Abstract
The colonic mucus layer serves as an important barrier and prevents colonic bacteria from invading the mucosa and cause inflammation. The regulation of colonic mucus secretion is poorly understood. The aim of this study was to investigate the role of the mucus barrier in induction of colitis. Furthermore, regulation of mucus secretion by luminal bacterial products was studied. The colon of anesthetized Muc2−/−, Muc1−/−, wild-type (wt), and germ-free mice was exteriorized, the mucosal surface was visualized, and mucus thickness was measured with micropipettes. Colitis was induced by DSS (dextran sodium sulfate, 3%, in drinking water), and disease activity index (DAI) was assessed daily. The colonic mucosa of germ-free and conventionally housed mice was exposed to the bacterial products LPS (lipopolysaccharide) and PGN (peptidoglycan). After DSS induction of colitis, the thickness of the firmly adherent mucus layer was significantly thinner after 5 days and onward, which paralleled the increment of DAI. Muc2−/− mice, which lacked firmly adherent mucus, were predisposed to colitis, whereas Muc1−/− mice were protected with significantly lower DAI by DSS compared with wt mice. The mucus barrier increased in Muc1−/− mice in response to DSS, whereas significantly fewer T cells were recruited to the inflamed colon. Mice housed under germ-free conditions had an extremely thin adherent colonic mucus layer, but when exposed to bacterial products (PGN or LPS) the thickness of the adherent mucus layer was quickly restored to levels observed in conventionally housed mice. This study demonstrates a correlation between decreasing mucus barrier and increasing clinical symptoms during onset of colitis. Mice lacking colonic mucus (Muc2−/−) were hypersensitive to DSS-induced colitis, whereas Muc1−/− were protected, probably through the ability to increase the mucus barrier but also by decreased T cell recruitment to the afflicted site. Furthermore, the ability of bacteria to regulate the thickness of the colonic mucus was demonstrated.
Keywords: mucin, dextran sulfate, colon, germ-free, lipopolysaccharide, peptidoglycan, ulcerative colitis
the colon is inhabited by a vast number of bacteria, which outnumber the human cells by 10 times and consists of more than 500 different species (16). Under normal conditions, these bacteria reside within the colonic lumen in a symbiotic relationship with the host and are believed to provide the colonocytes with nutrients as they are converting undigested carbohydrates to short-chain fatty acids that can be used by the host as energy supply (5). How this enormous bacterial load can be controlled without overt immune responses from the innate and adaptive systems of the host is not well understood.
The colonic epithelium is covered by a continuous mucus layer, which can be divided into a loose, luminal mucus and an underlying mucus layer that adheres firmly to the mucosa (3). The major components of this mucus, the gel-forming mucins, are a part of the innate immunity and are well preserved during evolution (15). Fifteen different mucin genes have so far been described, and Muc1, 2, 3, 12, 13, and 17 are found in the colon (14). Of these, only Muc2 codes for a gel-forming mucin (10), whereas the others are membrane-bound with transmembrane regions and are thus most likely involved in cell signaling (14). Recent results show that a colonic mucus layer formed by Muc2 mucins creates a functional barrier between the host and luminal bacterial microbiota (13). By using immunohistochemistry, bacteria were found in the loosely adherent mucus layer whereas few or no bacteria were detected in the firmly adherent mucus layer closest to the epithelium (13). The physiological relevance of this barrier is clear from studies in Muc2-deficient mice, which spontaneously develop inflammation and are much more susceptible to infection by pathogens (7, 29). In addition to the barrier function, the mucus layer also functions as a shelter that is required to retain a bacterial colonization within the colon and prevents the bacteria from constantly being removed by peristalsis. Thus colonic mucus has dual functions, being a functional barrier to avoid contact of bacteria to the epithelium and simultaneously enabling bacteria persistence within the colon.
Inflammatory bowel disease is a group of chronic inflammatory conditions with unknown pathogenesis. The involvement of bacteria in the pathology of these conditions is, however, undisputed, and antibiotics are commonly used in treatment of inflammatory bowel disease. In most animal models, induction of colitis requires presence of colonic bacteria, and germ-free mice have been shown to be protected against colitis (25), supporting the theory that colitis involves bacterial infiltration of the mucosa.
