Abstract
Toll-like receptor 4 (TLR4) and its coreceptor, myeloid differentiation factor-2 (MD-2), are key in recognition of lipopolysaccharide (LPS) and activation of proinflammatory pathways. Here we tested the hypothesis that TLR4 and its coreceptor MD-2 play a central role in nonalcoholic steatohepatitis (NASH) and liver fibrosis in nonalcoholic fatty liver disease. Mice of control genotypes and those deficient in MD-2 or TLR4 [knockout (KO)] received methionine choline-deficient (MCD) or methionine choline-supplemented (MCS) diet. In mice of control genotypes, MCD diet resulted in NASH, liver triglycerides accumulation, and increased thiobarbituric acid reactive substances, a marker of lipid peroxidation, compared with MCS diet. These features of NASH were significantly attenuated in MD-2 KO and TLR4 KO mice. Serum alanine aminotransferase, an indicator of liver injury, was increased in MCD diet-fed genotype controls but was attenuated in MD-2 KO and TLR4 KO mice. Inflammatory activation, indicated by serum TNF-α and nictoinamide adenine dinucleotide phosphate oxidase complex mRNA expression and activation, was significantly lower in MCD diet-fed MD-2 KO and TLR4 KO compared with corresponding genotype control mice. Markers of liver fibrosis [collagen by Sirius red and α-smooth muscle actin (SMA) staining, procollagen-I, transforming growth factor-β1, α-SMA, matrix metalloproteinase-2, and tissue inhibitor of matrix metalloproteinase-1 mRNA] were attenuated in MD-2 and TLR4 KO compared with their control genotype counterparts. In conclusion, our results demonstrate a novel, critical role for LPS recognition complex, including MD-2 and TLR4, through NADPH activation in liver steatosis, and fibrosis in a NASH model in mice.
Keywords: endotoxin, fatty liver, inflammation, nictoinamide adenine dinucleotide phosphate, α-smooth muscle actin
nonalcoholic fatty liver disease (NAFLD) is an increasingly common cause of liver disease that can progress to nonalcoholic steatohepatitis (NASH) and culminate in end-stage liver disease, featuring liver damage with fibrosis and cirrhosis (1, 10, 36). The pathogenesis of NAFLD/NASH is poorly understood. Increased hepatocyte fat deposition, apoptosis, mitochondrial dysfunction, abnormal peroxisome and microsome functions, and insulin resistance is associated with steatosis and have been observed during NAFLD (6, 32, 36, 44). More recently, a key role for inflammation was identified in NAFLD/NASH (6, 14, 28, 32, 38). Inflammation is imposed on steatosis by recruitment and activation of inflammatory cells in the liver, which contributes to steatohepatitis, a hallmark of clinically progressive NAFLD to NASH (1, 10, 14). Later, liver resident macrophages, Kupffer cells, and stellate cells induce liver remodeling with extensive fibrosis and liver cirrhosis, which dictates the final stages of the NAFLD/NASH (1, 10, 14). While the sequence of events, including fat accumulation, inflammation, and fibrosis, is not clearly delineated, inflammation seems to play a leading role in NAFLD/NASH progression (1, 10, 14, 36). Activation of inflammatory cascades can be induced by a variety of danger signals; recent studies have suggested that bacterial overgrowth and endotoxemia play a role in the pathogenesis of NAFLD/NASH (5, 11, 22, 31). The importance of increased circulating and portal blood levels of lipopolysaccharide (LPS) has been established in the pathogenesis of alcoholic liver disease (27), a disease that is histologically indistinguishable from NAFLD (36).
Pattern recognition toll-like receptor 4 (TLR4) is expressed on many cell types in the liver, including Kupffer cells, stellate cells, and hepatocytes (37). Initially discovered as a receptor for LPS, a cell wall component of gram-negative bacteria also referred to as endotoxin, TLR4 is implicated in recognition of heparan sulfate, fibrinogen, hyaluronan, high-mobility group box 1 (HMGB1), and potentially several other danger signals (18, 19). The diversity of TLR4 ligands, and the fact that the mediators that drive the systemic inflammatory response in the setting of sepsis or sterile tissue injury are strikingly similar (15), suggests that TLR4 and its coreceptors may play a key role in persistent inflammatory diseases for which the clear pathogen etiology has not been well established, including the NAFLD/NASH.
