Abstract
Antibody diversity is generated by a random gene recombination process with the inherent risk of the production of autoreactive specificities. The current view suggests that B cells expressing such specificities are negatively selected at an early developmental stage. Using the knock-in model system of the 3–83 autoreactive B-cell antigen receptor (BCR) in combination with precursor-BCR (pre-BCR) deficiency, we show here that the 3–83 BCR mediates efficient generation of B cells in the presence, but not the absence, of a strongly recognized auto-antigen. Experiments with mixed bone marrow chimeras showed that combining the 3–83 BCR with the corresponding auto-antigen resulted in efficient reconstitution of B-cell development in immune-deficient mice. These results suggest that B cells are positively selected by recognition of self-antigens during developmental stages that precede receptor editing. Moreover, the data indicate that the pre- BCR functions as a specialized autoreactive BCR to initiate positive selection at a stage where the cells express immunoglobulin heavy but not light chains.
Keywords: Autoimmune, Proliferation, Self-reactive, Selection
Introduction
Antibody diversity is achieved by random recombination of immunoglobulin (Ig) variable (V), diversity (D) and joining (J) gene segments in developing B-cell precursors [1]. Antibodies are initially expressed as B-cell antigen receptors (BCRs) containing, in addition to the two identical heavy chains (HCs) and two identical light chains (LCs) of the antibody, the heterodimer Ig-α/Ig-β required for signaling [2]. BCR signaling is essential for the generation and selection of B cells, as the VDJ recombination process providing the basis for antibody diversity can also lead to the generation of B cells with self-reactive receptors [3–5]. Mechanisms such as receptor editing, which alters BCR specificity by secondary LC gene rearrangement, clonal deletion and anergy may operate to prevent the development of autoreactive B cells and the production of self-reactive antibodies [3, 6]. We have recently shown that the effects of polyreactive BCRs recognizing multiple self-antigens are similar to those of the precursor- (pre-) BCR, suggesting such receptors to be functionally equivalent. Consequently, both polyreactive BCRs and the pre-BCR induce autonomous signaling and expansion of B cell precursors in vitro [7]. The pre-BCR, in which the HC pairs with a surrogate LC consisting of the germ line-encoded subunits λ5 and VpreB, plays an essential role in the positive selection and expansion of precursor-B (pre-B) cells that express an HC protein [8, 9]. Accordingly, a severe B-cell developmental block is observed in mice deficient for components of the surrogate LC [10, 11]. Recently, we found that even a single-point mutation removing a conserved N-linked glycosylation site in the C1 domain of µHC prevented pre-BCR formation and function [12]. These data suggested that binding of the surrogate LC to carbohydrates in the C1 domain of µHC is required as an initial step of autonomous pre-BCR function that allows precursor B cells to proceed in development. Moreover, our results offered a mechanistic explanation for pre-BCR autoreactivity by suggesting recognition and binding between neighboring pre-BCR molecules.
Here, we investigate the hypothesis that autoreactivity is critically required for the positive selection of precursor B cells in vivo and that the central role of the pre-BCR is the initiation of selection signals that can be replaced by signals from autoreactive BCRs.