The colonic mucus has been investigated in several studies of patients with colonic inflammation and was found to be altered and less effective as a barrier (24, 27, 28). Despite its importance in preventing mucosal inflammation, little is known about the regulation of thickness of the colonic mucus layer. The mucus thickness has been difficult to estimate from histological sections because of dehydration and erosion occurring during the process; the actual state of mucus thickness during established colitis is still unclear. In the upper gastrointestinal tract, the thickness of luminal mucus layers is reported to be stimulated by nitric oxide and prostaglandins (22). We have previously found that the colonic mucus layer is significantly decreased in mice with an inactivated gene for Muc1, indicating a signaling role for this membrane-bound mucin (17). However, the stimuli resulting in secretion of colonic mucus gel are so far unknown, as is the downstream signaling mechanism for Muc1.
In this study, we explored the role of the mucus layer and the mucins Muc1 and Muc2 in induction of colitis. Furthermore, we investigated in vivo whether the mucus secretion was regulated by luminal bacterial products.
MATERIALS AND METHODS
Animals.
Mice weighing between 22 and 43 g were used. Male C57Bl/6 and NMRI mice were purchased from B & K Universal, Stockholm, Sweden. Homozygous Muc1-deficient mice (both sexes, background C57Bl/6, Mayo Clinic Collage of Medicine, Scottsdale, AZ) and homozygous Muc2-deficient mice (both sexes, on a C57Bl/6 background, Montefiore Medical Center, Bronx, NY) were used. All animals were kept under standardized conditions of temperature (21–22°C) and illumination (12 h light-12 h dark). They were kept in cages with mesh bottoms and had free access to tap water and pelleted food (Ewos, Södertälje, or Lactamin, Kinstad, Sweden). Male germ-free NMRI mice (22–30 g, Karolinska Institute, Stockholm, Sweden) were kept at standard conditions (temperature 21–22°C) and illumination (12 h light-12 h dark) in lightweight stainless steel isolators. They received autoclaved rodent diet R36 (Lactamin, Södertälje, Sweden). The germ-free status was checked weekly by culturing fecal samples, both aerobically and anaerobically at 20 and 37°C for up to 4 wk. All experimental procedures in this study were approved by the Swedish Laboratory Animal Ethical Committee in Uppsala and were conducted in accordance with guidelines of the Swedish National Board for Laboratory Animals.
Animal preparation for intravital microscopy.
The mice were anesthetized with spontaneous inhalation of isoflurane (Forene, Abbott Scandinavia, Solna, Sweden). The inhalation gas was administered continuously through a breathing mask (Simtec Engineering) and contained a mixture of 40% oxygen, 60% nitrogen, and ≈2.2% isoflurane. Body temperature was maintained at 37°C by means of a heating pad controlled by a rectal thermistor probe. A catheter containing heparin dissolved in isotonic saline was placed in the left carotid artery to monitor blood pressure, and the jugular vein was cannulated for continuous infusion of Ringer's solution at a rate of 0.35 ml/h. The colonic preparation for in vivo microscopy has been described in detail previously (3, 17). Briefly, exteriorization of the proximal part of the descending colon through a midline abdominal incision was followed by a lengthwise incision along the antimesenteric border by electric cautery. The animal was placed on its left side on a Lucite table with a part of the colon loosely draped over a truncated cone at the center of the table, with the mucosal surface facing upward. A mucosal chamber, with a hole in the bottom corresponding to the position of the cone, was placed over the mucosa exposing ∼0.07 cm2 of the mouse mucosa through the hole. The mucosal chamber did not touch the mucosa, to not impair blood flow, and the edges of the hole were sealed with silicon grease (Dow Corning High Vacuum Grease, Dow Corning, Weisbaden, Germany). The mucosal chamber was filled with 3 ml of 0.9% NaCl maintained at 37°C by means of circulating warm water in a jacket in the bottom of the chamber. The colonic mucosa was observed in a stereomicroscope (Leica MZ12, Leica, Heerbrugg, Switzerland) and was transilluminated with light from a 150-W light source guided by fiber optics.
Measurements of the mucus thickness in vivo.
