NRG-1+/− mice are only partly effective to stimulate ErbB4 signaling in response to TPS.
Behavioral analysis of full and PV-interneuron selective ErbB4 mutant mice
A recent study reported some behavioral abnormalities in mice in which the Erbb4 gene was conditionally ablated using Cre expressed under the control of the PV promoter (Wen et al., 2010). Since a comprehensive behavioral analysis has not been performed for mice lacking ErbB4 in all neurons, we sought to compare cohorts of ErbB4−/− and PV-Cre;ErbB4 mice with their respective wild-type littermate controls in a battery of tests designed to investigate rodent behaviors potentially pertinent for behavioral abnormalities found in individuals with schizophrenia and other psychiatric disorders. The battery of tests performed on these cohorts included open field, PPI of acoustic startle, elevated plus maze, fear conditioning, as well as saccharin preference and resident intruder tests. Because the ErbB4−/− cohort exhibited no alterations in the saccharin preference test (a paradigm used for the assessment of hedonic behavior) or the resident intruder test for aggressive behavior, we did not subject the PV-Cre;ErbB4 cohort to these tests.
Open field test
Spontaneous locomotor activity in response to novelty is widely used to model positive symptoms of schizophrenia. In the open field test, both ErbB4−/− and PV-Cre;ErbB4 cohorts exhibited significantly more locomotor activity than their respective normal littermate control groups (Fig. 4). This hyperactivity was apparent at all times during the 30 min observations. Full ErbB4 knock-out mice traveled a total distance of 6900 ± 550 cm, compared with 4600 ± 460 cm in the control group (n = 17 for both groups; p < 0.05, Student's t test). Similarly, PV-Cre;ErbB4 mice traveled a total distance of 6000 ± 480 cm compared with their littermate control group that traveled 4500 ± 420 cm (p < 0.05, Student's t test). Although the observed differences in total distance traveled between the full and PV interneuron-restricted Erbb4-null mice were suggestive of an effect of genotype, differences in strain backgrounds between these two mutants precluded a direct comparison. Notwithstanding, the finding that both strains of ErbB4 mutant mice were hyperactive strongly suggests that ErbB4 signaling in PV interneurons contributes substantially to the regulation of novelty-induced locomotor activity.
Figure 4.
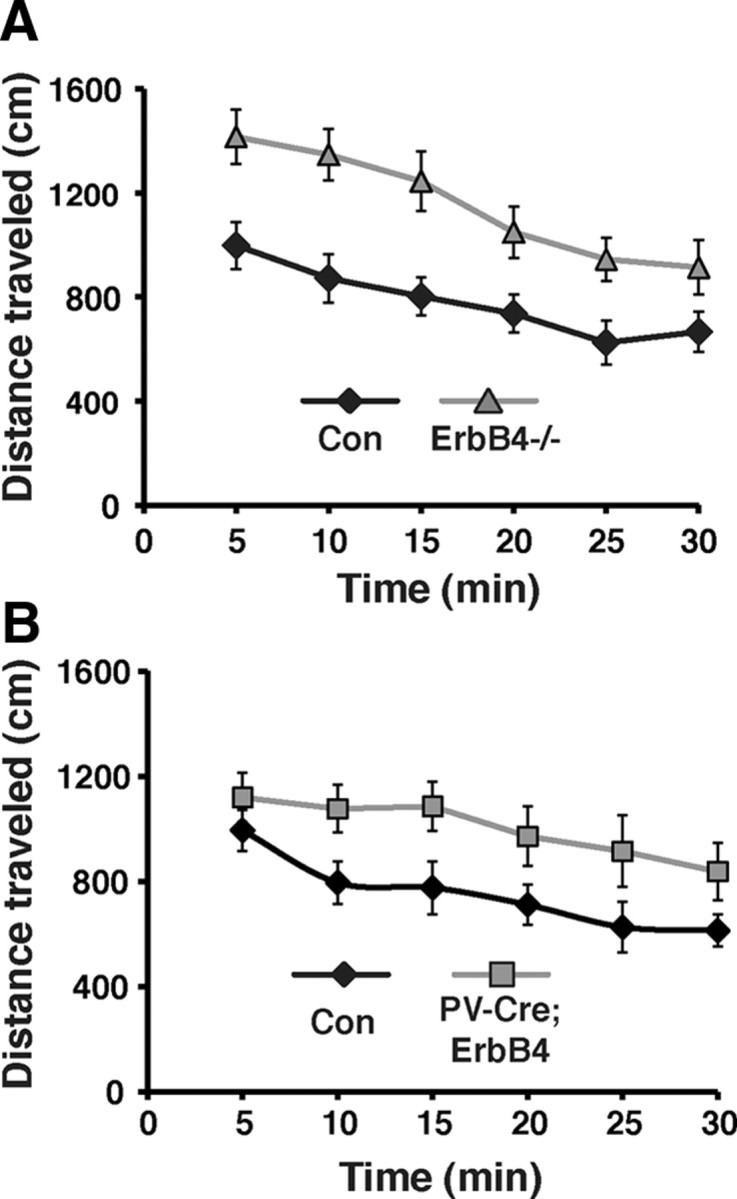
ErbB4−/− and PV-Cre;ErbB4 mice are hyperactive. A, B, Time course of spontaneous locomotor activity during the first 30 min following placement in the novel environment for ErbB4−/− (gray triangles) and its littermate control group (Con, black diamonds) (A) and for PV-Cre;ErbB4 mice (gray squares) and its respective littermate controls (Con, black diamonds) (B). Data points represent 5 min bins of locomotor activity. N = 17 for ErbB4−/− and its control group; N = 8 for PV-Cre;ErbB4 and 11 for its control group.