Results and discussion
Rescue of the developmental block in pre-BCR-deficient mice
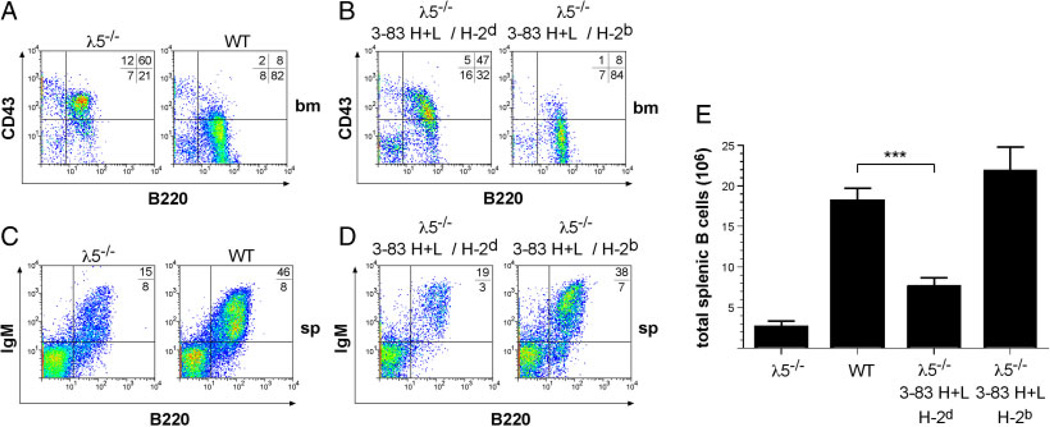
Based on our observations on the functional similarity between pre-BCRs and self-reactive BCRs in vitro, we hypothesized that if the pre-BCR was a specialized autoreactive receptor, then expressing an autoreactive BCR should overcome the developmental block in pre-BCR-deficient mice. To test this, we crossed 3–83Igi mice, in which the HC (3–83Hi) and LC (3–83κi) variable gene segments of the autoreactive BCR 3–83 are knocked into the IgH and Igκ loci respectively, with λ5-deficient mice [6, 10, 13]. The 3–83 BCR recognizes MHC class I proteins of different haplotypes with different affinities, with H-2Kb being strongly recognized, whereas the binding affinity to H-2Kd ranked as the lowest [14–16]. Thus, the 3–83 BCR is strongly autoreactive on the H-2b background and should rescue pre-BCR deficiency when combined with H-2b but not with H-2d. Indeed, flow cytometric analysis of bone marrow cells showed that autoreactive B cells (3–83Hi/3–83κi on the H-2b background) overcame the early developmental block in λ5-deficient mice (Figs. 1A and B, S1A). In contrast, on the H-2d background lacking the specific auto-antigen, the 3–83 BCR failed to efficiently rescue B-cell development. The majority of the B lineage cells in the bone marrow were pro-B cells, which, similar to λ5-deficient cells bearing WT Ig genes, express the early marker CD43 (Fig. 1A and B). In agreement with the rescue of B-cell development in the bone marrow, λ5-deficient mice expressing the 3–83 BCR on the H-2b background showed normal proportions of B cells in the spleen and restored B-cell numbers. On the H-2d background, however, B-cell numbers were significantly reduced, suggesting that 3–83 BCR expression alone is not sufficient to rescue B-cell development (Fig. 1C–E). Together, the above results suggest that an autoreactive BCR efficiently initiates B-cell development and rescues an otherwise severe developmental block caused by pre-BCR deficiency.
Figure 1.
The autoreactive BCR replaces the pre-BCR in B cell development. (A) Flow cytometric analysis of bone marrow (bm) cells from l5−/− and wild-type (WT) or (B) 3–83Hi/3–83κi/λ5−/− mice on different backgrounds: H-2d lacking and H-2b containing the auto-antigen. Numbers show the percentages of bone marrow cells in each quadrant. (C) Flow cytometric analysis of splenic cells of λ5−/−, WT and (D) 3–83Hi/3–83κi/λ5−/− mice on different backgrounds stained with anti-B220 versus anti-IgM. Numbers indicate percentages of B220+ cells. (E) Absolute numbers of total splenic B cells from the indicated mice were determined. For each group, at least three individuals were analyzed. Data represent mean+SD of total splenic B cells. ***indicates (p = 0.0002) in numbers of total splenic B cells. B cell numbers of WT mice (column 2) and 3–83Hi/3–83κi/λ5−/− mice on H-2b background (column 4) were not significantly different (p = 0.1761, Student’s t-test).