Mucus thickness was measured with micropipettes connected to a micromanipulator (Leitz, Wetzlar, Germany) with a digimatic indicator (IDC Series 543, Mitutoyo, Tokyo, Japan). Glass tubing (borosilicate tubing with 1.2 mm OD and 0.6 mm ID; Frederick Haer, Brunswick, ME) was pulled with a pipette puller (pp-83; Narishige Scientific Instrument Laboratories, Tokyo, Japan) to a tip diameter of 1–3 μm. To prevent mucus adhering to glass, the pipettes were siliconized by dipping the tip of the micropipette into a silicone solution (MS1107, 25% acetone) followed by drying at 100°C for 30 min. The luminal surface of the mucus gel was visualized by placing graphite particles (activated charcoal, extra pure, Merck, Darmstadt, Germany) on the gel, and the colonic epithelial cell surface was visible through the microscope. The micropipette was inserted into the mucus gel at an angle of ∼30° to the surface. The angle was measured with a protractor, and the same angle was maintained throughout an experiment. The distances traveled by the micropipette from the luminal surface of the mucus gel to the epithelial cell surface were measured and a mean value was calculated. The mucus thickness, which is the vertical distance between the cell surface and the luminal mucus surface, was then calculated. The mean value of four to five different measurements was taken as one thickness value. Prior to measurement of the thickness of the firmly adherent mucus, the degraded luminal mucus layer was removed by gentle suction with a thin polyethylene cannula connected to a syringe. This procedure was conducted under supervision through a stereomicroscope to avoid contact with the epithelium. In some of the experiments, lipopolysaccharide (LPS, 0.2 mg/ml) or peptidoglycan (PGN, 0.2 mg/ml) was added topically to the exposed colonic mucosa, and mucus thickness was measured 45 min later.
Induction of colitis.
Colitis was induced by giving 1 or 3% dextran sulfate sodium (DSS) (TdB Consultancy AB, Uppsala, Sweden), molecular weight 40 kDa, ad libitum in drinking water for up to 8 days.
Assessment of colitis.
The severity of colitis was assessed daily by using a disease activity index (DAI). DAI is the combined score of weight loss, stool consistency, and presence or absence of fecal blood divided by 3. The score ranges from 0 to 4. The DAI scoring system has been described in detail by Murthy et al. (20).
Histology.
Samples of the distal colon were fixed in 4% formalin, processed, embedded in wax, sectioned, and stained with hematoxylin-eosin or periodic acid-Schiff stain. T cell infiltration was investigated by using the monoclonal antibody against the T cell receptor (anti-CD3 mAb, Lab Vision, Fremont, CA).
Bacterial analysis.
Homogenized spleens (100 μl) were diluted with 900 μl peptone water and the samples were then serially diluted. A 100-μl aliquot of each dilution was spread on BHI agar (Oxoid, Basingstoke, UK) plates, which were incubated at 37°C for 48 h in anaerobic atmosphere (Gaspack system, BD, Sparks, MD).
Statistical analysis.
The results are expressed as means ± SE. For statistical evaluations of the in vivo mucus measurement analysis, ANOVA for multiple comparisons was performed when comparing data between groups. ANOVA was followed by Fisher's protected least significant difference test. The differences were regarded as significant at P < 0.05.
RESULTS
Colonic mucus layer decreased in thickness as inflammation progressed.
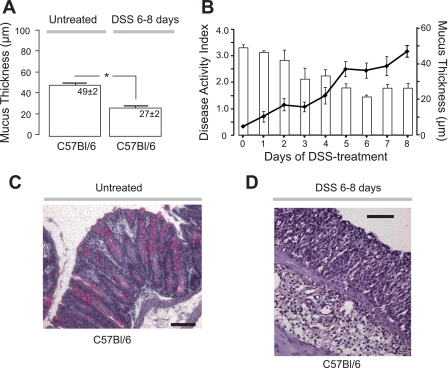
We investigated the thickness of the colonic firmly adherent mucus during onset and establishment of colitis in a DSS mouse model. After a week of treatment with 3% DSS (n = 9), the mucus layer was significantly thinner compared with untreated mice (Fig. 1A). When the mucus thickness was investigated each day following initiation of DSS treatment and during onset of inflammation (n = 27), it was found that the mucus thickness decreased progressively with time on DSS and reached a significantly lower plateau 5–8 days (n = 12) after induction (Fig. 1B). DAI increased simultaneously after addition of 3% DSS to the drinking water and was stabilized at a significantly higher plateau at day 5-8, which paralleled with the decrease in mucus thickness (Fig. 1B). Histological samples from untreated and DSS-treated C57Bl/6 mice revealed a profound mucosal infiltration of leukocytes in DSS treated but not in untreated mice (Fig. 1, C and D). Low concentration of DSS (1%) administered to the drinking water resulted in a slightly increased DAI, which remained at ∼1 for the investigated period of 9 days (1.2 ± 0.2 at day 9, n = 4). The thickness of the adherent mucus layer was not altered from what was observed in untreated mice on the same background (44 ± 3 μm; n = 4 and 49 ± 2 μm n = 11, respectively).