PPI of the startle response
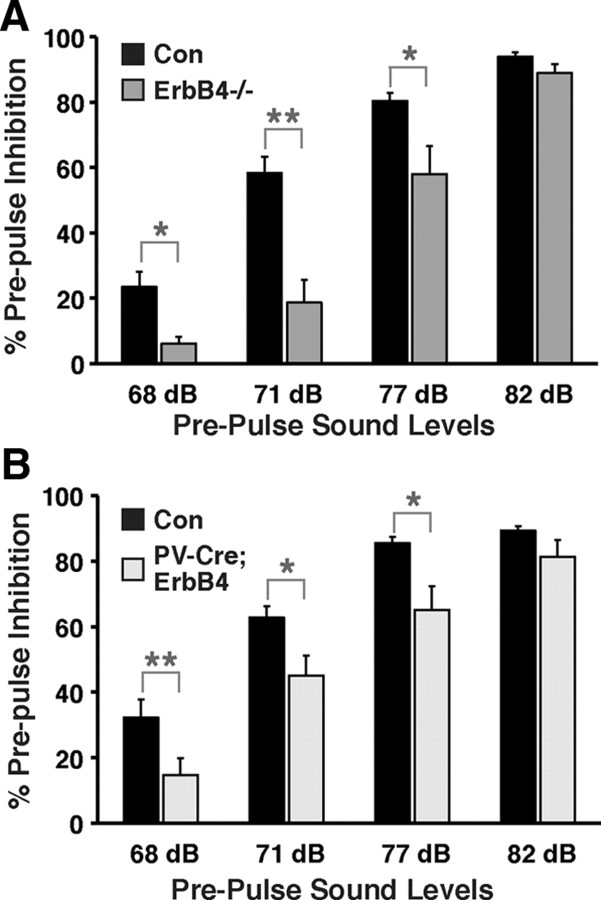
PPI is a widely used measure of sensorimotor gating in rodents and humans (Geyer, 2008), and deficits in this task have been reproducibly found in individuals with schizophrenia (Braff, 2010). Figure 5 shows that, like in the open field test, both ErbB4 mutants exhibited deficits in PPI. Two-way ANOVA with repeated measurements detected significant effects of genotype (p < 0.01) and genotype × prepulse sound level (p < 0.05). Post hoc analysis revealed that reductions in PPI levels were more pronounced at lower prepulse sound levels (68 dB: 6 ± 2% and 23 ± 5% for ErbB4−/− vs controls, p < 0.05; 15 ± 5% and 32 ± 5% for PV-Cre;ErbB4 vs controls; p < 0.01) than at higher prepulse sound levels (77 dB: 58 ± 8% and 80 ± 2% for ErbB4−/− vs controls, p < 0.05; 65 ± 7% vs 86 ± 2% for PV-Cre;ErbB4 vs controls; p < 0.05). At 82 dB, PPI was not significantly different between both ErbB4 mutant strains and their respective controls (94 ± 1% and 89 ± 3% for controls vs ErbB4−/−, p = 0.11; 89 ± 1% and 81 ± 5% for controls vs PV-Cre;ErbB4; p = 0.14). These data indicate that PPI deficits are more pronounced at smaller differentials between the prepulse and background sound levels (68 dB: Δ3 dB; 82 dB: Δ17 dB). Importantly, acoustic startle responses, per se, were not significantly different between mutants and their respective controls, affirming that all pertinent sensorimotor functions were unaffected (data not shown).
Figure 5.