Replacement of the pre-BCR by an autoreactive BCR
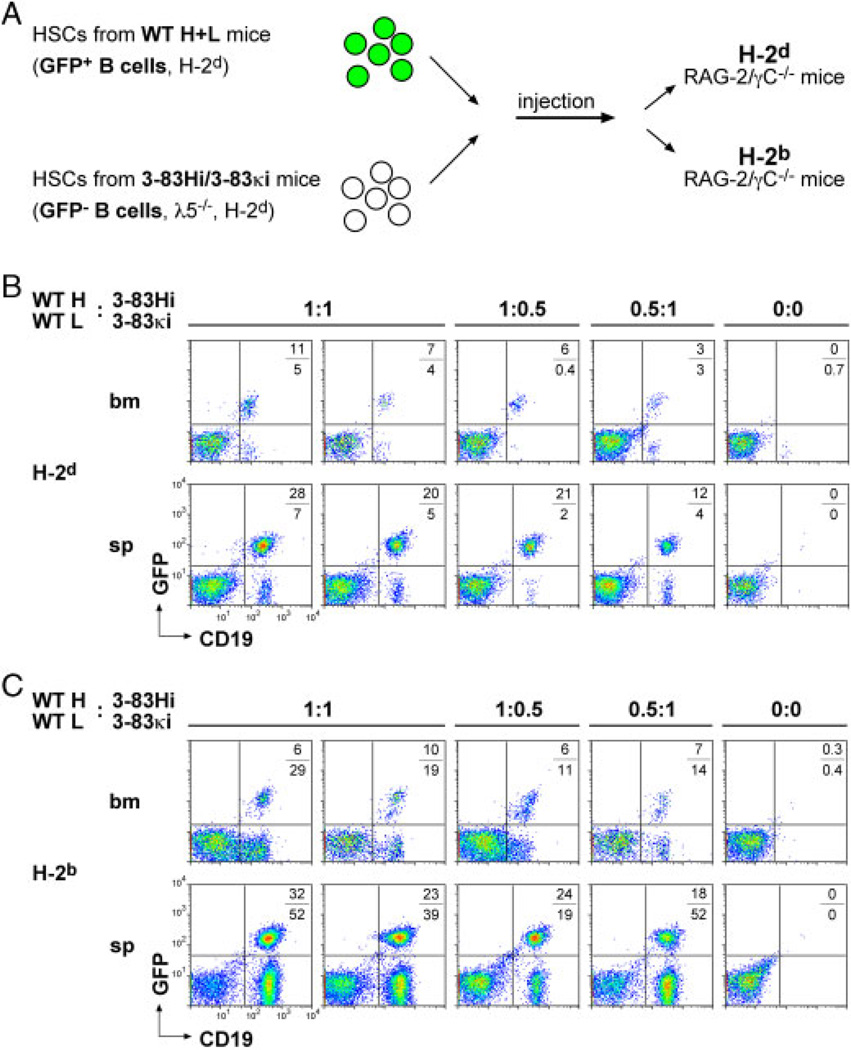
To further investigate the ability of autoreactive BCRs to drive early B-cell development, we injected HSCs from λ5-deficient 3–83Hi/3–83κi mice into immune deficient Rag-2/γC−/− mice [17]. The cells were mixed in various proportions with WT HSCs to test the capacity of autoreactive B cells to compete with WT cells (Fig. 2A). Since the donor (λ5-deficient or WT) mice had the same genetic background (H-2d), WT cells were tagged with green fluorescent protein (GFP) expressed from a transgene knocked into the mb1 locus [18]. On the H-2d background, the 3–83Hi/3–83κi derived B cells represented a minority in the spleen and bone marrow of the reconstituted mice, whereas WT B cells were efficiently generated (Fig. 2B). On the H-2b background however, the 3–83Hi/3–83κi derived B cells slightly outnumbered WT B cells (Fig. 2C). These results show that self-recognition provides developing B cells with a strong advantage, overcoming pre-BCR deficiency and enabling the cells to efficiently compete with WT cells.
Figure 2.
Autoreactivity restores pre-BCR deficiency in competitive BM chimeras. (A) Schematic illustration of hematopoietic stem cell (HSC) transfer into immune-deficient mice. GFP cassette inserted into the mb-1 gene, which encodes the BCR component Ig-α, was used to track WT B cells. (B, C) Flow cytometric analysis of bone marrow (bm) and splenic (sp) cells 5wk after HSC transfer. The background of the recipient Rag-2/γbC−/− mice is indicated. HSCs were injected in different ratios (1:1, 1:0.5, 0.5:1). In 0:0, PBS was injected as control. The percentages of GFP+ and GFP− cells are indicated. A total of 12 mice were analyzed (2 mice per HSC ratio on two different backgrounds).