Fig. 1.
The adherent mucus layer in wild-type mice decreased whereas disease activity index increased with days on dextran sulfate sodium (DSS). A: adherent mucus thickness (μm) in wild-type (C57Bl/6) mice without (untreated, n = 11) or with 3% DSS addition to the drinking water for 6–8 days (n = 9). B: disease activity index of the mice treated with DSS (solid lines) and the decreasing mucus thickness at different time points during DSS treatment (open bars) (n = 27). C and D: histological tissue sections stained with hematoxylin-eosin from untreated and DSS-treated C57Bl/6 mice, respectively. *P < 0.05; bars correspond to 100 μm.
Mice lacking a colonic mucus layer developed severe colitis and sepsis.
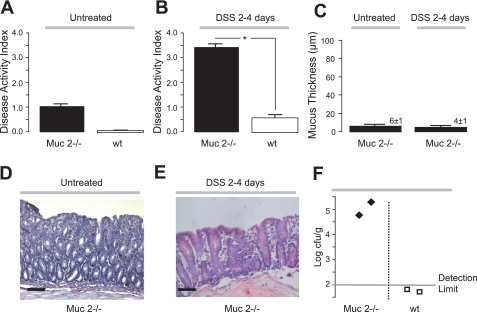
To understand how mucus thickness correlates with the degree of inflammation during colitis, the severity of DSS-induced colitis was determined in a mouse model known to be deficient in colonic mucus, the Muc2−/− mouse (13). Muc2−/− mice have been reported to develop mild colitis with increasing age (7, 29), and in the present study signs of mild colitis were present already before the DSS treatment (Fig. 2A). When DSS was given, Muc2−/− mice (n = 5) developed severe colitis very quickly and DAI was significantly increased compared with DSS-treated wild-type (wt) mice already after 2–4 days (Fig. 2B). As a consequence, the treatments had to be terminated and experiments performed at day 2-4 because of ethical considerations. An extremely thin firmly adherent colonic mucus layer could be detected in untreated Muc2−/− mice (n = 9) (Fig. 2C), most probably corresponding to the size of the carbon particles used to detect the luminal surface and thus demonstrating the lowest detection level of this method for mucus measurement. Furthermore, DSS treatment of Muc2−/− mice also showed lack of adherent mucus (Fig. 2C), indicating that no compensation of other secreted mucins occurred during inflammation as also shown previously using biochemical methods (13). Histological sections revealed that DSS treatment of Muc2−/− resulted in a vastly damaged mucosa, compared with untreated Muc2−/− mice (Fig. 2, D and E). In addition, anaerobic bacteria, detected by bacterial culturing, were found in the spleens of DSS-treated Muc2−/− but not in DSS-treated wt mice, indicating that a mucus barrier is required during colitis and protects against bacterial translocation (Fig. 2F). Thus a more severe inflammation and bacterial translocation were seen in mice lacking colonic mucus, indicating that during colitis, the colonic mucus layer is still important as a barrier toward invading bacteria.
Fig. 2.
Muc2-deficient mice lack adherent colonic mucus and develop a more severe colitis. Disease activity index in wild-type (wt; n = 8) and Muc2-deficient mice (n = 5) before (A) and 2–4 days following DSS treatment (B). C: adherent mucus thickness (μm) of untreated (n = 9) and DSS-treated Muc2-deficient mice (n = 5). Please note that the DSS treatment had to be interrupted already after 2–4 days because of severe rectal bleeding and weight loss in Muc2-deficient mice. D and E: histological samples stained with periodic acid-Schiff (PAS) from untreated and DSS-treated Muc2-deficient mice, respectively. F: bacterial penetrations estimated by bacterial culturing of spleens isolated from DSS-treated wt (n = 2) or Muc2−/− mice (n = 2). *P < 0.05; bars correspond to 100 μm.
Muc1−/− mice exhibited ameliorated colitis with lower T cell infiltration and increased mucus thickness.