ErbB4−/− and PV-Cre;ErbB4 mice show deficits in PPI of the acoustic startle response. A, B, Percentage inhibition of the startle response as a function of increasing prepulse sound levels (68, 71, 77, 82 dB) for ErbB4−/− and their controls (Con) (N = 8 for both groups; A) and for PV-Cre;ErbB4 and their controls (N = 12 for both groups; B). A 65 dB background sound was presented throughout the sessions. *p < 0.05; **p < 0.01.
Elevated plus-shaped maze
To evaluate possible alterations in fear and anxiety-related behaviors in ErbB4 mutant mice, we tested their performance in the elevated plus maze. As shown in Figure 6, ErbB4−/− mice spent significantly more time in the open arms during the 5 min trials than their wild-type littermates (open arm: 97 ± 20 s for ErbB4−/− vs 34 ± 18 s for controls; closed arm: 197 ± 17 s for ErbB4−/− vs 279 ± 29 s for controls; p < 0.05 for both arms). By contrast, PV-Cre;ErbB4 mice spent as much time in the closed arms as their control littermates (open arm: 13 ± 3 s for PV-Cre;ErbB4 vs 20 ± 7 s for controls, p = 0.47; closed arm: 266 ± 6 s for PV-Cre;ErbB4 vs 256 ± 8 s for controls; p = 0.4). Moreover, ErbB4−/− mice entered the open arms approximately twice as often as their wild-type controls (on average, 13.9 vs 6.9 visits) and also visited the closed arms approximately twice as often (18.3 vs 10.4 visits). The increased overall activity of ErbB4−/− mice in the elevated plus-shaped maze, as assessed by the increase in the number of entries into either arm, is generally considered a built-in control measure for general activity, and therefore consistent with the corresponding data from the open field test. Together, these results suggest reduced anxiety in ErbB4-null mice. However, unlike in the open field and PPI tests, defective ErbB4 signaling in PV interneurons apparently does not account for the effects observed in full ErbB4−/− mice.
Figure 6.
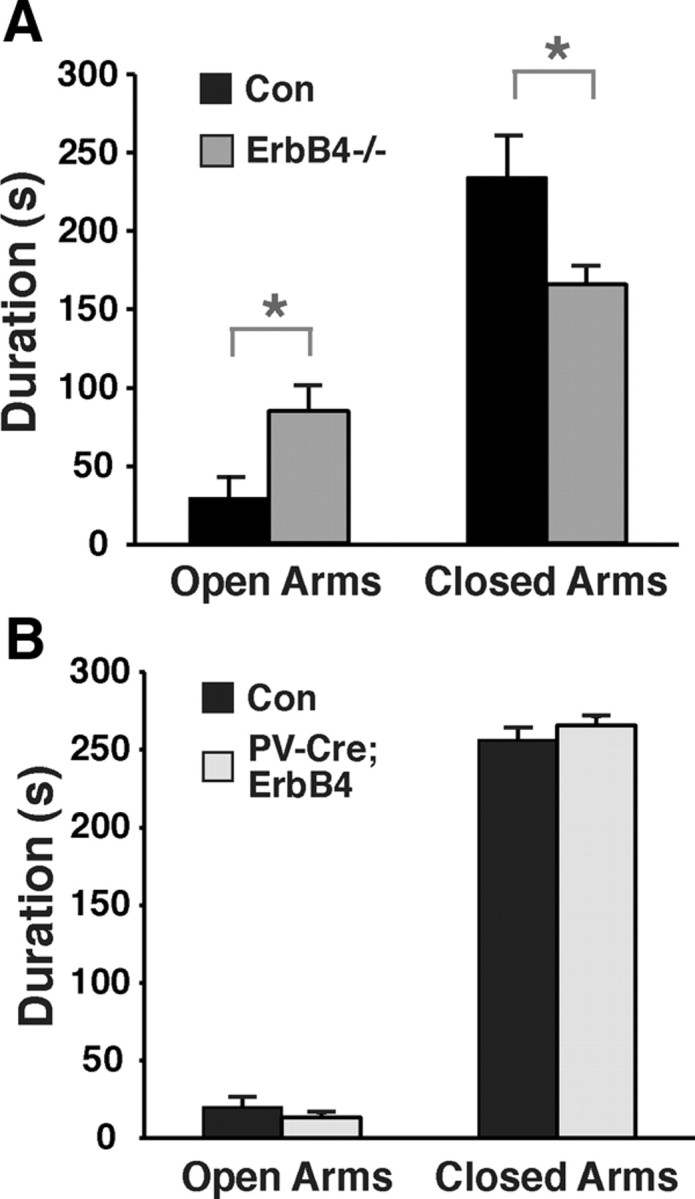
Reduced anxiety in ErbB4−/− but not PV-Cre;ErbB4 mice. A, B, Time spent in open and closed arms of the elevated plus maze during 5 min trials for ErbB4−/− (N = 7 for both groups; A) and for PV-Cre;ErbB4. N = 11 for controls (Con) and 8 for PV-Cre;ErbB4; B]. *p < 0.05.
