Abstract
Selective glycolytic inhibition (GI) promotes electromechanical alternans and triggered beats in isolated cardiac myocytes. We sought to determine whether GI promotes triggered activity by early afterdepolarization (EAD) or delayed afterdepolarizations in intact hearts isolated from adult and aged rats. Dual voltage and intracellular calcium ion (Cai2+) fluorescent optical maps and single cell glass microelectrode recordings were made from the left ventricular (LV) epicardium of isolated Langendorff-perfused adult (∼4 mo) and aged (∼24 mo) rat hearts. GI was induced by replacing glucose with 10 mM pyruvate in oxygenated Tyrode's. Within 20 min, GI slowed Cai2+ transient decline rate and shortened action potential duration in both groups. These changes were associated with ventricular fibrillation (VF) in the aged hearts (64 out of 66) but not in adult hearts (0 out of 18; P < 0.001). VF was preceded by a transient period of focal ventricular tachycardia caused by EAD-mediated triggered activity leading to VF within seconds. The VF was suppressed by the ATP-sensitive K (KATP) channel blocker glibenclamide (1 μM) but not (0 out of 7) by mitochondrial KATP block. The Ca-calmodulin-dependent protein kinase II (CaMKII) blocker KN-93 (1 μM) prevented GI-mediated VF (P < 0.05). Block of Na-Ca exchanger (NCX) by SEA0400 (2 μM) prevented GI-mediated VF (3 out of 6), provided significant bradycardia did not occur. Aged hearts had significantly greater LV fibrosis and reduced connexin 43 than adult hearts (P < 0.05). We conclude that in aged fibrotic unlike in adult rat hearts, GI promotes EADs, triggered activity, and VF by activation of KATP channels CaMKII and NCX.
Keywords: fibrosis, aging, early afterdepolarizations, triggered activity, calcium cycling, optical mapping
glycolysis accounts for ∼5% of total cellular ATP production in the beating heart under aerobic conditions (19, 52). However, studies in isolated cardiac myocytes have shown that selectively inhibiting glycolytic ATP production (12, 20), without affecting mitochondrial ATP production or redox state (2, 21), can lead to electromechanical alternans and triggered action potentials. This is consistent with other experimental evidence supporting a functional coupling between glycolytic ATP production and membrane protein function, including intracellular calcium ion concentration (Cai2+) transport by the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) (52), Nai+ transport by Na-K-ATPase (42), and regulation of sarcolemmal ATP-sensitive K (KATP) channels (48).
For each glucose molecule, glycolysis generates two pyruvate, two ATP, and two reduced NADH molecules, according to the reaction: glucose + 2[NAD]+ + 2[ADP] + 2[P]i → 2pyruvate + 2NADH + 2ATP + 2H2O. Replacing glucose with pyruvate inhibits forward glycolytic flux while providing mitochondria with abundant substrate (pyruvate) for oxidative phosphorylation, resulting in selective glycolytic inhibition (GI) without impairing overall cellular energy production (47).
Reduction of glycolytic ATP supply to the SERCA2a pump and other membrane proteins could lead to 1) reduced Cai2+ transient decline rate, 2) shortening of the action potential duration (APD) due to activation of ATP-sensitive potassium (KATP) channels (37, 48), and 3) a shift toward cellular pro-oxidant state (4, 14, 15, 52) events that together may promote phase three early afterdepolarizations (EADs) initiated by forward mode Na-Ca exchanger (NCX) during late systole (38). Furthermore, reduced SERCA2a activity and the associated elevation of cytosolic Cai2+ coupled with a pro-oxidant shift may promote activation of Ca-calmodulin kinase II (CaMKII), which increases the l-type calcium current and the late Na current (1, 8, 24) to facilitate EADs (40, 46, 50). Unlike isolated myocytes, intact ventricular muscle may be shielded from the arrhythmogenic consequences of these changes by the sink effect of adjacent well-coupled myocytes, which prevent a single myocyte from manifesting an afterdepolarization unless a large number of its neighbors are also similarly inclined (51). Consistent with this scenario, we recently showed in experimental and simulation studies that oxidative stress sufficient to initiate EADs and triggered activity in isolated myocytes (50) failed to cause arrhythmias in young adult rat ventricles (32). However, a high incidence of EADs, triggered activity, and ventricular fibrillation (VF) occurred in aged fibrotic rat ventricles, in which fibrosis and other aging-related changes alter the source-sink relationship in a manner facilitating emergence of EAD-mediated arrhythmias (31, 32). These findings led us to hypothesize that the arrhythmogenic effects of GI observed at the isolated myocyte level (2, 20) might not manifest as arrhythmias in normal well-coupled ventricular tissue from young adult rat hearts, but might emerge in aged rat hearts in which the source-sink relationship has been altered by fibrosis and other aging-related factors. We show that in aged, but not young adult, rat hearts, GI promotes EADs, triggered activity, and VF by a mechanism involving activation of KATP channels, CaMKII, and NCX.
METHODS AND MATERIALS
The research protocol was approved by the Institutional Animal Care and Use Committee and followed the guidelines of the American Heart Association (AHA).
Langendorff preparation.