At least for LPS recognition, TLR4 forms a complex with its coreceptor, myeloid differentiation factor-2 (MD-2) (18, 21). MD-2 is a glycoprotein that binds to both LPS and the extracellular domain of TLR4 (2, 40, 41). Ligand engagement of the TLR4-MD-2 complex results in activation of a plethora of downstream signaling pathways that generate a variety of cellular responses. TLR4/MD-2-mediated activation of MAP kinases and NF-κB leads to activation of the proinflammatory cascade and oxidative stress through the components of the NADPH complex (25). Activation of the inflammatory cascade and induction of reactive oxygen species are particularly relevant to NAFLD/NASH, since inflammatory cytokines, such as TNF-α and oxidative stress, through NF-κB activation, contribute not only to liver inflammation but also to insulin resistance, a hallmark of NAFLD (20, 44).
Here, we investigated the hypothesis that MD-2 and TLR4 receptor complex play a role in the development of NAFLD/NASH. Using MD-2 and TLR4 KO mice and methionine choline restriction as a tool to evaluate mechanisms of inflammation and liver damage in mice (28, 44), we found a mechanistic role for MD-2 and TLR4 receptor complex in several steps of the pathogenesis of NAFLD/NASH, including liver inflammation, steatosis, and fibrosis.
MATERIALS AND METHODS
Animals and experimental protocol.
Three-month-old female mice were employed. MD-2-deficient or TLR4-deficient animals [knockout (KO)] (a kind gift from Dr. K. Miyake from Tokyo, Japan) were backcrossed with C57BL/6 and genotyped by PCR of tail DNA. MD2 KO mice were tested for microsatellite (99% identical with C57Bl/6J), and littermate controls were used (n = 6–8 mice/group). For TLR4 KO mice, all tests were initially performed using mice backcrossed (x) with C57Bl/6 at generation x = 6 (tested for microsatellite and showed >96% similarity with C57Bl/6J) and later confirmed using mice at backcross generation x = 8; given the similarity of the results, the data were compiled, and overall there were n = 14–16/group. Testing shown in Figs. 1C, 1D, and 2F was performed on TLR4 KO mice at generation eight (x = 8) after backcrossing. C57Bl6J mice were used as controls for all TLR4 KO mice, based on their genetic proximity to the C57Bl6 strain and in agreement with recommendations for genetic background use from Jackson Laboratory (43).
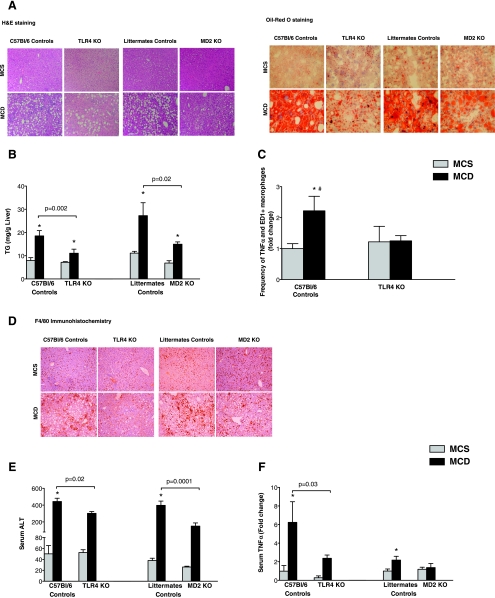
Fig. 1.
Deficiency in myeloid differentiation factor-2 (MD-2) and toll-like receptor 4 (TLR4), members of the lipopolysaccharide (LPS) recognition complex, protects from methionine choline-deficient (MCD) diet-induced liver injury. Mice of control genotypes and those deficient [knockout (KO)] in TLR4 (TLR4 KO) and MD-2 (MD-2 KO) were fed MCD or methionine choline-supplemented (MCS) diets for 8 wk. Liver tissue was subjected to hematoxylin and eosin (H&E) (A, left) and OilRed O (A, right) staining and F4/80 immunohistochemistry (D); one representative slide from n = 6–16 mice/group is shown. Liver triglycerides (TG) (B) and serum alanine aminotransferase (ALT) (E) levels were determined as described in materials and methods. Serum TNF-α level (F) was determined using the Multiplex assay. Liver macrophages were isolated and stained for TNF-α and the macrophage marker CD68 (ED-1) after cell permeabilization; fluorescence-activated cell sorter analysis of changes in frequency of TNF-α/CD68 double-positive cells compared with the MCS-fed genotype control is shown (C). *P < 0.05 compared with the corresponding MCS group (B, C, E, and F). #P < 0.05, MCD WT compared with MCD TLR4 KO.