Cued and contextual fear conditioning
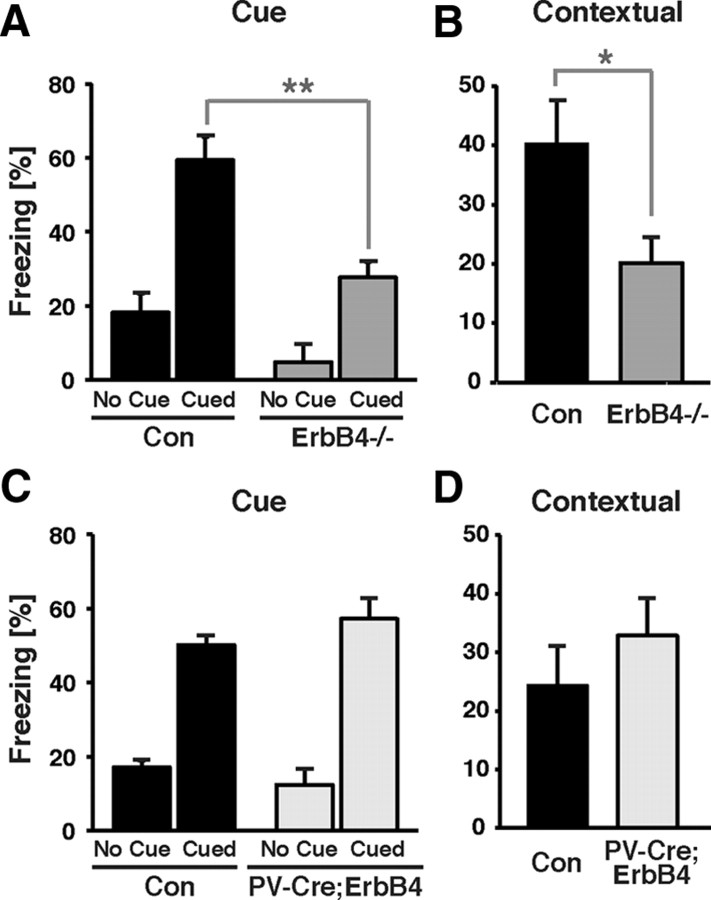
To more directly assess the role of ErbB4 in fear-related behaviors, and to reveal possible differences between full and PV-interneuron selective ErbB4 mutants, we tested our cohorts in cued and contextual fear conditioning. Cued fear conditioning is generally believed to be largely dependent on the amygdala, while contextual fear conditioning involves both the amygdala and the hippocampus (Phillips and LeDoux, 1992). Interestingly, before and during the two training trials, freezing levels were consistently lower for ErbB4−/− mice compared with controls (naive, 3% vs 10%; first trial, 8% vs 21%; second trial, 21.2% and 33.5%; F(2,42) = 7.04; p < 0.05, two-way ANOVA). However, no interaction between genotype and trial was found (F(2,42) = 0.23; p = 0.80, two-way ANOVA), suggesting that the difference in freezing between WT and mutant mice is not related to training, i.e., the ability of ErbB4−/− mice to acquire conditioned fear. Unlike full ErbB4 mutant mice, PV-Cre;ErbB4 mice and their controls performed similarly during training. As shown in Figure 7, in the testing phase, ErbB4−/− mice exhibited significantly lower freezing responses when presented with the conditioned stimulus in a completely new context, as well as when returned to the training environment (cue: 59 ± 6% for controls vs 28 ± 4% for ErbB4−/−, p < 0.01; context: 40 ± 7% for controls vs 20 ± 4% for ErbB4−/−, p < 0.05; Student's t test). In stark contrast, neither cued nor contextual fear conditioning was different between the PV-Cre;ErbB4 group and their controls (cue: 50 ± 4% for controls vs 57 ± 6% for PV-Cre;ErbB4, p = 0.31; context: 24 ± 7% for controls vs 33 ± 6% for PV-Cre;ErbB4, p = 0.39; Student's t test). Together, the results from the elevated plus maze and fear conditioning tests suggest that the NRG/ErbB4 signaling pathway affects neural circuits involved in the regulation of anxiety and fear-related behaviors, but that ErbB4 effects on PV interneurons play a minor role in these processes.