Male Fisher 344 young 3- to 4-mo-old (N = 18) and aged 24- to 30-mo-old (N = 66) rats were used in this study. The hearts of the anesthetized rats were removed, and the ascending aorta was cannulated for retrograde perfusion through the coronary ostia as we previously described (32, 36). Briefly, the isolated hearts were immersed in cold oxygenated Tyrode's and the entire duration of the cannulation of the aorta and securing the perfusion tubing in place took 3 to 4 min. The cannulated hearts were then mounted in the tissue bath, and oxygenated Tyrode's perfusion continued at 37 ± 0.5 degrees Celsius. The composition of the Tyrode's solution (in mM) was: 125 NaCl, 4.5 KCl, 1.8 NaH2PO4, 24 NaHCO3, 1.8 CaCl2, 0.5 MgCl2, 5.5 glucose, and 100 mg/l albumin.
Optical mapping.
The hearts were stained with RH237 and Rhod-2 AM for simultaneous dual voltage (V) and Cai2+ fluorescent optical imaging, respectively, as we described previously (32). Cytochalasin D (5 μmol/l) was added to the perfusate to eliminate motion artifact during optical recordings (32). Cai2+ transient decay rate constant was determined by a monoexponential fit during the relaxation, and the Cai2+ level during repolarization coinciding in time with the emergence of EAD was related to peak systolic Cai2+ transient amplitude (32). Continuous single cell glass microelectrode recordings were made at the onset of ventricular tachycardia (VT) from the frequent epicardial focal site of origin of the VT as determined by optical mapping (32). Once initiated, VF was terminated within 10 s by an electrical shock (coil electrode placed in the tissue bath). Typically, within minutes of VF termination, new episodes of VT/VF reoccurred repeatedly providing us the opportunity to capture the onset of VT/VF during combined acquisition of microelectrode and optical mapping data. The repeated spontaneous episodes of VFs in each heart allowed us to successfully capture and optically map the onset of eight GI-induced VF.
Combined glass microelectrode recordings and dual V-Cai2+ optical mapping.
To define the cellular mechanisms of the focal VT (leading to VF, see below), which most often arose from the base of the left ventricular (LV) epicardial surface, we first terminated the VF with electrical shock and then continuously recorded with a glass microelectrode from the same region of origin of the VT in an attempt to capture a new onset of VT/VF. Six to eight episodes of VFs were studied in each aged heart. In few experiments we also made triple recordings simultaneously to define the cellular mechanism of the focal VT and the underlying calcium dynamics.
Dynamic APD restitution.
In 10 aged and 10 adult hearts, we recorded with a glass microelectrode from 4 to 6 epicardial cells in each heart at the base and the midwall on the LV epicardial surface during pacing at a cycle length (CL) of 250 ms before and 30 min after GI. The pacing electrode was located at the base of the LV epicardial surface. The results on AP data were pooled in each age group, since there were no significant base versus midwall differences in AP properties. We constructed dynamic APD restitution curves for 90 percent repolarization (APD90) before and after GI using single cell glass microelectrode. Pacing started at a CL of 250 ms and then decreased by 20 ms until 160 and then by 10 ms (29, 30, 32). Conduction velocity (CV) was estimated in both age groups before and after GI (N = 8 in each group) during optical AP recordings while pacing from the base of the LV epicardial surface at a CL of 250 ms. CV was calculated by dividing the conduction time from LV base to LV apex by the distance from LV base to the LV apex.
Metabolic and pharmacological interventions.
In this portion of the study we used an additional 35 aged rats to test the efficacy of drugs against GI-mediated VF. We did not test adult rats in this part of the study, since no spontaneous VF occurred during GI in the adult rat hearts (see below). Selective GI was induced by substituting 5.5 mM glucose with 10 mM pyruvate in oxygenated, otherwise normal Tyrode's solution (12). To determine whether GI-induced VF was mediated through alteration of cellular redox state, the reducing agent N-acetyl-cysteine (NAC; 2 mM) was tested in eight aged rats. In four hearts, NAC antioxidant therapy was initiated 15 min before and continuing throughout the GI period (preventive therapy). In an additional four hearts, NAC was administered after the onset of GI-mediated arrhythmias (suppressive therapy, N = 4). Because elevated cytosolic Cai2+ levels activating CaMKII (8, 32) could potentially mediate GI effects, we also examined the preventive (N = 5) and suppressive (N = 4) effects of CaMKII inhibiter KN-93 (1 μM). The preventive effect of the inactive form (KN-92, 1 μM) (8) on GI-mediated VT/VF was tested in four aged hearts, respectively. In six aged hearts after the conclusion of GI-mediated initiation of VF and optical mapping studies, the hearts were perfused for 90 min with normal Tyrode's solution containing glucose present but no pyruvate to determine reversibility of GI-induced VF in the aged hearts.
Because a decrease in cellular ATP level and the associated shortening of the APD after GI (48) may result either from sarcolemmal KATP channel or mitochondrial KATP channel (23, 27), we used the selective mitochondrial KATP channel blocker 5-hydroxydecanoic acid (5-HD; 1 μM; N = 6 for preventive and N = 3 for suppressive N = 3) (13) and the potent sarcolemmal and mitochondrial KATP channel blocker glybenclamide (1 μM; N = 6) (23, 27) to determine the site of KATP channel activation after GI.
SEA0400 (a generous gift of Prof. András Varró, Szeged, Hungary) was used to block NCX blocker. All other chemicals were obtained from Sigma-Aldrich.
Endocardial and midmyocardial cryoablation.
To determine the role of the LV epicardial mapped surface in GI-mediated arrhythmias we cryoablated endo- and midmyocardial structures while leaving a thin rim of surviving epicardial cell layers as we previously described (32). Briefly, cryoablation was performed in the aged rats (N = 5) using a 7-cm SurgiFrost cryoablation probe (ATS Medical, Minneapolis, MN). The probe was placed in the LV cavity and its temperature decreased to −55°C for ∼5 min (31, 32).