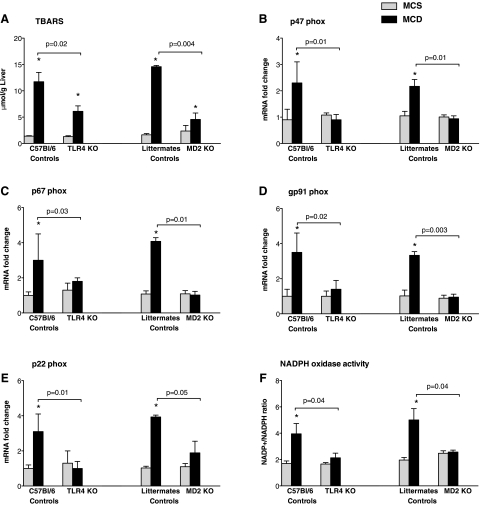
Fig. 2.
Deficiency in LPS recognition complex prevents MCD diet-induced upregulation in the expression of NADPH complex and protects from lipid peroxidation. Mice of genotype control, TLR4 KO, and MD-2 KO were fed MCD or MCS diets for 8 wk. Liver thiobarbituric acid reactive substances (TBARS) levels (A) were analyzed as described in materials and methods. Expression of liver p47phox (B), p67phox (C), gp91phox (D), and p22phox (E) was quantified by quantitative PCR (qPCR) using specific primers and normalization against the housekeeping gene 18S. NADPH oxidase activity was determined by measuring NADP+-to-NADPH ratios as described in materials and methods (F). *P < 0.05 compared with the corresponding MCS group.
This study was approved by the Institutional Animal Use and Care Committee at the University of Massachusetts Medical School. All animals were cared for in accordance with the Institutional Animal Care and Use Committee regulations at the University of Massachusetts Medical School. The mice were fed a methionine choline-deficient (MCD) diet or methionine choline-supplemented (MCS) diet; the latter control diet was identical in composition to the MCD diet but was supplemented with l-methionine (1.7 g/kg) and choline bitartrate (14.48 g/kg) (Dyets, Bethlehem, PA) for 8 wk; all mice had unrestricted access to water.
Preparation of serum and tissue.
Serum was separated from whole blood and frozen at −80°C. Livers were rapidly excised, and separate aliquots were: 1) snap-frozen in liquid nitrogen and stored at −80°C, 2) stored in RNA stabilization reagent (RNAlater; Qiagen, Hilden, Germany), and 3) fixed in 10% neutral-buffered formalin or frozen in optimum cutting temperature (OCT) media for histology.
Histopathological analysis.
Sections of formalin-fixed, paraffin-embedded livers were stained with: 1) hematoxylin and eosin to assess for histological features of steatohepatitis or 2) picrosirius red stain to evaluate for hepatic collagen deposition. The OilRed O tissue staining method on OCT-embedded frozen sections was used to quantify the steatosis. Liver sections were also subject to immunohistochemical staining for macrophages with monoclonal F4/80 antibody (Abcam, Cambridge, MA) and α-smooth muscle actin (α-SMA) with a monoclonal antibody against α-SMA (Lab Vision, Fremont, CA) using a labeled streptavidin-biotin immunoenzymatic antigen detection system (UltraVision Mouse Tissue Detection System Anti-Mouse-HRP/DAB; Lab Vision).
Biochemical assays.
Serum alanine aminotransferase (ALT) was determined using a kinetic method [ALT Liquid, Advanced Diagnostics (South Plainfield, NJ) and D-TEK (Bensalem, PA)]; liver thiobarbituric acid reactive substances (TBARS) and triglycerides were measured as previously described, using commercial kits (39). Serum endotoxin was quantified using LAL assay (detection limit 0.1 EU/ml; Cambrex, Walkersville, MD).
Isolation of liver mononuclear cells and flow cytometry analysis.
Animals received anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg); the livers were perfused with Hank's balanced saline solution (HBSS) followed by in vivo digestion with 0.33 mg/ml Liberase RI Enzyme (F. Hoffmann-La Roche, Basel, Switzerland) in HBSS. The liver mononuclear cells (LMNCs) were purified from whole liver cell suspension obtained after tissue disruption using centrifugation at slow speed (500 g) and subsequent isolation in Percoll 40/70 gradient density at 800 g; LMNC were harvested from the gradient interface. The cells were further washed in saline supplemented with 2% FBS, stimulated with a cocktail of PMA (50 ng/ml), ionomycin (1 μg/ml), and brefeldin A (10 μg/ml) in RPMI 1640 + 10% FBS for 4 h, and stained for surface CD68 and intracellular TNF-α using specific fluorescent-labeled antibodies and CytoFix/CytoPerm Kit (BD Bioscience, San Jose, CA). The cells were gated by size and granularity, and their fluorescence was analyzed using the LSR flow cytometer.
Cytokine measurements.