Figure 7.
ErbB4−/− mice, but not PV-Cre;ErbB4 mice, exhibit deficits in fear conditioning. A–D, Freezing behavior, plotted as the fraction of time spent motionless, was recorded for 3 min following presentation of the CS to assess cued fear conditioning (A, C) and for 5 min following return to the training chamber to measure contextual fear conditioning (B, D). Generalized fear (no cue) was assessed for 2 min before presentation of the CS. ErbB4−/− mice exhibit reduced cued and contextual fear responses (A, B; N = 8 for both groups), whereas responses in PV-Cre;ErbB4 mice were not different from controls (Con) (C, D; N = 11 for controls and 8 for PV-Cre;ErbB4). *p < 0.05, **p < 0.01.
ErbB4 and PV expression is mostly non-overlapping in the amygdala
To further substantiate the notion that ErbB4 deficiency in PV interneurons is unlikely to contribute to altered amygdalar function observed in full ErbB4 mutants, we analyzed expression of ErbB4 and PV in the amygdala. PV mRNA expression is generally sparse in basolateral (BLA) and basomedial (BMA) areas, two of the major amygdalar nuclei (Fig. 8) (Allen Mouse Brain Atlas, 2009). By comparison, ErbB4 mRNA signal density in the BLA is moderately higher and is extraordinarily high in the BMA. We used immunofluorescence histology to determine the extent of coexpression of PV and ErbB4. To unambiguously identify ErbB4- and PV-expressing cell bodies, we resorted to a mouse line in which tamoxifen-inducible Cre recombinase expressed from the Erbb4 locus and translated from a fusion transcript between ErbB4 and Cre drives the recombination and subsequent high-level expression of tdTomato fluorescent protein from the Rosa26 locus (Erbb4-2A-CreERT2). This mouse has been shown to faithfully report the expression pattern of ErbB4 (Madisen et al., 2010). As shown in Figure 8, the general pattern of cell bodies expressing ErbB4 mRNA and tdTomato following Cre-mediated recombination was very similar, with high densities of fluorescent cell bodies in the BMA, and a much sparser distribution in the BLA. Strikingly, the overlay between tdTomato and PV demonstrates that, while some colocalization was observed in the BLA where both ErbB4 and PV are sparse, virtually all tdTomato expression in the BMA was in cells immunonegative for PV. We conclude that the majority of ErbB4-expressing cells in the amygdala are not PV interneurons. This finding provides a rational basis for the observed differences in fear-related behaviors between full and PV-restricted ErbB4 mutant mice.
Figure 8.
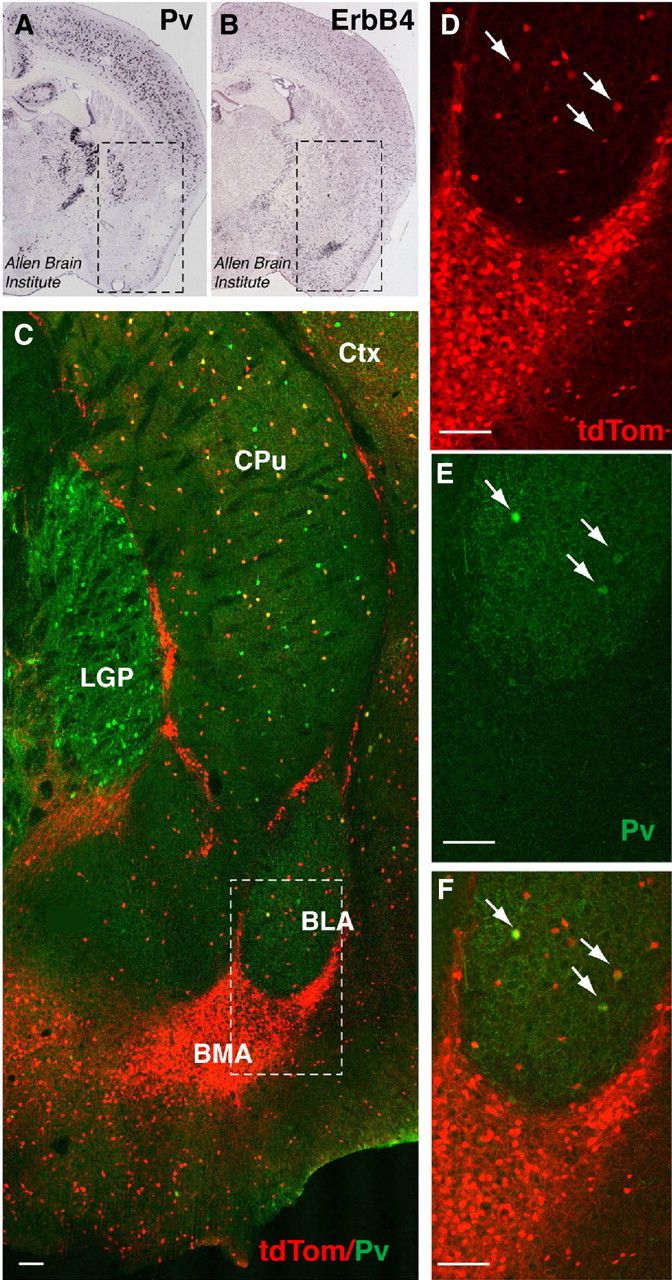
ErbB4 is abundantly expressed in the amygdala and shows only modest colocalization with PV. A, B, In situ hybridization of PV and ErbB4 in coronal sections of P56 mice (Allen Brain Atlas, 2009). Boxed areas correspond to the region shown in C and include the amygdala (ventrally), caudate/putamen, and the lateral global pallidus (dorsally). PV mRNA signals are dense in the lateral globus pallidus (LGP), sparse in the BLA, and not detectable in the BMA. By contrast, ErbB4 mRNA signals are very dense in the BMA and more sparsely distributed in most other areas. C, Co(immuno)fluorescence micrograph image of tdTomato (tdTom; red), reporting ErbB4 expression in adult Ai14 × ErbB4-2A-CreERT2 mice, and endogenous PV (green). The boxed area is magnified in D–F. While few tdTomato-labeled cells in the BLA coexpress PV (arrows), virtually none of the large number of the ErbB4-expressing cells in the BMA and other intercalated areas coexpress PV. CPu, Caudate–putamen; Ctx, cerebral cortex; LGP, lateral globus pallidus. Scale bar, 100 μm.