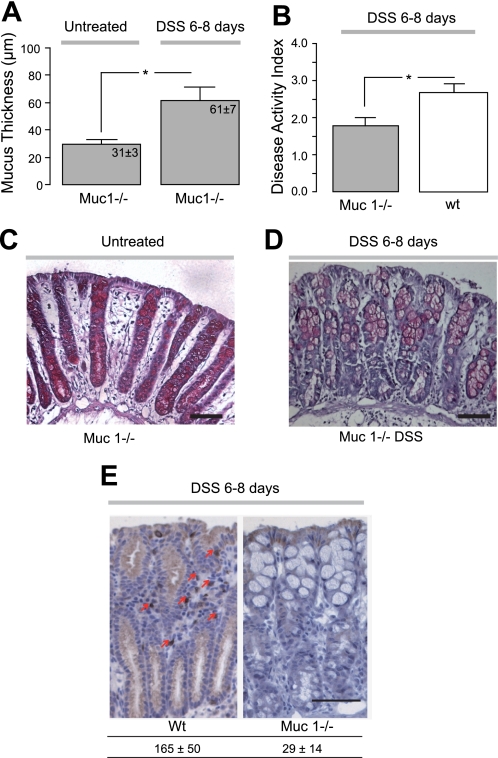
A previous study reports a thinner firmly adherent colonic mucus thickness in Muc1−/− mice (17). If progression of inflammation is a result of the thinner mucus layer observed in wt mice during DSS treatment, we hypothesized that Muc1−/− would develop a more severe colitis since they already from the start had thinner adherent mucus. Untreated Muc1−/− mice (n = 7) had a significantly thinner firmly adherent colonic mucus layer compared with wt control mice (n = 11) (Figs. 3A and 1A, respectively), in agreement with earlier observations (17). However, after DSS treatment for 6–8 days, these mice (n = 7) exhibited increased thickness of the firmly adherent colonic mucus layer to significantly higher levels than what was observed in untreated wt mice (Figs. 3A and 1A, respectively). In addition, DAI was significantly lower in Muc1−/− compared with wt treated with DSS (Fig. 3B), and histological evaluation did not reveal damage to the mucosa (Fig. 3, C and D). Since Muc1 has been implicated having a role in the immune system (6, 23), histological evaluation of lymphocytes with an anti-CD3 antibody (against the T cell receptor) was performed and revealed significantly fewer infiltrating T cells in the DSS-treated Muc1−/− colonic mucosa compared with wt (Fig. 3E). This suggests a regulating role of Muc1 mucin for the immune system.
Fig. 3.
Muc1-deficient mice are partly protected against DSS colitis by increased adherent mucus thickness and decreased T cell infiltration. A: adherent colonic mucus thickness (μm) in Muc1-deficient mice with (n = 7) or without (n = 7) DSS treatment for 6–8 days. B: disease activity index in wild-type (n = 9) or Muc1-deficient mice (n = 7) after DSS treatment. *P < 0.05. C and D: representative histological samples stained with hematoxylin-eosin from Muc1-deficient mice with or with DSS treatment. E: immunohistological staining with an anti-CD3 mAb (as indicated by arrows) and quantification of infiltrated T cells in the DSS-treated wild-type (n = 3) or Muc1-deficient (n = 5) colonic mucosa (number of infiltrated T cells per mm2 colonic mucosa). Significantly fewer T cells were observed in the mucosa of Muc1−/− mice. Bars correspond to 100 μm.
Germ-free mice virtually lack adherent colonic mucus, but the mucus thickness is restored by bacterial products.
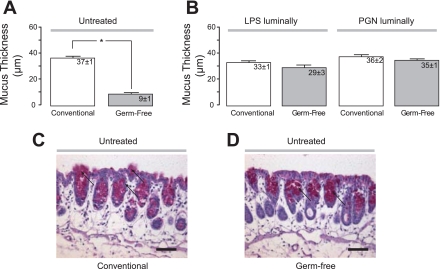
To investigate the role of the commensal microbiota in mucus secretion, colonic mucus thickness was measured in germ-free mice and compared with conventionally housed mice on the same background. Interestingly, we found that the firmly adherent colonic mucus layer was very thin in the germ-free mice (9 ± 1 μm, n = 11) compared with what was observed in conventionally housed mice (37 ± 1 μm, n = 9, NMRI, Fig. 4A). When the bacterial products LPS and PGN were added to the luminal saline solution covering the colonic mucosa of conventional mice (n = 5, n = 4, respectively), no effect on the thickness of the mucus layer could be detected (Fig. 4B). However, when LPS or PGN were administered to the exposed colonic mucosal surface of germ-free mice (n = 7, n = 4, respectively), the thickness of the firmly adherent mucus layer was restored to normal levels already after 40 min of exposure (Fig. 4B). PAS staining of histological sections confirmed that mucus was stored within colonic goblet cells in both conventionally stalled mice and germ-free mice, but no secretion of mucus could be detected in the germ-free mice (Fig. 4, C and D).