Autoreactivity is required for the efficient generation of B cells
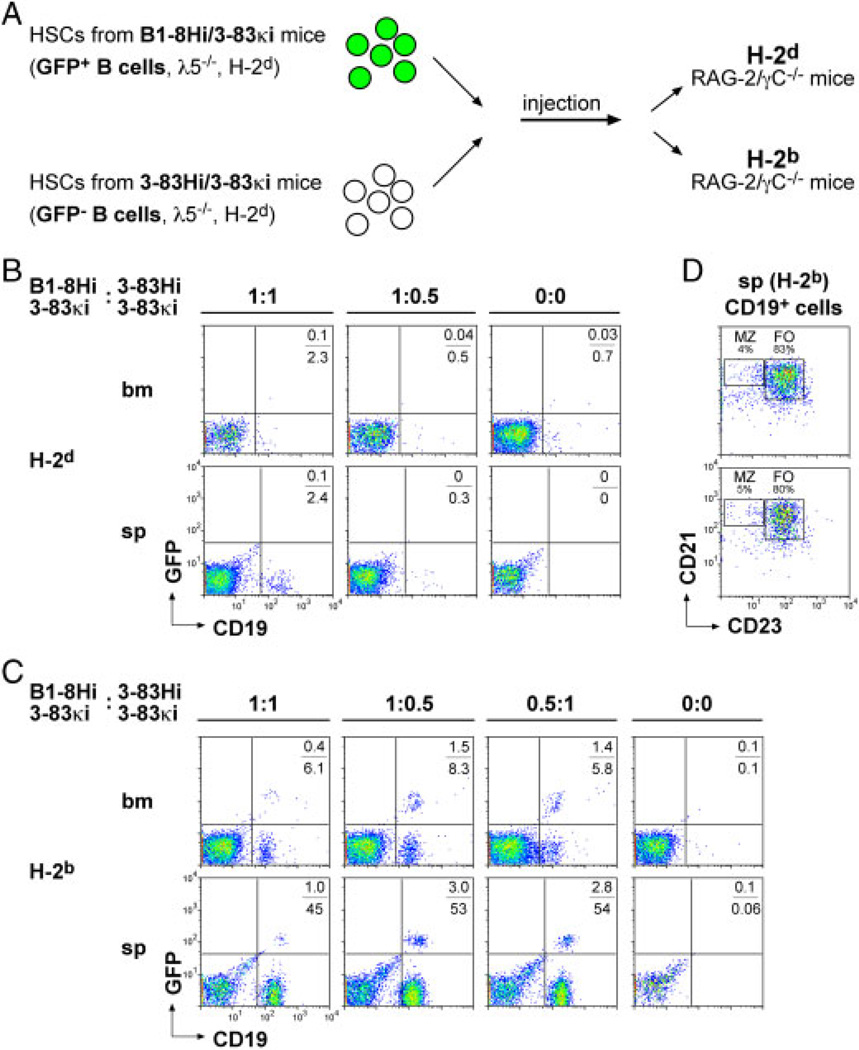
The functional similarity between the pre-BCR and autoreactive BCRs suggests that pre-BCR expression provides immediate autoreactivity to all µHC-positive WT pre-B cells. In the above experiments, developing B cells expressing two different sources of autoreactivity competed with one another: B cells whose autoreactivity is provided by the pre-BCR (WT cells) and those whose autoreactivity is based on the 3–83Hi/3–83κi BCR with its cognate antigen. To assess the specific contribution of 3–83Hi/3–83κi BCR expression in the presence or absence of auto-antigen on B-cell development, we investigated the development of B cells expressing the 3–83Hi/3–83κi BCR in comparison to B cells expressing an unrelated non-autoreactive BCR. Thus, the 3–83Hi/3–83κi HSCs were mixed prior to injection with HSCs from mice expressing the 3–83κi LC together with the HC knock-in B1–8Hi to generate an unrelated BCR (B1–8Hi/3–83κi) [13]. The donor mice, 3–83Hi/3–83κi or B1–8Hi/3–83κi, were λ5-deficient and since both were of the same genetic background (H-2d), the only difference between the injected cells is the HC of the BCR (Fig. 3A). The HSC mixtures were injected into Rag-2/γC−/− mice having different backgrounds and B-cell development was analyzed 5wk after injection. The results show that, on the H-2d background lacking the auto-antigen, neither of the injected HSC populations was able to initiate efficient B-cell development (Fig. 3B). This is most likely due to the λ5-deficiency. On the H-2b background, in contrast, elevated numbers of 3–83Hi/3–83κi B cells were detected suggesting that 3–83Hi/3–83κi B cells developed efficiently in the presence of the cognate auto-antigen (Fig. 3C). Previous reports showed that autoreactive B cells develop mainly into marginal zone B cells [19]. However, analysis of CD21 and CD23 expression revealed that the majority of cells were follicular B cells, suggesting normal development of 3–83Hi/3–83κi B cells on the H-2b background (Figs. 3D, S1B). B1–8Hi/3–83κi/GFP B cells showed slightly improved development on the H-2b background as compared with the H-2d background where almost no GFP-positive cells could be detected (Fig. 3B and C). It is not clear whether this effect was due to the different backgrounds or whether the efficient development of the 3–83Hi/3–83κi B cells on the H-2b background might have improved the generation of the co-injected B1–8Hi/3–83κi B cells.