Discussion
The major findings of this study are as follows: (1) ErbB4−/−, PV-Cre;ErbB4, and NRG-1 hypomorphic mice all show enhanced hippocampal LTP; (2) ErbB4 mediates the effects of NRG-1 and TPS on LTP reversal; (3) ErbB4 signaling in PV interneurons is critical to reverse LTP by neuronal stimuli; and (4) ErbB4−/− mice exhibit numerous behavioral deficits, some of which they share with, and others that are not reproduced by, PV-Cre;ErbB4 mice. Our findings firmly establish NRG/ErbB4 signaling in PV interneurons as an important counterbalance to processes promoting hippocampal LTP, and as a powerful modulator of behaviors relevant to schizophrenia and other psychiatric disorders. Intriguingly, deficits in synaptic plasticity have been postulated to contribute to functional dysconnectivity in schizophrenia (Stephan et al., 2006; Uhlhaas and Singer, 2010). Abnormal regulation of LTP in NRG-1 and ErbB4 mutant mice could therefore conceivably contribute to behavioral phenotypes observed by us and other groups.
Hippocampal synaptic plasticity in ErbB4-null mice
We chose to compare the involvement of ErbB4 in hippocampal LTP using ErbB4-null and PV-Cre;ErbB4 mice to account for the intricate spatial and temporal expression pattern of ErbB4 in the rodent cortex on the one hand, and the prominent postnatal expression of ErbB4 in PV interneurons on the other hand. ErbB4 is expressed throughout development and in the adult brain (Lai and Lemke, 1991; Gerecke et al., 2001; Fox and Kornblum, 2005), and its expression pattern in the adult hippocampus indicates a complex distribution of ErbB4 expression in distinct interneuron subclasses (Yau et al., 2003; Neddens and Buonanno, 2010). During embryonic development, ErbB4 is involved in interneuron migration (Anton et al., 2004; Flames et al., 2004), and the number of GAD67- and PV-immunoreactive interneurons in the hippocampus of ErbB4−/− mice is reduced by 24% and 31%, respectively (Fisahn et al., 2009; Neddens and Buonanno, 2010). However, Erbb4 ablation in PV-Cre;ErbB4 mice presumably occurs late during perinatal development (>P13), after GABAergic neuronal migration, due to the late onset of PV expression in the rodent cortex (del Río et al., 1994; Madisen et al., 2010). It is therefore unlikely that increased LTP and deficits in LTP reversal observed in both ErbB4−/− and PV-Cre;ErbB4 mice reflect aberrant neurodevelopmental processes. This idea is also consistent with the ability of the ErbB receptor inhibitor PD158780 to acutely increase LTP levels and to block LTP reversal by TPS (Kwon et al., 2005). Notwithstanding, both NRG-1/ErbB4 signaling during development and in other interneuron types in the adult brain conceivably affect the balance of excitatory and inhibitory transmission, and could contribute to the behavioral abnormalities observed in full, but not PV-Cre;ErbB4, mice.