Fig. 4.
Germ-free mice lack firmly adherent colonic mucus, but this mucus layer is restored by bacterial products. A and B: thickness of the adherent mucus in the colon in NMRI mice housed conventionally (n = 9) or under germ-free conditions (n = 11) either with luminal saline or the bacterial products LPS (conventional; n = 5, germ-free; n = 7) or peptidoglycan (PGN; conventional; n = 4, germ-free; n = 4). The mucus thickness is expressed in μm; *P < 0.05. C and D: representative histological samples from a conventional and a germ-free mouse stained with PAS solution. Arrows point at PAS-stained mucins. Bars correspond to 100 μm.
DISCUSSION
In this study we investigated how the firmly adherent mucus barrier is altered during inflammation, and how bacterial products influence this mucus barrier. We found that during DSS induction of colitis the thickness of the mucus gel decreased at the same time as the mice developed increasing symptoms of colitis, assessed by DAI. Mice deficient in Muc2 lacked a colonic mucus layer and were more susceptible to colitis. The thickness of the adherent mucus increased by DSS treatment in Muc1−/−, and these mice developed a very mild colitis. This mild colitis might be due to an increased mucus barrier but also a decreased ability to recruit T cells to the inflamed area. In addition, we found that germ-free mice had a very thin colonic mucus barrier, but luminal exposure to the bacterial products LPS and PGN quickly restored the firmly adherent mucus layer thickness to levels observed in conventionally housed mice.
The inner firmly adherent colonic mucus layer has been reported to be a functional barrier that separates the colonic epithelium from the commensal bacterial microbiota (13). Concurrently, the bacteria colonize the outer, loosely adherent parts of the mucus. Although the mucus serves as nutrients to the bacteria, the bacterial microbiota provides the host with vitamin K and the colonocytes with nutrients as they ferment undigested carbohydrates (5). Thus during colonic homeostasis, a symbiosis between the gut microbiota and the host is maintained that is favorable for both parties. During colonic inflammation, the balance is shifted and bacteria are found within the colonic tissue as well as in mucosa draining lymph nodes, which further activates the immune system and aggravates the inflammation (1). The barrier function of the mucus layer is thus overridden, as the bacteria come in direct contact with and invade the mucosa. Whether or not the failing mucus barrier is a cause or a result of the inflammation is not known. One of the most commonly used animal models of colitis is DSS-induced inflammation, which resembles ulcerative colitis in terms of localization of inflammation as well as clinical symptoms. The mechanism through which DSS induces colonic mucosal inflammation is not completely known, but recent results indicate that sulfate groups of the DSS molecules destabilize the mucus and make it more permeable to bacteria (12). Effect of DSS on the barrier function was observed already after 4–12 h, whereas no effect on mucus thickness nor clinical symptoms of colitis could be detected at that point (12). Indeed, in the present study we found that colonic mucus thickness decreased whereas DAI increased during DSS induction of colitis and reached significance at day 5 following DSS induction. This could either be due to DSS destroying the mucus gel allowing bacteria to reach the epithelia or the result of inflammation of the epithelium that negatively affects the mucus production by the goblet cells.
The gel-forming component of the colonic mucus has previously been shown to be the mucin Muc2 (13). In agreement with this observation, we measured no mucus layer in mice deficient in Muc2, demonstrating that expression of other gel-forming mucins was not upregulated in this model. These genetically modified mice spontaneously developed a mild colitis, as previously reported (29). If the mechanism behind DSS induction of colitis is due to a destruction of the mucus barrier, one could predict that no further inflammation would develop in Muc2−/− since these mice already lack a mucus gel layer. However, when Muc2−/− were challenged with DSS 3%, they rapidly developed serious colonic and extraintestinal symptoms within a couple of days with rectal bleeding and reluctance to move and eat. Bacterial translocation was confirmed in spleens from these mice in contrast to DSS-treated wt mice. As a consequence, the treatments had to be terminated and experiments performed at day 2–4 because of ethical considerations. DSS treatment and inflammation did not result in mucus secretion in the Muc2−/−mice, demonstrating that no compensation of other secreted gel forming mucins occurred during inflammation. These results implicate that the DSS has a direct toxic effect and that the inflammation observed by DSS is not restricted to decreasing the barrier function of the mucus layer. Since a more severe inflammation and bacterial translocation were seen in mice lacking colonic mucus, the colonic mucus layer is still important as a barrier toward invading bacteria during colitis.