Figure 3.
Autoreactivity is required for efficient generation of B cells. (A) Schematic illustration of HSC transfer into immune-deficient mice. GFP expression was used to track B cells from expressing the non-autoreactive BCR containing the B1–8 HC. (B, C) Flow cytometric analysis of bone marrow (bm) and splenic (sp) cells 5wk after HSC transfer. The background of the recipient Rag-2/γC−/− mice is indicated. As in Fig. 2, different ratios (1:1, 1:0.5, 0.5:1) of HSCs or PBS were injected. (D) Flow cytometric analysis of CD19+ splenic B cells from two different mice on H-2b background. Cells were stained with anti-CD21 versus anti-CD23. Percentages of follicular (FO) and marginal zone (MZ) B cells are indicated. A total of 12 mice were analyzed (2 mice per HSC ratio on two different backgrounds).
To rule out any effect of the mb1-GFP knock-in that we used to identify B1–8Hi/3–83κi B cells, we repeated the reconstitution experiments using mb1-GFP 3–83Igi mice and found no impact of the mb1-GFP knock-in (Fig. S2A–C). Self-reactive B cells can change their specificity by receptor editing [20]. Thus, we analyzed the B-cell repertoire from mice of different backgrounds using the anti-idiotype 54.1 antibody and found that the majority of CD19+ cells did not express the 3–83BCR, suggesting efficient receptor editing of self-reactive B cells on the H-2b background (Fig. S2D).
Together, the present data show that recognition of self-antigens at an early stage of development promotes positive selection and efficient generation of B cells. Thus, the pre-BCR appears to act as an invariantly autoreactive receptor [7, 14], whose activity generates the required signals in developing B cells that express a µHC to continue development. Therefore, the association of any µHC protein with the inherently autoreactive germ line-encoded surrogate LC may enable B cells to develop properly. Although a contribution of the HC to the autoreactivity of a given pre-BCR is conceivable, this may argue against selection of particular HCs at the pro-/pre-B stages of development [21, 22].
In the absence of pre-BCR expression, only those B cells that express an autoreactive BCR may receive the signals required for survival and further development [23]. In agreement with this, pre-BCR-deficient early B cells expressing the 3–83 BCR showed efficient B-cell development only on the H-2b background containing the specific auto-antigen. Importantly, our data are in accordance with the previous work using autoreactive BCRs or antibody-mediated crosslinking of antigen receptor signaling subunits in pre-BCR-deficient mice [24, 25].
Expression of the autoreactive 3–83 BCR by conventional transgenes blocked B-cell development but did not result in extended expansion of autoreactive B cells in the bone marrow [6, 26]. Presumably, this was due to the fact that transgenic autoreactive BCRs were not expressed from their physiological loci and therefore could not be efficiently removed by the recombination machinery. Similarly, transgenic expression of the surrogate LC blocked B-cell development but did not lead to increased pre-B cell numbers in the transgenic animals [27]. In contrast, our approach using site-specific knock-ins for autoreactive BCRs leaves these regulatory mechanisms mostly unaffected and allows a better assessment of the role of self-recognition in B cells. Thus, our data support a view in which self-reactive immature B cells do not undergo rapid apoptosis, at least as long as they have the ability to change their specificities by receptor editing.