An important unresolved question remains as to the specific role of inhibitory interneurons and GABAergic transmission in mediating LTP inhibition by NRG-1/ErbB4. While electrophysiological recordings in the initial studies were performed in the presence of bicuculline (Huang et al., 2000; Kwon et al., 2005), an inhibitor of fast GABAergic transmission, recent reports differ with regards to the role of fast GABAergic transmission for LTP inhibition by NRG-1 (Chen et al., 2010; Pitcher et al., 2011). Consistent with Huang et al. (2000) and Pitcher at al. (2011), we found that GABAA receptor blockade does not affect LTP reversal by NRG-1 or TPS (Kwon et al., 2005). Because of the interneuron-restricted expression of ErbB4 in the hippocampus and the finding that selective ablation of Erbb4 in pyramidal neurons does not affect NRG-1-mediated inhibition of LTP induction (Chen et al., 2010), the most parsimonious scenario still places the first events leading up to LTP inhibition or reversal on ErbB4-expressing PV-interneurons. Based on our previous findings that NRG-1 acutely triggers dopamine release in the dorsal hippocampus, and that LTP reversal by NRG-1 and TPS critically depends on the activation of dopamine D4 receptors (Kwon et al., 2008), we propose that NRG/ErbB4 signaling in PV interneurons engages this dopaminergic signaling pathway to regulate synaptic plasticity; a direct effect of NRG on dopaminergic terminals cannot presently be excluded. Future studies will be necessary to work out the mechanism by which this occurs.
ErbB4 and behavior
Based on the intricate expression pattern of ErbB4 during development and in the adult brain, we decided to compare the behavioral outcomes between ErbB4−/− and PV-Cre;ErbB4 mice. Interest in identifying the potential role of PV-expressing interneurons in mediating NRG/ErbB4 effects on behavior stems from multiple observations, including (1) the extensive coexpression of PV and ErbB4 in the PFC and, albeit to a lesser extent, the hippocampus (Fisahn et al., 2009; Fazzari et al., 2010; Neddens and Buonanno, 2010); (2) the notion that PV interneurons are involved in gamma oscillations whose power is reduced in schizophrenia (Kwon et al., 1999; Wilson et al., 2008); and (3) the finding that the number of PV mRNA and protein-expressing cells is reduced in the PFC of individuals with schizophrenia (for review, see Lewis et al., 2005). Intriguingly, acute NRG-1/ErbB4 signaling potently augments the power of kainate-induced hippocampal gamma oscillations, while slices from ErbB4−/− mice exhibit reduced gamma power. Moreover, the number of PV interneurons is reduced by 31% in the hippocampus of ErbB4−/− mice (Fisahn et al., 2009).
Our study represents the first comprehensive behavioral analysis of mice lacking ErbB4 in all cells except the heart. Only one study investigated the effects of selectively ablating Erbb4 throughout the CNS by crossing floxed Erbb4 with Nestin-Cre mice (Golub et al., 2004). The authors interestingly observed that adult mutant mice were hypoactive in a novel environment. A second study, using hGFAP-Cre to ablate both Erbb2 and Erbb4 genes in the CNS, found no difference in horizontal activity, but interpretation of this finding is complicated by the fact that removal of Erbb2 is also expected to affect signaling via ErbB3 and EGF receptors (Barros et al., 2009). In contrast, we (this work) and others (Wen et al., 2010) have shown that ErbB4−/− and PV-Cre;ErbB4 mice are hyperactive in the open field test. To what extent these seemingly disparate findings represent differences in gene targeting strategies (i.e., unconditional vs floxed alleles), strain backgrounds, or other methodological details, is currently not understood. Nevertheless, the increasing evidence for a role of the NRG-1/ErbB4 pathway in regulating dopaminergic function (for review, see Buonanno, 2010), together with data indicating that NRG-1 hypomorphic mice are also hyperactive (Gerlai et al., 2000; Stefansson et al., 2002; Duffy et al., 2008; O'Tuathaigh et al., 2010) and that activity in the open field is a good indicator of nigrostriatal dopaminergic state, provides a possible neurochemical link between ErbB4 and spontaneous locomotor activity.