Histological analysis.
Percent tissue fibrosis was determined from routinely prepared histological sections stained by Masson's trichrome method for collagen as we previously described (28). Briefly, using a grid that divided the field of view into 100 squares, the number of collagenous tissue (blue stain) at the 100 intersection points in the grid was scored as 1 (present) or 0 (absent). Results are expressed as percent occupied by fibrosis to the total area examined. Four to five longitudinal sections were made from the LV anterior base and midwall and the posterior LV and two to three longitudinal sections in the RV in five aged and five adult hearts. Necrotic tissue in cryoablated hearts was visualized grossly using 1% triphenyl tetrazolium chloride (TTC) that stains viable myocardium red, whereas necrotic tissue fails to stain (32). Distribution of gap junction connexin (Cx)43 was evaluated by immunohistochemical staining for Cx43 as described previously (35). Briefly, after the conclusion of the electrophysiological studies, the hearts were fixed in 4% neutral buffered formalin. Five transmural sections (5 micrometer thick) were made through the base and the midwall of the LV epicardium in each of the four aged and four adult hearts stained for Cx43. The paraffin embedded sections were stained with Cx43 using a modified immunocytochemical ABC method. The slides were then incubated with the primary Cx43 antibody overnight at 4 degrees centigrade The secondary antibody, biotinylated mouse IgG, was then applied for 10 min, followed by horseradish peroxidase for 30 min and then the chromagen diaminobenzidine for 10 min. Finally the slides were counterstained in weak hematoxylin for 5 min and coverslipped. Immunostaining was performed in pairs, with adult and aged tissues isolated from identical LV sites. Each sample was examined under an Olympus microscope (BX60). Ten to 12 fields of longitudinally sectioned fibers from each animal were then analyzed using Image-Pro Plus (Media Cybernetics, Silver Spring, MD) to quantify Cx43 positive spots. The amount of Cx43 was expressed as a percentage of the total cellular and extracellular area.
Statistical analyses.
Significant differences in the incidence of VF (dichotomous comparisons) were determined using Fisher exact test. Likelihood ratio test was used to determine significance of site-specific origination of focal activity in the LV. Changes in Cai2+ decline rate constant, τ, and AP properties were determined using repeated-measures ANOVA. Differences among individual means were verified subsequently by Newman-Keuls post hoc tests. Since normality of τ-to-APD ratio distribution cannot be assumed to exist, we therefore used the bootstrapping methods to detect significant differences (7, 25). To compare the ratios, we therefore used bootstrap methods with random resampling (1,000 times) with replacement. If the actual value was greater than 975 of the 1,000 values of the random re-sampling, we concluded that the F value was statistically significant at the 0.025 level (2-sided test for α = 0.05) (7, 25). P ≤ 0.05 was considered significant. All data are presented as means ± SD.
RESULTS
Effects of GI on cardiac rhythm in intact aged and adult hearts.
In control experiments, no spontaneous arrhythmias occurred in four aged and four adult hearts perfused for up to 3 h with oxygenated Tyrode's perfusion containing glucose. When glycolysis was inhibited by replacing glucose with pyruvate in the arterial perfusate, however, VF occurred spontaneously during sinus rhythm (CL = 374 ± 120 ms) after a mean of 22 ± 8 min in 29 out of 31 aged rat hearts, but in none of the 18 young adult hearts exposed to GI for up to 2 h (P < 0.001). VF was preceded by a transient period of VT at a CL = 71 ± 25 that arose suddenly from sinus rhythm, as shown in Fig. 1A. Optical mapping (Fig. 1B) showed that the VT had a focal mechanism, originating from the base of the LV epicardium in 24 out of 35 episodes (69%) in 10 mapped hearts. The focal VT propagated repeatedly as single wavefront from base to apex (Fig. 1B) but within 2 s degenerated to VF (mean CL, 48 ± 12 ms; Fig. 1, A and B). Transition from VT to VF was characterized by a wavebreak midway between the base and the apex (Fig. 1, B and C). The two ends of the wavefronts then propagated laterally to join together at the base of the heart and re-enter through the site of the initial block inscribing a figure 8 type reentry (Fig. 1B, snapshot 128 ms). Wavebreak resulted from spatially discordant APD alternans when a site with short APD propagated to a site with long APD as shown in Fig. 1D optical AP recordings. In the remaining 31% of the VF episodes, the origin of the VT preceding the VF could not be determined. In these episodes a single wavefront entered the mapped region either from the apex (7 episodes, 20%) or the left lateral LV (4 episodes, 11%). The effects of GI were reversible. Replacing pyruvate with glucose suppressed the spontaneous episodes of VF within 30 min (N = 10).
Fig. 1.
Initiation of ventricular tachycardia (VT)/ventricular fibrillation (VF) in an aged heart exposed to glycolytic inhibition (GI). A: a pseudo-ECG showing the last 2 sinus beats before the sudden onset of the VT that leads to VF. B: snap shots of the first and the last beat of the VT and the first beat of the VF. VT is caused by a focal activation arising from the base of the heart that propagates without block (yellow arrows; snap shots, 0 to 68 ms). However, the beat after the last VT beat undergoes functional conduction block toward the apex (snap shots, 102) and shown schematically by double horizontal lines in C. The front, however, continues to propagate laterally on both sides of the block, causing Fig. 8 reentry and subsequent transition from VT to VF as shown in A. D: optical action potentials recorded from base to apex (numbered 1 to 10) during the last 4 VT beats and at the onset of VF showing that the site of block corresponds to the site of long action potential duration (APD; double horizontal red lines) during the emergence of spatially discordant APD alternans. In each snap shot the activation time is in milliseconds (bottom right) with time zero (arbitrary) coinciding with the onset of beat 1 and subsequent VT beats. The red color in the snap shots represents depolarization (Dep) and the blue repolarization (Rep) as shown in E. Downward pointing arrows indicate the direction of propagation. L, long; S, short.