Presumably, the signals generated by the pre-BCR or autoreactive BCRs initiate a series of cell divisions leading to a significant increase in cell number. However, although essential, these signals are most likely not sufficient for efficient generation of early B cells, as their survival and proliferation is also regulated by growth factors available in the microenvironment. For instance, IL-7 is essential for the generation of murine pre-B cells and the IL-7 receptor synergizes with the pre-BCR to activate pre-B cell cycling [28, 29]. This dual regulation of early B-cell generation might be important to prevent an uncontrolled proliferation of pre-B or autoreactive B cells, while allowing a certain magnitude of cell cycling, which is followed by the rearrangement of the LC genes. Thus, regulating the concentration of growth factors in the microenvironment or altering the responsiveness of developing B cells to these factors seems to control the switch from proliferation to differentiation (i.e. LC gene rearrangement) in response to pre-BCR or autoreactive BCR signaling. Conversely, combining autoreactive BCRs with elevated expression of growth factors such as IL-7 might lead to lymphoproliferative and/or autoimmune diseases as suggested by transgenic over-expression of IL-7 [30]. It would be interesting to test whether the BCRs of these immature B-cell lymphomas possess increased autoreactivity and whether this is involved in the increased lymphoproliferation. Altogether, understanding the positive role of autoreactivity in precursor B-cell proliferation not only highlights the importance of pre-BCR expression for early B-cell selection but might also help to explain the molecular mechanisms that underlie the development of autoimmune and lymphoproliferative diseases.
Concluding remarks
Our study demonstrates the importance of autoreactivity for proper B-cell development with the pre-BCR being an invariantly autoreactive receptor. In the presence of a strongly recognized antigen the self-reactivity of the pre-BCR can be substituted by an autoreactive BCR to allow efficient generation of B cells. Thus, it is conceivable that at the early immature B-cell stage, cells bearing an autoreactive BCR may continue to proliferate and to recombine their LCs just as their pre-B cell predecessors do. After having changed their autoreactive specificity by receptor editing, such BCRs may get stably expressed on the surface of immature B cells, which then proceed in development. Our results are reminiscent of a hypothesis published by Niels Jerne in the very first issue of this journal 40 years ago, in which he proposed the selection of escape mutants through the initial expansion and subsequent negative selection of progenitor cells expressing germ line encoded autoreactive receptors as a mechanism of somatic antibody diversification [31].
Materials and methods
Mice
Mb1-lox-GFP mice [18], λ5−/− mice [10], 3–83Igi mice carrying the pre-rearranged 3–83Hi/33–83κi Ig gene segments [14] and mice carrying the B1–8Hi/3–83κi Ig gene segments [15] were used in this study. All mice used for the generation of HSCs were backcrossed on H-2d background. Rag-2/λC−/− mice [17], either on Balb/C or BL/6 background, were used as recipient mice for adoptive transfer experiments. 7–10 wk-old mice were injected with 300 µL of a 10 mg/mL 5-Fluor-Uracil stock solution (Sigma-Aldrich). After 3 days, HSCs were isolated from the bone marrow. After 10 days in culture, 1 × 105 cells of two different HSC populations were injected into Rag-2/γC−/− mice expressing either H-2Kd or H-2Kb. Mice were analyzed 4–5 wk after HSC transfer. Animal experiments were done in compliance with the guidelines of German law and the Max-Planck-Institute of Immunobiology and Epigenetics.
Culture conditions
HSCs were grown in Iscove’s medium (Biochrom) supplemented with 2% of heat inactivated FCS (PAN Biotech), 10mM l-glutamine, 100U/mL penicillin, 100U/mL streptomycin (GIBCO), 50mM 2-mercaptoethanol, 0.03% primatone (Sigma-Aldrich), 4.2mg/mL insulin (Sigma-Aldrich), IL-6, IL-3 and c-kit-ligand.
Flow cytometry
The expression of H-2d and H-2b was determined by flow cytometry using the specific monoclonal antibodies H-2Dd-PE and H-2Kb-FITC (BD). Cells were stained with anti-B220/CD45R-PerCP (RA3-6B2, BD), anti-CD43-PE (S7, BD), anti-CD19-PE/-PerCP (1D3, BD), anti-CD21-APC (7G6, BD), anti-CD23-PE/biotin (B3B4, BD/PharMingen), anti-IgM-Cy5 (Jackson Immunoresearch) and anti-idiotype 54.1 (kindly provided by D. Nemazee). Flow cytometric analysis was performed with FACS-Calibur (BD).
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 4 software using Student’s t-test as the statistical hypothesis test.
Supplementary Material
Acknowledgements
The authors thank U. Stauffer, N. Joswig and C. Johner for mouse work and further assistance. They thank E. Hobeika for the mb1-lox-GFP mice, P. Nielsen, D. Nemazee and M. Reth for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (SFB620 and SFB746).