We have previously found that Muc1, a membrane bound non-gel forming mucin, regulates the thickness of the colonic mucus in a still unknown way, since the adherent mucus was thinner in Muc1−/− mice (17). On the basis of these findings, we hypothesized that Muc1−/− mice would be more susceptible to DSS colitis. Interestingly, DSS treatment of Muc1−/− mice resulted in increased adherent mucus thickness and milder symptoms of colitis compared with DSS-treated wt mice (Fig. 3, A and B). These somewhat surprising results most probably reflect pleiotropic effects of Muc1. In the literature, Muc1 has been reported to be a part of the gastric mucosal barrier to infection by Helicobacter pylori (18). Muc1−/− mice displayed increased bacterial colonization of the stomach and greater TNF-α and keratinocyte chemoattractant transcript levels compared with Muc1+ mice following experimental H. pylori infection (9, 19). This effect might be caused by epithelial Muc1 functioning as a steric hindrance and decoy receptor that prevents direct contact between the mucosa and the luminal bacteria (19). Epithelial adherence of H. pylori is of outermost importance for its pathogenicity, which depends at least in part on the ability of bacteria to inject peptidoglycan and CagA into epithelial cells and thereby initiate inflammation (4). Patients with symptomatic H. pylori infections most often carry bacteria expressing the Cag pathogenicity islet (cag PAI), which is a cluster of genes coding for the type IV secretion apparatus and CagA (4). However, type IV secretion systems involved in interactions with eukaryotic cells are not normally observed in the bacterial strains that form the normal intestinal microbiota. Muc1-deficient mice do not suffer from colonic inflammation as a result of infiltration of the commensal flora (Fig. 3). This observation and the low amount of Muc1 in colon suggest that Muc1 does not have an important role in separating colonic epithelium from bacteria during colonic homeostasis.
The mild clinical symptoms observed in Muc1-deficient mice in response to DSS could partly be explained by the increased adherent mucus thickness found in these mice. However, evaluation of immunohistological sections of DSS-treated Muc1−/− revealed defect T cell recruitment in the colon compared with DSS-treated wt mice. These results suggest that Muc1 mucin can have a regulatory role in the immune system (6, 23). In addition, Muc1-deficient mice have been found to express slightly higher levels of both Muc2 and Muc3 mucin transcripts in the crypts in the ileum (21). Mice in which the genes coding for both Muc1 and Cftr were inactivated showed that the protein levels of Muc3(17) was twice as high compared with mice with only inactivated Cftr (17). Hence, Muc3 seems to be upregulated in Muc1-deficient mice, and therefore might in addition to the increased mucus thickness and defect T cell recruitment contribute to the decreased bacterial translocation.
Mice lacking a functional Cftr channel, as in cystic fibrosis, develop mucus obstruction of their intestine and failure to thrive. However, in Cftr−/− mice also lacking the Muc1 mucin, the intestinal accumulation of mucus was milder, Muc 3 mucins were increased, and the animals were healthier (11, 21). There is still no explanation for these observations, but they suggest that certain forms of Muc1 can stimulate mucus release in the intestine. Toll-like receptor signaling activates NF-κB, and a binding site of this transcription factor has been detected on the Muc2 gene promoter site (2). Some of the Toll-like receptor ligands are bacterial products such as LPS and PGN, and the influence of bacteria on mucus secretion has been implied in vitro since LPS was shown to stimulate secretion of Muc5ac and Muc5b mucins in a goblet cell line (26). Furthermore, administration of LPS to germ-free rats increased expelled luminal mucus, an observation that might mirror increased mucus secretion but could also be a result of mucus degradation (8). In the present study, when the thickness of the adherent colonic mucus was measured in germ-free mice and compared with conventionally housed mice on the same background, we found that the colonic mucus layer was virtually absent in the germ-free mice compared with conventionally housed mice. Thus in the absence of bacteria, the colonic mucus seems not to be required as a barrier between host and lumen and consequently the mucus layer is very thin. Indeed, as soon as the bacterial products and Toll-like receptor (TLR) ligands LPS (TLR4) and PGN (TLR2) were administered in vivo, the thickness of the firmly adherent mucus barrier was quickly restored, probably because of release of prestored mucus from goblet cells. Histological sections confirmed that mucus was stored within colonic goblet cells in conventionally stalled as well as in germ-free mice, but no mucus being secreted could be detected in the germ-free mice. Since DSS treatment was shown to acutely decrease the barrier function of the mucus (12), this could facilitate for TLR ligands to reach the mucosa and thus increase mucus secretion as observed in DSS-treated Muc1−/− mice. Furthermore, these results may indicate that colonic luminal bacteria can affect the host by stimulating mucus release. A recent study reports the ability of Muc2 mucins in regulating commensal and pathogen numbers in the colon (7). The present study expands this observation since secretion of Muc2 mucins was shown to be regulated by bacterial products LPS and PGN, unraveling bacterial regulation of mucus thickness.