Deficits in sensorimotor gating have been observed in all ErbB4 mutant mouse strains thus far tested in PPI, including full, PV-restricted, and double Erbb2/Erbb4 mutant mice (Chen et al., 2008; Barros et al., 2009; this work). Moreover, some studies showed reduced PPI in different NRG-1 hypomorphic mouse strains targeting all isoforms (ΔEGF) or specific subsets of NRG-1 variants (for review, see van den Buuse, 2010), in particular of NRG-1 type III, the predominating NRG-1 isoform in the adult brain (Chen et al., 2008). In aggregate, these findings attest to the significant contribution of the NRG/ErbB4 signaling pathway to neural processes underlying normal gating responses. PPI deficits are widely regarded endophenotypic for schizophrenia and PPI is sensitive to treatments generating hyperdopaminergic and hypoglutamatergic states (Braff, 2010; van den Buuse, 2010). The finding that PV interneuron-restricted ErbB4 mutant mice replicate the hyperactivity and sensorimotor-gating phenotypes of full ErbB4−/− mice emphasizes the significant contribution of the NRG-1/ErbB4 pathway for normal function of PV interneurons.
While both ErbB4−/− and PV-Cre;ErbB4 mice shared hyperactivity and PPI deficits, a divergent phenotypic outcome was observed in behavioral tasks assessing anxiety and fear. While Erbb4−/− mice exhibited deficits in the elevated plus maze and in cued and contextual fear conditioning, PV-Cre;ErbB4 mice were not different from their control littermates in these tasks. Only one other study investigated the effects of genetically ablating ErbB4 signaling on emotional learning and reported reduced contextual fear conditioning in mice with targeted ErbB4 mutations in PV interneurons, a finding that was interpreted as indicative of abnormal LTP regulation in the hippocampus of these mice (Chen et al., 2010). While we presently cannot satisfactorily explain the discrepancy between our groups, the fact that we observed deficits in both cued and contextual fear conditioning in ErbB4−/− mice, together with the widespread expression of ErbB4 in the amygdala, in particular in the BMA, is consistent with the notion that NRG/ErbB4 signaling in the amygdala is important for emotional learning. The lack of such deficits in PV-Cre;ErbB4 mutant mice is consistent with the relatively sparse distribution of PV interneurons in the amygdala and our finding that most ErbB4-expressing neurons in the amygdala do not coexpress PV. More refined histological analyses of ErbB4 expression in the amygdala and Erbb4 targeting strategies will be required to identify the pertinent neuron types and signaling processes involved in NRG/ErbB4-mediated regulation of fear behavior.
Footnotes
This work was supported by the NICHD intramural program (to all authors). We thank Drs. Cary Lai and Kent Loyd for the floxed ErbB4 mouse strain, Dr. Novak for the NRG-1ΔEGF mouse strain, and Dr. Hongkui Zeng for the ErbB4-2A-CreERT2 mouse strain. We are grateful to Daniel Abebe for expert assistance with mouse husbandry, to Drs. Vincent Schram and Carolyn Smith from the NICHD and NINDS microscopy core facilities for expert assistance with microscopy, and to Dr. Joerg Neddens for critical reading of the manuscript.
References
- Allen Brain Atlas [Internet] Seattle (WA): Allen Institute for Brain Science; ©2009. Available from: http://www.brain-map.org. [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Müller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. Prepulse inhibition of the startle reflex: a window on the brain in schizophrenia. Curr Top Behav Neurosci. 2010;4:349–371. doi: 10.1007/7854_2010_61. [DOI] [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu RC, Gingrich JA, Small S, Moore H, Dwork AJ, Talmage DA, Role LW. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, Lu YS, Zhu XH, Li SJ, Wu CY, Wang XM, Lai C, Xiong WC, Mei L, Gao TM. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río JA, de Lecea L, Ferrer I, Soriano E. The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain Res Dev Brain Res. 1994;81:247–259. doi: 10.1016/0165-3806(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci. 2008;122:748–759. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
- Erickson SL, O'Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Luján R, Lloyd K, Lerma J, Marín O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marín O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotox Res. 2008;14:71–78. doi: 10.1007/BF03033576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific ErbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, Buonanno A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol. 2004;472:156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology. 2011;36:488–496. doi: 10.1038/npp.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knock-out mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, Lewis DA, McBain CJ, Buonanno A. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol Psychiatry. 2011;70:636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Harte M, O'Leary C, O'Sullivan GJ, Blau C, Lai D, Harvey RP, Tighe O, Fagan AJ, Kerskens C, Reynolds GP, Waddington JL. Schizophrenia-related endophenotypes in heterozygous neuregulin-1 ‘knockout’ mice. Eur J Neurosci. 2010;31:349–358. doi: 10.1111/j.1460-9568.2009.07069.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol. 1999;159:494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Jr, Vazdarjanova A, Xiong WC, Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18:371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]