Serum TNF-α level was determined using the Pierce Multiplex Cytokine Array (Pierce, Woburn, MA).
mRNA analysis.
Total RNA extraction from liver tissue and mRNA quantification using SYBR Green-based real-time quantitative polymerase chain reaction was performed as previously described (39). All specific mRNA levels were normalized against the housekeeping gene, 18S, in the same sample. The specific PCR primer sequences for target genes 18S, p22phox, p47phox, p67phox, and gp91phox have been published previously (27); additional genes studied here are listed in Table 1.
Table 1.
PCR primers
| Target Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| α-SMA | gtc cca gac atc agg gag taa | tcg gat act tca gcg tca gga |
| Procollagen-I α1 | gct cct ctt agg ggc cac t | cca cgt ctc acc att ggg g |
| TGF-β1 | att cct ggc gtt acc ttg | ctg tat tcc gtc tcc ttg gtt |
| MMP-2 | ttt gct cgg gcc tta aaa gta t | cca tca aac ggg tat cca tct c |
| TIMP-1 | ctt ggt tcc ctg gcg tac tc | acc tga tcc gtc cac aaa cag |
α-SMA, α−smooth muscle actin; TGF-β1, transforming growth factor-β1; MMP-2, matrix metalloproeinase-2; TIMP-1, tissue inhibitor of matrix metalloproteinase-1.
NADPH oxidase assay.
Whole liver tissue extract were performed as we previously described (39); protein content was quantified using Bio-Rad Protein Assay (500–0006; Bio-Rad Laboratories, Hercules, CA). NADP+/NADPH concentrations from tissue extracts with comparable protein amounts were determined using the EnzyChrom NADP+/NADPH assay kit (ECNP-100) (BioAssay Systems, Hayward, CA), as recommended by the manufacturer.
Statistical analysis.
Statistical significance was determined using the nonparametric Kruskal-Wallis and Mann-Whitney tests; for ALT assays, given the usage of two distinct kits for analysis in different experiments, the statistical significance of the data was confirmed using the nonparametric Wilcoxon test. Data are presented as means ± SE and were considered statistically significant at a P value <0.05.
RESULTS
MD-2 or TLR4 deficiency protects from MCD diet-induced liver fat deposition and inflammation.
Inflammation is a major component of NASH (1, 10, 36). In the related condition of alcoholic steatohepatitis (ASH), endotoxin has been shown to contribute to activation of the inflammatory cascade leading to liver damage (27). MD-2 and TLR4 complex is the major receptor for endotoxin (18). Given the common pathophysiological features of ASH and NASH, we aimed to identify the role of MD-2-TLR4 complex in an experimental model of NASH using mice deficient in MD-2 or TLR4 and their genotype control counterparts. Feeding a MCS diet resulted in no signs of hepatic steatosis or inflammation in any of the mice (Fig. 1). In contrast, mice of control genotypes fed a MCD diet for 8 wk developed significant hepatic steatosis; MD-2- and TLR4-deficient mice on MCD diet showed lower liver fat accumulation, identified after OilRed O staining, compared with the mice of control genotypes (Fig. 1A). Consistent with the development of hepatic steatosis, liver triglyceride levels were significantly increased in MCD diet-fed control genotype mice but to a significantly lower extent in MD-2- or TLR4-deficient mice (Fig. 1B). These findings suggested that TLR4-MD-2 complex deficiency is partially protective against MCD-induced liver steatosis.
Feeding of MCD diet leads to accumulation of inflammatory cells into the liver in mice of control genotypes, and to a lesser extent in MD-2 or TLR4 KO mice, as indicated by the increase in content of F 4/80+ cells in the livers of MCD-fed animals compared with MCS diet-fed controls (Fig. 1C). Furthermore, the proportion of TNF-α-producing CD68+ macrophages was increased in MCD-fed compared with MCS-fed genotype controls (Fig. 1D). More importantly, TLR4 deficiency protected from MCD diet-induced accumulation of the TNF-α-producing CD68+ macrophages in the liver (Fig. 1D). A significant increase in serum ALT, suggesting ongoing liver damage, was observed in the MCD diet-fed control genotype mice, and this correlated well with the steatohepatitis; however, the ALT increase was significantly attenuated in MD-2- and TLR4-deficient mice (Fig. 1E). TNF-α has been suggested as a central proinflammatory cytokine that is produced by activated inflammatory cells and mediates insulin resistance and hepatocyte apoptosis in liver disease (7, 38). Consistent with activation of the inflammatory cascade, serum TNF-α level was increased in MCD diet-fed control genotype mice compared with the MCS diet-fed controls (Fig. 1F). In contrast, MCD-induced TNF-α was significantly lower in MD-2- or TLR4-deficient MCD diet-fed mice (Fig. 1F). These data suggested that TLR4-MD-2 complex deficiency is partially protective against MCD-induced liver inflammation and damage.