In conclusion, this study shows that decreased mucus thickness correlates with disease progression in the DSS mouse model of colitis. Although mice deficient in Muc2 that lacked a colonic mucus layer were more susceptible to colitis, the Muc1−/− mice developed a very mild colitis. The mild colitis might be due to an increased colonic mucus barrier but also a decreased ability to recruit T cells to the affected region. In addition, the adherent colonic mucus was extremely thin in germ-free mice, but was quickly restored by luminal exposure of the bacterial products LPS and PGN to normal levels, indicating the ability of bacteria to regulate the colonic environment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors are funded by grants from The Swedish Research Council (55X-08646, 57P-20680, 57X-20675, 7461, 21027), Sahlgren's University Hospital (LUA-ALF), the Swedish Foundation for Strategic Research, Vinnova (CIDaT), Nanna Svartz Foundation, and Ruth and Richard Julins Foundation.
REFERENCES
- 1. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ahn DH, Crawley SC, Hokari R, Kato S, Yang SC, Li JD, Kim YS. TNF-alpha activates MUC2 transcription via NF-kappaB but inhibits via JNK activation. Cell Physiol Biochem 15: 29–40, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol 10: 1573–1581, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 307: 1915–1920, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Beatson RE, Taylor-Papadimitriou J, Burchell JM. MUC1 immunotherapy. Immunotherapy 2: 305–327, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6: e1000902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enss ML, Muller H, Schmidt-Wittig U, Kownatzki R, Coenen M, Hedrich HJ. Effects of perorally applied endotoxin on colonic mucins of germfree rats. Scand J Gastroenterol 31: 868–874, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Guang W, Ding H, Czinn SJ, Kim KC, Blanchard TG, Lillehoj EP. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. J Biol Chem 285: 20547–20557, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gum JR, Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem 269: 2440–2446, 1994 [PubMed] [Google Scholar]
- 11. Hinojosa-Kurtzberg AM, Johansson ME, Madsen CS, Hansson GC, Gendler SJ. Novel MUC1 splice variants contribute to mucin overexpression in CFTR-deficient mice. Am J Physiol Gastrointest Liver Physiol 284: G853–G862, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Johansson ME, Gustafsson JK, Sjöberg KE, Petersson J, Holm L, Sjövall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5(8): e12238 doi:10.1371/journal.pone.0012238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12: 319–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA 104: 16209–16214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mai V, Morris JG., Jr Colonic bacterial flora: changing understandings in the molecular age. J Nutr 134: 459–464, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Malmberg EK, Noaksson KA, Phillipson M, Johansson ME, Hinojosa-Kurtzberg M, Holm L, Gendler SJ, Hansson GC. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol 291: G203–G210, 2006 [DOI] [PubMed] [Google Scholar]
- 18. McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest 117: 2313–2324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGuckin MA, Every AL, Skene CD, Linden SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M, Ferrero R, Sutton P. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133: 1210–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci 38: 1722–1734, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Parmley RR, Gendler SJ. Cystic fibrosis mice lacking Muc1 have reduced amounts of intestinal mucus. J Clin Invest 102: 1798–1806, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillipson M, Johansson ME, Henriksnas J, Petersson J, Gendler SJ, Sandler S, Persson AE, Hansson GC, Holm L. The gastric mucus layers: constituents and regulation of accumulation. Am J Physiol Gastrointest Liver Physiol 295: G806–G812, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Poh TW, Bradley JM, Mukherjee P, Gendler SJ. Lack of Muc1-regulated beta-catenin stability results in aberrant expansion of CD11b+Gr1+ myeloid-derived suppressor cells from the bone marrow. Cancer Res 69: 3554–3562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35: 353–359, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66: 5224–5231, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 221: 42–49, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Strugala V, Dettmar PW, Pearson JP. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn's disease. Int J Clin Pract 62: 762–769, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56: 343–350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006 [DOI] [PubMed] [Google Scholar]