Cellular mechanism of the focal VT leading to VF.
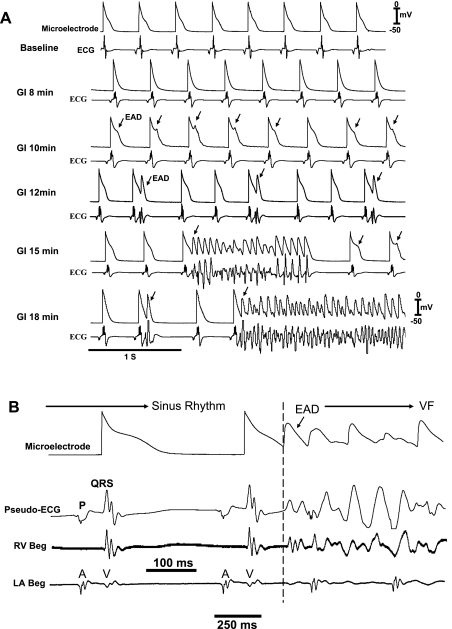
To gain insight into the cellular mechanism of focal VT preceding VF in aged hearts, VF was terminated with an electrical shock. Since focal VT typically originated at the base of the LV epicardium, a myocyte in this region was impaled with the roving glass microelectrode to capture the onset of the next VT/VF episode (N = 18). As shown in Fig. 2A, EADs arose initially and caused single triggered beats (12 min) registering as ventricular ectopic beats on the ECG, which after a mean of 22 ± 6 min led to short runs of triggered activity (nonsustained VT) and then VF, respectively (Fig. 2A). EADs arose from a mean membrane potential of −56 ± 12 mV (N = 18) at a time when the ECG, LV, and left atrial electrograms were isoelectric, indicating absence of an outside electrical source to drive the impaled cell exhibiting the EADs (Fig. 2B).
Fig. 2.
Time course of emergence of epicardial early afterdepolarizations (EADs), triggered activity, and VF in an aged heart exposed to GI. A: simultaneous microelectrode (top) and pseudo-ECG (bottom) recordings at baseline and at increasing time (18 min) after GI. EADs emerge 10 min after GI after APD shortening (8 min post-GI); EAD-mediated single triggered APs arise after 12 min causing premature ventricular depolarization as seen on the pseudo-ECG, which then evolve into short runs of triggered activity causing VT (15 min), which then degenerates to VF (18 min post-GI). B: epicardial EAD emerges when the ECG and the right ventricular (RV Beg) and left atrial (LA Beg) electrograms manifest isoelectric interval, indicating absence of electrical activity elsewhere in the heart.
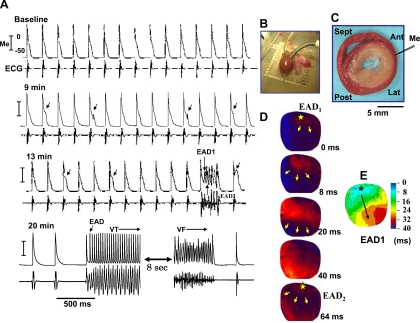
To exclude the possibility that breakthrough excitation from underlying tissue might be the cause of epicardial EADs, we cryoablated the endo- and midmyocardial tissues in five aged hearts, sparing only a thin rim of LV surviving epicardial tissue as shown in Fig. 3. GI still caused epicardial cells to manifest EADs, triggered activity, and VF in all of five cryoablated hearts studied, indicating that the endo- and midmyocardial cells including endocardial Purkinje fibers are not the sole source of EADs and that epicardial cells have an intrinsic ability to generate EADs. However, unlike noncryoablated hearts, VF in cryoablated aged hearts was not sustained beyond 10 s and self-terminated spontaneously, perhaps due to reduced tissue mass (17).
Fig. 3.
The emergence of epicardial EADs, triggered activity, and VF in a cryoablated aged heart exposed to GI. A: a pair of simultaneous microelectrode (Me; top) and pseudo-ECG (bottom) recordings at increasing time after the onset of GI. Arrows indicate EADs and short run of triggered activity causing VT, which eventually degenerates to VF 20 min post-GI period. B: placement of ablation catheter in the LV. C: triphenyltetrazolium chloride-stained cross-sectional view of the cryoablated ventricle with whitish areas corresponding to necrotic tissue with a thin rim of viable epicardial tissue (reddish area) that survives in the subepicardial myocardium. D: snap shots of the first and second (EAD1 and EAD2) triggered beats that arise from the base of LV (*). E: isochronal map of EAD1. Ant, Sep, Lat, and Post are the anterior, septal, lateral, and posterior wall of the LV, respectively. Yellow arrows indicate the direction of propagation.
Effects of GI on APD, Cai2+ cycling, and CV.