MD-2 and TLR4 deficiency attenuates oxidative stress.
Increased lipid peroxidation and oxidative stress are key in development of steatosis in NAFLD (20). We identified significantly higher levels of liver TBARS, indicative of lipid peroxidation, in MCD diet- compared with the MCS diet-fed genotype control mice (Fig. 2A). Consistent with our hypothesis that MD-2-TLR4 complex plays a role in NASH, we found significantly reduced induction of TBARS in the livers of MCD diet-fed MD-2- and TLR4-deficient mice (Fig. 2A).
NADPH oxidases play an important role in the generation of reactive oxygen radicals (25, 30). The classic NADPH complex is composed of at least six components, which include two trans-membrane flavocytochrome b components (gp91phox and p22phox) and four cytosolic components (p47phox, p67phox, p40phox, and Rac-1 protein) (30). TLR4-mediated signals are strong inducers of NADPH transcription and functional activity (25). Investigation of NADPH oxidase expression revealed a significant upregulation of the cytoplasmic components of the NADPH oxidase, including p47phox (Fig. 2B) and p67phox (Fig. 2C), in MCD diet-fed animals of control genotypes. The membrane-associated components of the NADPH complex, gp91phox (Fig. 2D) and p22phox (Fig. 2E), were also upregulated at the mRNA level in the livers of MCD diet- compared with the MCS diet-fed mice of control genotypes. Deficiency in MD-2 or TLR4 abrogated the MCD-induced upregulation of all of the NADPH oxidase subunits (Fig. 2, B-E), suggesting that NADPH-mediated oxidative stress is dependent on MD-2 and TLR4 expression in this model. To test for the biological significance of the mRNA increase in the NADPH subunits, we evaluated the NADPH oxidase activity. Consistent with the increased mRNA levels of NAPDH oxidase complex components, NADPH oxidase activity was elevated, as suggested by the increased NADP+-to-NADPH ratio in livers of MCD-fed compared with MCS-fed mice of control genotypes (Fig. 2F). More importantly, we identified that both TLR4 KO and MD-2 KO mice were protected from the MCD diet-induced activation of NADPH oxidase (Fig. 2F). Collectively, these results indicated that MD-2-TLR4 complex-induced signals contribute to liver pathology via NADPH-dependent lipid peroxidation and oxidative stress in the MCD diet-induced NASH model.
MD-2 and TLR4 deficiency protects from NASH-associated liver fibrosis.
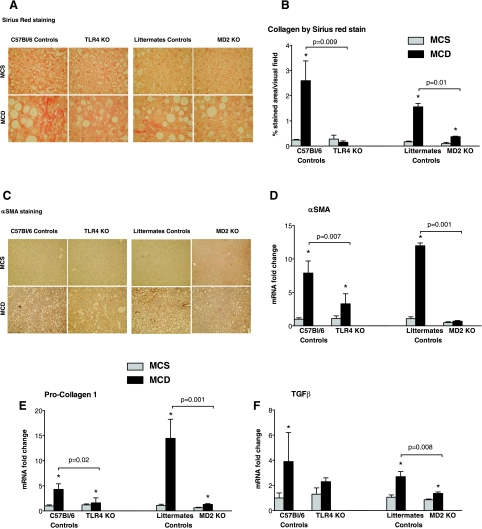
A key clinical challenge in human NASH is its progression to fibrosis and cirrhosis (1, 10, 36). In contrast to livers of MCS diet-fed control genotype animals, Sirius red (Fig. 3, A and B) and α-SMA immunohistochemistry (Fig. 3C) staining revealed that administration of MCD diet resulted in signs of fibrosis (Fig. 3, A-C). On the contrary, we found no substantial Sirius red (Fig. 3, A and B) or α-SMA (Fig. 3C) staining in either MD-2- or TLR4-deficient MCD diet-fed mice. Genes associated with fibrosis, including α-SMA (Fig. 3D), procollagen-1 (Fig. 3E), and transforming growth factor (TGF)-β (Fig. 3F), were significantly upregulated at the RNA level in MCD diet-fed control genotypes, but not or less extent in MD-2- and TLR4-deficient mice.
Fig. 3.