Abbreviations
- HC
Ig heavy chain
- LC
Ig light chain
- pre-BCR
precursor-BCR
Footnotes
Supporting Information available online
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Schlissel MS. Regulating antigen-receptor gene assembly. Nat. Rev. Immunol. 2003;3:890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 2.Reth M. Antigen receptors on B lymphocytes. Annu. Rev. Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 3.Nemazee D, Weigert M. Revising B cell receptors. Exp. Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 6.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 7.Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, Wardemann H, Jumaa H. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat. Rev. Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 9.Burrows PD, Stephan RP, Wang YH, Lassoued K, Zhang Z, Cooper MD. The transient expression of pre-B cell receptors governs B cell development. Semin. Immunol. 2002;14:343–349. doi: 10.1016/s1044-5323(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Mundt C, Licence S, Melchers F, Martensson IL. VpreB1/VpreB2/lambda 5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. Immunol. 2002;168:6286–6293. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- 12.Ubelhart R, Bach MP, Eschbach C, Wossning T, Reth M, Jumaa H. N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat. Immunol. 2010;11:759–765. doi: 10.1038/ni.1903. [DOI] [PubMed] [Google Scholar]
- 13.Pelanda R, Schaal S, Torres RM, Rajewsky K. A prematurely expressed Ig(kappa) transgene, but not V(kappa)J(kappa) gene segment targeted into the Ig(kappa) locus, can rescue B cell development in lambda5-deficient mice. Immunity. 1996;5:229–239. doi: 10.1016/s1074-7613(00)80318-0. [DOI] [PubMed] [Google Scholar]
- 14.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 15.Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 16.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. Exp. Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colucci F, Soudais C, Rosmaraki E, Vanes L, Tybulewicz VL, Di Santo JP. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl protooncogene in murine NK cell differentiation. J. Immunol. 1999;162:2761–2765. [PubMed] [Google Scholar]
- 18.Pelanda R, Hobeika E, Kurokawa T, Zhang Y, Kuppig S, Reth M. Cre recombinase-controlled expression of the mb-1 allele. Genesis. 2002;32:154–157. doi: 10.1002/gene.10070. [DOI] [PubMed] [Google Scholar]
- 19.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker DJ, Boyle NE, Klinman NR. Predominance of nonproductive rearrangements of VH81X gene segments evidences a dependence of B cell clonal maturation on the structure of nascent H chains. J. Immunol. 1991;147:1406–1411. [PubMed] [Google Scholar]
- 22.Ye J, McCray SK, Clarke SH. The transition of pre-BI to pre-BII cells is dependent on the VH structure of the mu/surrogate L chain receptor. EMBO. J. 1996;15:1524–1533. [PMC free article] [PubMed] [Google Scholar]
- 23.Keenan RA, De Riva A, Corleis B, Hepburn L, Licence S, Winkler TH, Martensson IL. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 24.Papavasiliou F, Jankovic M, Nussenzweig MC. Surrogate or conventional light chains are required for membrane immunoglobulin mu to activate the precursor B cell transition. J. Exp. Med. 1996;184:2025–2030. doi: 10.1084/jem.184.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata K, Nakamura T, Kitamura F, Kuramochi S, Taki S, Campbell KS, Karasuyama H. The Ig alpha/Igbeta heterodimer on mu-negative proB cells is competent for transducing signals to induce early B cell differentiation. Immunity. 1997;7:559–570. doi: 10.1016/s1074-7613(00)80377-5. [DOI] [PubMed] [Google Scholar]
- 26.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fokko van Loo P, Dingjan GM, Maas A, Hendriks RW. Surrogate-light-chain silencing is not critical for the limitation of pre-B cell expansion but is for the termination of constitutive signaling. Immunity. 2007;27:468–480. doi: 10.1016/j.immuni.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 29.Wei C, Zeff R, Goldschneider I. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. J. Immunol. 2000;164:1961–1970. doi: 10.4049/jimmunol.164.4.1961. [DOI] [PubMed] [Google Scholar]
- 30.Mertsching E, Grawunder U, Meyer V, Rolink T, Ceredig R. Phenotypic and functional analysis of B lymphopoiesis in interleukin-7-transgenic mice: expansion of pro/pre-B cell number and persistence of B lymphocyte development in lymph nodes and spleen. Eur. J. Immunol. 1996;26:28–33. doi: 10.1002/eji.1830260105. [DOI] [PubMed] [Google Scholar]
- 31.Jerne NK. The somatic generation of immune recognition. Eur. J. Immunol. 1971;1:1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.