GI shortened APD90 in both age groups but had no effect on the other AP parameters (Table 1). GI also prolonged the Cai2+ transient decay time constant (τCa; Table 1), with greater slowing in the aged compared with young adult hearts (P < 0.01; Table 1). In aged hearts, τCa also exhibited significant heterogeneity over the LV epicardial surface (base vs. apex) both before and after GI (Fig. 4A). Overall, the ratio of τCa to APD90 was significantly (P < 0.01) greater in aged compared with young adult hearts (Fig. 4E and Table 1). CV from LV epicardial base to apex and measured during pacing at a CL of 250 ms was significantly slower in the aged compared with adult hearts both before and after GI.
Table 1.
Effects of GI on action potential & Cai2+ transient in aged and adult rat hearts
| Adult |
Aged |
|||||
|---|---|---|---|---|---|---|
| Parameters | Baseline | GI | P | Baseline | GI | P |
| RMP, mV | −83 ± 0.1 | −83 ± 13 | NS | −82.6 ± 0.7 | −83 ± 0.8 | NS |
| APA, mV | 108 ± 9 | 104 ± 13 | NS | 102 ± 8 | 108 ± 12 | NS |
| APD90, ms | 92 ± 10 | 76 ± 4 | <0.05 | 98 ± 11 | 81 ± 12 | <0.05 |
| APD50, ms | 28 ± 11 | 24 ± 8 | NS | 30 ± 9 | 26 ± 8 | NS |
| dV/dtmax, V/s | 168 ± 20 | 176 ± 21 | NS | 170 ± 26 | 172 ± 16 | NS |
| Slope APD90 R | 1.1 ± 0.2 | 1.1 ± 0.5 | NS | 1.3 ± 0.5 | 1.3 ± 0.6 | NS |
| Cai2+ τ, ms | 71 ± 2 | 80 ± 3 | P < 0.05 | 82 ± 6 | 101 ± 8* | P < 0.05 |
| τ/APD90 | 0.71 ± 0.07 | 0.98 ± 0.08* | P < 0.05 | 0.82 ± 0.09 | 1.28 ± 0.19*† | P < 0.05 |
| CV, cm/s | 40 ± 12 | 41 ± 13 | NS | 28 ± 10 | 26 ± 14‡ | NS |
Values are means ± SD.
GI, glycolytic inhibition; Cai2+, intracellular calcium ion concentration; RMP, resting membrane potential; APA, action potential amplitude (in mV); APD90 and APD50, action potential duration to 90% and 50% repolarization, respectively; dV/dtmax, maximum slope of phase zero AP; τ, calcium transient decline rate constant; CV, conduction velocity; NS, not significant.
Significantly (P < 0.05) larger than baseline in both groups;
significantly (P < 0.05) larger than adult GI;
significantly (P < 0.05) larger than adult GI. All measurements were made at a PCL of 250 ms. APD90 restitution curves were constructed during dynamic pacing starting at PCL of 250 ms to 120 ms in 20- to 10-ms decrements.
Fig. 4.
Calcium transient decay time constant (τ) in young and aged hearts exposed to GI. A: regional differences in τ in an aged heart before (baseline) and after (blue) GI during pacing at a cycle length (PCL) of 250 ms. B: a superimposed recording of intracellular calcium ion concentration (Cai2+) transients and microelectrode recording at the base of an aged heart before and after GI. C: superimposed LV basal single cell microelectrode recording and underlying Cai2+ transients. Arrows indicate the percent Cai2+ transient amplitude relative to peak amplitude at membrane potential of −60 mV in both groups before and after GI. In aged hearts the level (81%) was significantly higher than in adult hearts after GI.
The combined effect of shortened APD90 and prolonged τCa during GI led to Cai2+ being much more elevated over the voltage range at which EADs arose (around −60 mV). In aged hearts, Cai2+ remained at 70 ± 13% of the peak Cai2+ transient value, compared with 48 ± 4% in young adult hearts (P < 0.05; Fig. 4E). Elevated Cai2+ during repolarization may facilitate EAD formation (32, 36, 44) by stimulating forward mode of NCX (34, 38). Consistent with this idea, the emergence of EADs coincided in time with a mean Cai2+ level of 70 ± 13% of the peak systolic Cai2+ transient amplitude (N = 6) as shown in Fig. 5A by triple microelectrode and dual optical V-Cai2+ mapping. In contrast, when the APs were not associated with EADs, the Cai2+ level at −60 mV (mean EAD takeoff voltage) was 35 ± 5% (P < 0.01; Fig. 5, A and B).
Fig. 5.
Simultaneous microelectrode and dual optical voltage (V)-Cai2+ transient recordings at the onset of VT/VF in an aged heart exposed to GI. A: simultaneous dual optical voltage (V) and Cai2+ transients, ECG, and microelectrode (Me) recordings from the site of the origin of the focal VT located at the base of the LV as shown by the V-Cai2+ snap shots in B. Two consecutive triggered beats are shown in B with their corresponding isochronal maps in C. Notice that the cellular EAD recorded with the microelectrode in A coincides in time with a Cai2+ transient level that is 68% of the peak systolic Cai2+ transient amplitude, whereas the AP without an EAD (upward pointing arrow) has a corresponding Cai2+ level that is 45% of the peak Cai2+ transient amplitude. D: another example of simultaneous optical V-Cai2+ showing that the simultaneous shortening of the APD and slowing of the Cai2+ transients decline rate is associated with EADs arising at a Cai2+ level of 80% of the peak Cai2+ transient amplitude. O-AP, optical action potential.
The effects of KATP channel block of GI-mediated VF.
The KATP channel blocker glibenclamide (1 μM) reversed APD shortening by GI and suppressed the VF in five out of seven aged hearts (P < 0.05; Tables 2 and 3). In contrast, the selective mitochondrial KATP channel blocker 5-HD (1 μM) had no effect on GI-mediated VF in all seven aged hearts studied and failed to reverse APD shortening caused by GI (Tables 2 and 3).