Deficiency in TLR4 and MD-2 protects from MCD diet-induced liver fibrosis. The livers of MCD and MCS diet-fed genotype controls and MD-2 KO and TLR4 KO mice were stained with Sirius red (A) or α-smooth muscle actin (α-SMA) immunohistochemistry (C); shown here are representative images from n = 6–16/group. Sirius red positive areas were quantified using Image J software (B). Genes associated with fibrosis, including α-SMA (D), procollagen-1 (E), and transforming growth factor (TGF)-β (F), were quantified by qPCR using specific primers and normalization against the housekeeping gene 18S; data (C-F) are shown as means ± SE from n = 6–16/group; *P < 0.05 compared with the corresponding MCS group.
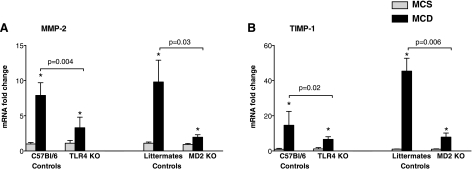
Liver fibrosis involves inflammation-driven tissue remodeling; matrix metalloproteinases (MMP) and their specific tissue inhibitors (TIMPs) closely regulate the metabolism of the extracellular matrix (1, 8, 14). The expression of MMP-2 (Fig. 4A) and TIMP-1 (Fig. 4B) were increased in livers of MCD- compared with MCS diet-fed mice of control genotypes; the induction of these genes was significantly attenuated in the absence of MD-2 or TLR4 expression.
Fig. 4.
Expression of tissue remodeling factors is impaired in mice deficient in MD-2 and TLR4. The livers of MCD and MCS diet-fed genotype controls and MD-2 KO and TLR4 KO mice were analyzed for matrix metalloproteinase (MMP)-2 and tissue inhibitor of metalloproteinase (TIMP)-1 expression by qPCR using specific primers and normalization against the housekeeping gene 18S; data are shown as means ± SE from n = 6–16/group; *P < 0.05 compared with the corresponding MCS group.
DISCUSSION
Diet-induced NASH in mice mimics several features of human NASH, including steatosis, inflammation, and fibrosis (1, 10, 36). In this study, we demonstrate for the first time that deficient integrity of the danger receptor complex, including TLR4 or its coreceptor MD-2, is protective from MCD diet-induced liver steatosis and inflammation and correlates with attenuated liver injury and histological features of NASH. To this extent, our novel data also indicate that the deficiency in MD-2 or TLR4 confers protection from development of liver fibrosis in MCD diet-induced NASH.
To date, several research groups have identified that LPS, in the context of a multihit model, plays a role in development of NAFLD/NASH (10, 22, 29); the details of LPS implication per se are yet to be fully defined. Here we provide novel data indicating that danger sensing via MD-2 and TLR4 is key in the pathogenesis of NASH. Ligand recognition by the TLR4-MD-2 complex, which binds LPS to deliver intracellular signals, occurs as a result of complementary functions of MD-2 and TLR4. Neither MD-2 nor TLR4 alone can account for optimal LPS recognition (2, 40, 41). MD-2 binds LPS, however, it lacks a transmembrane domain and cannot result in intracellular signaling alone (2, 40, 41). The recently discovered crystal structure of the TLR4-MD-2 complex demonstrates the critical role of MD-2 in LPS binding and LPS-induced TLR4 activation resulting in TLR4-MD-2 complex and conformational changes to initiate intracellular signaling through the intracellular domain of TLR4 (16). Our data suggest a major role for TLR4 and MD-2 in liver damage, as indicated by profound attenuation of features of NASH in their absence. The exact ligand(s) of TLR4/MD-2 in NASH is yet to be defined. A candidate ligand is endotoxin, most likely derived from the gut (29). This hypothesis is supported by recent reports in other models of NAFLD and is also consistent with the causal role of gut-derived endotoxin in ASH, which shares many pathological features of NASH (5, 11, 22, 27). We found a moderate but significant increase in serum endotoxin levels in MCD diet-fed mice of control genotypes (data not shown); this observation is similar to that described in the portal circulation of LPS-insensitive C3H/HeJ mice (29).
In evaluation of the role of TLR4-MD-2 complex in the pathogenesis of NASH, there is a need to consider that, while TLR4 recognizes exogenous danger signals, such as LPS, it also can sense multiple endogenous danger signals (19), including, but possibly not limited to, heat shock proteins (3), fibrinogen (35), fibronectin (23), and HMGB1 (26). Our results suggest protection from murine NASH when the recognition of ligands by TLR4-MD-2 complex is impaired; the role of endogenous danger signals in experimental or human NASH is yet to be evaluated.