Table 2.
Effects of glybenclamide, 5-HD, and SEA0400 on GI-induced shortening of APD in aged hearts
| GI | GI + 5-HD | GI + SEA0400 | GI + Glybenclamide | |
|---|---|---|---|---|
| APD50, ms | 30.4 ± 4.3 | 29.4 ± 6.3 | 29.9 ± 8.0 | 32.6 ± 6.3 |
| APD90, ms | 84.6 ± 8.7 | 85.6 ± 4.7 | 67 ± 4.4 | 96.8 ± 11.4* |
| APA, mV | 100 ± 7.5 | 97.6 ± 5.8 | 100 ± 5.1 | 100.6 ± 11.8 |
| dV/dtmax, V/s | 176 ± 11.4 | 170 ± 15.3 | 180 ± 27.7 | 171.6 ± 7.7 |
Values are means ± SD.
5-HD, 5-hydroxydecanoate.
P < 0.05 compared with GI.
Table 3.
Effects of pharmacological agents on GI-induced VF in aged hearts
| Glybenclamide | 5-HD | KN-93 | KN-92 | NAC | SEA0400 | |
|---|---|---|---|---|---|---|
| n | 7 | 7 | 9 | 6 | 8 | 6 |
| P | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.01 | P < 0.01 | P < 0.09* |
| VF suppression | ||||||
| Yes | 5 | 0 | 5 | 0 | 0 | 3 |
| No | 2 | 7 | 4 | 6 | 8 | 3 |
N, number of hearts.VF, ventricular fibrillation; NAC, N-acetyl-cysteine.
In 3 hearts, SEA0400-induced severe sinus bradycardia; ventricular pacing at a cycle length of 350 ms restored the protective effect of SA0400 against GI-mediated VF in all 3 hearts. Inclusion of these 3 hearts rendered the protective effect of SEA0400 against GI-induced VF (6 out of 6 hearts) significant (P < 0.01).
The influence of CaMKII inhibition on GI-mediated VF.
To evaluate whether CaMKII, activated by the Cai cycling alterations induced by GI, contributed to EAD-mediated arrhythmias and VT/VF (8), we tested the effects of the CaMKII inhibitor KN-93 (45) on GI-mediated VF, using its inactive form KN-92 as a control. As shown in Table 3, KN-93 (1 μM) suppressed GI-mediated EADs in five out of nine aged hearts studied (P < 0.05) without affecting Cai2+ transient decline rate constant or APD (data not shown). In contrast, KN-92 (1 μM) had no effect on GI-mediated VF (Table 3) or on Cai2+ transient or APD in any of the six aged hearts studied (data not shown). These findings suggest that CaMKII activation may contribute to GI-mediated EADs and VT/VF in aged hearts, but its effects are not overriding or absolutely necessary.
Redox state and GI-mediated VT/VF.
Although GI shifts the cellular redox balance to a pro-oxidant state (21), we investigated the role of the reducing agent NAC (16) on GI-mediated VF. As shown in Table 3, NAC (2 mM) had no effect on GI-mediated VF either when given before (15 min) GI onset (N = 4) or after GI had already induced VF (N = 4).
Effects of SEA0400 in preventing GI-mediated VF.
The influence of SEA0400 (2 μM) was evaluated in six aged hearts. In three hearts, SAE0400 had no effect on the sinus heart rate, but in three hearts it slowed heart rate from a mean of 180 ± 16 beats/min to 95 ± 14 beats/min. SEA0400 prevented GI-induced VF in all three hearts with no sinus slowing, but failed to protect against GI-induced VF in the three hearts with severe sinus bradycardia. Pacing of the latter hearts at a CL of 350 ms restored the protective effect of SEA0400 against GI-induced VF.
Tissue fibrosis and Cx43 immunostaining.
Quantitative analysis using Masson's trichrome stain showed significantly greater fibrosis in the aged compared with young adult ventricles (averaging 35 ± 10% vs. 3.4 ± 1%; P < 0.001). Fibrosis in aged hearts was heterogeneous in distribution, with dense subendocardial fibrosis and intermediate levels of fibrosis in the midventricular and subepicardial myocardium (Fig, 6), consistent with our previous findings (32). Quantitative analysis of Cx43 immunostaining at the base and the midwall of the LV showed significantly less Cx43 positive spots in the aged compared with adult ventricles (0.8 ± 0.02% vs. 1.7 ± 0.4%; P < 0.05), as shown in Fig. 7.
Fig. 6.
Quantitative tissue fibrosis in aged and adult ventricles. A and B: trichrome-stained cross-sectional views of the ventricles in adult (top) and aged (bottom) hearts. Notice the highly fibrotic (blue stain) subendocardial region of the aged hearts; C and D are higher magnification sections made at the LV epicardial base in adult and aged hearts, respectively. E: mean percent ventricular fibrosis in adult and aged hearts. F: specific LV epicardial (G) and endocardial areas of percent fibrosis in aged hearts. NS, not significant. *P < 0.01.
Fig. 7.
Consecutive longitudinal sections stained for connexin (Cx)43 and for fibrosis with Masson trichrome stain in adult (left) and in an aged (right) rat hearts. In the hearts the 2 consecutive sections are made at the base the LV epicardial surface. Notice the abundance of Cx43 positive spots in the adult hearts (black arrows) with minimal or no fibrosis (bottom left). In contrast, notice in the aged heart the paucity of Cx43-stained positive spots (black arrows, top right) with extensive fibrosis (bottom left).