We identified that MD-2 and TLR4 deficiency is protective in NASH due to interference with inflammation and oxidative stress. The elements of protections included prevention of inflammatory cell infiltration into the liver, diminished proinflammatory cytokine production, impaired upregulation of the liver mRNA levels of all components of the NADPH oxidase complex, and impaired function of the NADPH complex. Our observation of increased expression of the phagocyte-specific NADPH complex and increased NADPH activity in MCD-fed animals of control genotypes and lack of such effects in TLR4 or MD-2 KO animals suggests a communication between TLR4/MD-2 and NADPH oxidase activation in NASH. Several research groups have reported the key role of the proinflammatory effects of Kupffer cells (29) and TLR4 receptor (29) in NASH-associated liver inflammation; our data are in agreement with those reports. Kupffer cells are rich in TLR4-MD-2 receptor complex (37) and are a major source of NADPH in the liver. The critical role of the Kupffer cells p47phox NADPH oxidase component has been reported in alcoholic liver disease (17).
The most important clinical challenge in NASH is the progression to liver fibrosis, which often leads to cirrhosis and liver failure (1, 10, 36). Here, we present our novel observation that MD-2-TLR4 complex plays a central role in induction of fibrosis in the MCD diet-induced NASH model. The current understanding on the pathogenic mechanisms of NASH favors a model in which steatosis, and later steatohepatitis, are induced as a result of fatty acid overload and inflammation, leading to subsequent activation of stellate cells resulting in liver fibrosis (1, 10, 36). The critical step in the generation of liver fibrosis is the activation of stellate cells resulting in α-SMA and collagen deposition (1, 10, 24, 34, 36). Stellate cell activation is induced by multiple insults, including endotoxin, TNF-α, and TGF-β (34). Stellate cells express MD-2 and TLR4 and, thus, can be directly stimulated through the TLR4-MD-2 complex to produce a profibrotic transformation, including expression of α-SMA and deposition of collagen-1 (8, 34, 37). We found increased expression of α-SMA and collagen-1 at the mRNA and protein levels in livers of MCD diet-fed animals of control genotypes that was abrogated in both MD-2-deficient and in TLR4-deficient mice. A recent finding that TLR4-MD-2 fusion protein could prevent LPS-induced stellate cell activation highlights the importance of the TLR4/MD-2-dependent mechanisms of stellate cell activation (33), suggesting that inhibition of TLR4/MD-2 is a potential therapeutic target. It remains to be determined whether the protective effect of deficiency of MD-2 and TLR4 on fibrosis is solely related to the lack of TLR4/MD-2 activation in stellate cells or a combined lack of TLR4 signaling in stellate and Kupffer cells. We identified increased TNF-α, a product of activated Kupffer cells, in MCD diet-induced NASH, which can induce stellate cell activation (6). Importantly, TNF-α levels were significantly attenuated in the absence of MD-2 or TLR4 expression. NADPH oxidase-dependent oxidative stress has been reported to have a crucial role in hepatic stellate cell activation by angiotensin II and leptin (4, 9). Although TLR4/MD-2 deficiency attenuated both the fibrosis and the NADPH oxidase activity, further experiments are needed to prove causality between NADPH oxidase activity and fibrosis in NASH. However, our novel data and the availability of relatively safe and well-established therapeutics to manipulate the NADPH oxidase-dependent oxidative stress brings hope for future NADPH-based therapeutic interventions in steatohepatitis (12).
Our study is based on two distinct models of genetically modified mice: TLR4 KO and MD-2 KO. Although both types of animals exhibit impaired recognition of LPS because of deficient assembly of the recognition complex, we did observe some subtle differences between TLR4 KO and MD-2 KO mice upon developing MCD diet-induced NASH. For example, the extent of liver fibrosis, indicated by the Sirius red positive areas, was more pronounced in MD-2 KO compared with TLR4 KO, etc. The origin of the lack of full overlap between the TLR4- and MD-2-owed extent of protection against NASH likely lies in the final effect of these molecules on ligand recognition and/or downstream signaling events. MD-2 is an important component of LPS recognition; however, it may, or may not, be implicated in the recognition of the entire repertoire of TLR4 ligands. Alternatively, TLR4 with or without MD-2 may signal differently, or TLR4-MD-2 complex receptor may function in two separate modes: one in which full signaling occurs and one limited to MyD88-dependent signaling (13). We had previously reported a critical role of TLRs and the common TLR adaptor, MyD88, in other models of liver inflammation and injury (39); the exact signaling events downstream from TLR4-MD-2 complex in NASH are yet to be fully understood. Nevertheless, it is important to note that both TLR4 KO and MD-2 KO genotypes offered only partial protection against MCD diet-induced NASH, suggesting the possibility that TLR4/MD-2-independent events may be involved in the pathogenesis of NASH.