Body and heart weight.
The body and hearts weights of the aged rats were significantly (P < 0.01) heavier than adults rats (421 ± 41 vs. 331 ± 59 g and 1.4 ± 0.3 and 0.9 ± 0.1 g, respectively). Ratio of heart to body weight in milligrams per gram was 3.32 ± 0.8 in the aged versus 2.71 ± 0.64 (P < 0.05), indicating mild to moderate myocyte hypertrophy as shown in Figs. 6 and 7.
DISCUSSION
Major findings.
The predisposition of aged, but not young adult, hearts to EAD-mediated triggered activity, VT and VF during the relatively mild metabolic stress of GI constitutes the major finding of this study. GI caused simultaneous shortening of the APD and slowing of Cai2+ decline rate, resulting in maintained elevation of Cai2+ during phase 3 repolarization, a condition that has been previously shown to promote late phase 3 EADs in fibrotic rabbit ventricles (34) and in canine pulmonary veins (38) and atria (3).
Although we have observed EADs to occur both during phase 2 and during late phase 3, the phase 2 EADs did not cause triggered beats. However, as the period of GI prolonged, the highly arrhythmogenic late phase 3 EADs predominated, leading to triggered activity, VT and VF.
Maintained elevation of Cai2+ enhances the forward mode NCX, generating a net depolarizing inward current (26, 38). The ability of NCX inhibitor SEA0400 to prevent GI-mediated EADs supports this scenario. However, the protective effect of NCX inhibition was rate dependent and was lost when the SEA0400 significantly slowed the sinus rate. This may indicate that the EAD-mediated arrhythmias during bradycardia were less dependent on NCX and more dependent on the reactivation of the l-type Ca current and reduced repolarization reserve associated with bradycardia (56).
Although GI had qualitatively similar influences on APD and Cai2+ in both age groups, the slowing of the Cai2+ transient decline rate was quantitatively greater and more heterogeneous in aged hearts. However, this difference in Cai2+ decline rate could not fully explain the differential arrhythmic response to GI in the aged versus young adult hearts, because slowing of the Cai2+ decline rate with the SERCA2a inhibitor thapsigargin (5 μM) failed to promote VF in the absence (N = 4) and presence (N = 4) of GI all eight adult hearts studied (data not shown). Similarly, the difference between the two age groups could not be explained by differences in APD restitution, since the slopes of the restitution curves were not different between two age groups both at baseline and after GI. However, it is possible that other aging-related electrophysiological remodeling factors contributed to the greater susceptibility of aged hearts to EAD-mediated arrhythmias during GI. For example, GI caused significantly greater disparity in APD90 and the calcium transient duration in the aged compared with young hearts (Table 1), which may have contributed to the greater vulnerability of the aged hearts to GI-mediated VT/VF.
The role of fibrosis.
The most striking histological difference between the aged and the adult rat ventricles was the presence of extensive fibrosis in the aged hearts, consistent with previous reports (10, 36). Aging is also associated with reduced gap junctional Cx43, as shown previously by Spach and Dolber (41) and observed in aged rat hearts (Fig. 6). Both increased fibrosis and gap junction loss reduce the electrical coupling between myocytes, making it more likely that the relatively weak currents producing EADs can overcome the source-to-sink mismatch imposed by adjacent normally repolarizing tissue (11, 55). It is of interest to note the relative preferential origination of EADs (69% of the episodes) from epicardial cells located at the base of the LV, which had an intermediate level of fibrosis (i.e., ∼35%) compared with other regions. In a previous study (32), simulations suggested that intermediate fibrosis provides a more favorable sink-source relationship for EAD formation and propagation at the tissue level, since with too much fibrosis (e.g., LV endocardium) EAD-mediated triggered beats fail to propagate, whereas with too little fibrosis (e.g., right ventricle, LV apex, septum), the source-sink mismatch remains too great for EADs to form.
The role of KATP channels.
APD shortening during GI was prevented by the sarcolemmal KATP channel blocker glibenclamide, implicating activation of sarcolemmal KATP channels as the mechanism of APD shortening. This finding is consistent with previous studies showing that glycolytically derived ATP preferentially regulates sarcolemmal KATP channels (48). Since glibenclamide blocks mitochondrial as well as sarcolemmal KATP channels, we also tested the effects of selective mitochondrial KATP channel blocker 5-HD, which did not reproduce the effects of glibenclamide at preventing either APD shortening or EAD-mediated arrhythmias. Despite preventing EAD-mediated arrhythmias in aged hearts, glibenclamide had no effect on Cai2+ dynamics (22, 54), strongly suggesting that APD shortening plays a critical role in GI-mediated EADs and VF.
The roles of CaMKII and oxidative stress.
Consistent with studies in isolated cardiac myocytes subjected to GI (21), the reducing agent NAC did not prevent EADs, suggesting that changes in the redox state of the cell did not play a major role, even though oxidative stress is known to induce EAD-mediated VT/VF in aged rat hearts via oxidative activation of CaMKII (32). Nevertheless, CaMKII activation, which promotes EADs by altering L-type Ca2+ current and enhancing late Na+ current (9, 45, 49, 50), did appear to contribute to EAD-mediated arrhythmias during GI, since CaMKII inhibition with KN-93 (but not its inactive form KN-92) was partially effective in preventing GI-mediated EADs and VF in five of nine aged hearts.
Clinical implications.