In conclusion, we found that danger receptor TLR4 and its coreceptor, MD-2, are critical in the development of steatosis, liver damage, inflammation, and fibrosis in the MCD diet-induced NASH in mice. The significant attenuation of steatohepatitis and the protection from fibrosis in the presence of MD-2 or TLR4 deficiency suggest that danger signals provided by MD-2-TLR4 complex play a central role in this model of NASH.
GRANTS
This work was supported by National Institutes of Health Grants 1R01DK-075635 (to G. Szabo) and 1R01AI-51405 (to E. Kurt-Jones), Public Health Service Grant DK-32520, and UMass CFAR Grant 5P30 AI-42845.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. K. Miyake (Division of Infectious Genetics, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan) for providing the KO animals.
Current address for I. Hritz: 2nd Department of Medicine, Semmelweis University, Budapest, Hungary.
REFERENCES
- 1. Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol 17: 863–869, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med 198: 1035–1042, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular hsp70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112: 1383–1394, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 48: 983–992, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Byrne CD. Fatty liver: role of inflammation and fatty acid nutrition. Prostaglandins Leukot Essent Fatty Acids 82: 265–271, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol 103: 1036–1042, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Das SK, Vasudevan DM. Genesis of hepatic fibrosis and its biochemical markers. Scand J Clin Lab Invest 68: 260–269, 2008 [DOI] [PubMed] [Google Scholar]
- 9. De Minicis S, Seki E, Oesterreicher C, Schnable B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology 48: 2016–2026, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis 11: 75–104, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int 28: 1026–1033, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fortuño A, Bidegain J, Robador PA, Hermida J, López-Sagaseta J, Beloqui O, Díez J, Zalba G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertension. Hypertension 54: 744–750, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol 6: 565–570, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Jou J, Choj SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 28: 370–379, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol 83: 546–552, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130: 906–917, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-indiced liver disease. J Clin Invest 106: 867–872, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyake K. Endotoxin recognition molecules, Toll-like receptor 4-MD-2. Semin Immunol 16: 11–16, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 19: 3–10, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Musso G, Gambino R, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanism and therapeutic opportunities. Antioxid Redox Signal In press [DOI] [PubMed] [Google Scholar]
- 21. Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3: 667–672, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Nolan JP. The role of endotoxin in liver injury. Gastroenterology 69: 1346–1356, 1975 [PubMed] [Google Scholar]
- 23. Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka S, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 37: 1043–1055, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 45: 1108–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 47: 571–579, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol 30: 315–327, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Ruiz AG, Casafont F, Crespo J, Cayón A, Mayorga M, Estebanez A, Fernadez-Escalante JC, Pons-Romero F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg 17: 1374–1380, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 43: 163–167, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Schnabl B, Brandl K, Fink M, Gross P, Taura K, Gäbele E, Hellerbrand C, Falk W. A TLR4/MD-2 fusion protein inhibits LPS-induced pro-inflammatory signaling in hepatic stellate cells. Biochem Biophys Res Commun 375: 210–214, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13: 1324–1332, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4 J. Immunol 167: 2887–2894, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Syn WK, Teaberry V, Choi SS, Diehl AM. Similarities and differences in the pathogenesis of alcoholic and non-alcoholic steatohepatitis. Semin Liver Dis 29: 200–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology 44: 287–298, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986–1998, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Velayudham A, Hritz I, Dolganiuc A, Mandrekar P, Kurt-Jones E, Szabo G. Critical role of toll-like receptors and the common TLR adaptor, MyD88, in induction of granulomas and liver injury. J Hepatol 45: 813–824, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Visintin A, Halmen KA, Khan N, Monks BG, Golenbock DT, Lien E. MD-2 expression is not required for cell surface targeting of Toll-like receptor 4 (TLR4). J Leukoc Biol 80: 1584–1592, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Visintin A, Iliev DB, Monks BG, Halmen KA, Golenbock DT. MD-2. Immunobiology 211: 437–447, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis 27: 44–54, 2007 [DOI] [PubMed] [Google Scholar]
- 43. www.informatics.jax.org. Access www.informatics.jax.org/silver for Silver LM. 1995. Mouse Genetics: Concepts, and Applications, Oxford University Press; and http://jaxmice.jax.org/manual/index.html for Resource: The Importance of Genetic Background.
- 44. Yu J, Ip E, DelaPeña A, Hou JY, Sesha J, Pera N, Hall P, Kirsch R, Leclercq I, Farrell GC. COX-2 induction in mice with experimental nutritional steatohepatitis: role as pro-inflammatory mediator. Hepatology 43: 826–836, 2006 [DOI] [PubMed] [Google Scholar]