VF is the most common cause of sudden cardiac death, which prematurely claims the lives of ∼300,000 persons every year in the US (39). Since animal models of spontaneous VF arising from otherwise stable background cardiac rhythms are rare, the isolated aged rat heart model may be of value in evaluating how altered metabolic, oxidative, and ionic factors predispose to spontaneous VF, which would result in sudden cardiac death in an intact animal. Although the clinical relevance of GI may be questioned, we point out that replacement of glucose with pyruvate is a relatively mild form of GI. Normally the heart prefers fatty acids over glucose, and a variety of diseased cardiac conditions, including hypertrophy, heart failure, and diabetes, are characterized by a reduction in glucose oxidation (5, 18, 33). Importantly, a major consequence of chronic oxidative stress in the heart is GI (4), which may amplify the alterations in Cai2+ cycling and APD shortening to exert adverse arrhythmic consequences. The evidence linking oxidative stress to GI may be relevant to the increased risk of atrial fibrillation (AF) as well as shown by us (36) and others in animal models and in humans (6, 43). Strategies to enhance glycolysis and/or reverse tissue fibrosis may be new therapeutic targets to reduce the risk of VF and AF in diseased and aged hearts.
Limitations.
When the EADs were recorded at the LV base using an intracellular microelectrode, the proximity to the site of origin of focal VT is only approximate. However, because the tissue is a syncytium, the membrane voltage cannot vary tremendously over a length scale shorter than the tissue electrical space constant, typically 1 to 2 mm, which includes many thousands of myocytes. Therefore, even though the microelectrode is impaling a single myocyte, it reflects the voltage of thousands of adjacent myocytes as well (51). As the EAD propagates further away from its site of origin into surrounding tissue, the take-off potential becomes lower and lower as the impulse encounters progressively more repolarized tissue. Therefore, the EAD take-off potential may be more positive than our estimate of −56 mV, if the recording site was several millimeters or more away from at the true site of origin. However, because we observed a similar EAD take-off potential in the cryoablated hearts, which excludes a site of origin more than 1 mm below the epicardial surface, we do not think that the error is likely to be too large. As indicated in results, we cannot exclude the possibility that EADs could also originate in intact hearts from deeper myocardial cells and endocardial Purkinje fiber network. In fact 31% of the VF episodes in the intact hearts originated from the outside of the mapped region. The origin of these triggers could have been the endocardial Purkinje fiber network. However, our cryoablation studies destroying the entire mid- and endocardial layers indicate that epicardial cells are capable of generating EAD-mediated triggered activity and VF. The contribution of epicardial cells in the genesis of EADs is further suggested by the presence of isoelectric interval on the ECG during the emergence of epicardial EADs, indicating the absence of electrical activity elsewhere in the heart preceding the emergence of epicardial EADs. It may be argued that the observed differences in the arrhythmic outcomes between the adult and aged groups could be an artifact of the use of the excitation-contraction uncoupler CytoD, which have been shown to have different effects on APD in failing versus normal myocytes (53). However, the fact that GI caused APD shortening in both groups suggests that the observed differences in the two groups were not the result of a preferential effect of CytoD on aged compared with young/adult hearts. CytoD also reduces energy demand, which makes the energetic consequences of GI less severe. This suggests that the effects of GI might be further exacerbated in a working heart preparation, although previous studies have shown global ATP and creatine phosphate levels are well maintained when glucose is replaced by pyruvate in this setting (47).
GRANTS
This study was supported by AHA Western States Affiliate Research Fellowship Award (0725218Y) and a Grant-in-Aid (0555057Y); National Institutes of Health Grants P01 HL-78931 and R01 HL-103662; the Laubisch, Kawata and Medtronic-Zipes Endowments; and the Electrocardiographic Heart Beat Organization (Los Angeles, CA).
DISCLOSURES
There is no conflict of interest between the authors and any entity with respect to the subject matter discussed in this paper.
ACKNOWLEDGMENTS
We thank Dr. Alan Garfinkel for statistical consultation, Anna Frid for data analyses, Matthew Uy, and Pargol Samanianpour, Nicole Petrochuk, Shuzen Zhang, and Longsheng Hong for technical assistance. Aneesh Bapat was a research fellow supported by the Sarnoff Cardiovascular Research Foundation.
REFERENCES
- 1. Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther 287: 996–1006, 1998 [PubMed] [Google Scholar]
- 2. Blatter LA, Kockskamper J, Sheehan KA, Zima AV, Huser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol 546: 19–31, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 107: 2355–2360, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Corretti MC, Koretsune Y, Kusuoka H, Chacko VP, Zweier JL, Marban E. Glycolytic inhibition and calcium overload as consequences of exogenously generated free radicals in rabbit hearts. J Clin Invest 88: 1014–1025, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donthi RV, Ye G, Wu C, McClain DA, Lange AJ, Epstein PN. Cardiac expression of kinase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J Biol Chem 279: 48085–48090, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases 1. Circulation 112: 1266–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Efron B, Tibshirani R. Statistical data analysis in the computer age. Science 253: 390–395, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol 148: 16–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi H, Wang C, Miyauchi Y, Omichi C, Pak HN, Zhou S, Ohara T, Mandel WJ, Lin SF, Fishbein MC, Chen PS, Karagueuzian HS. Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol 13: 801–808, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Huelsing DJ, Spitzer KW, Pollard AE. Electrotonic suppression of early afterdepolarizations in isolated rabbit Purkinje myocytes 1. Am J Physiol Heart Circ Physiol 279: H250–H259, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol 524: 795–806, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem 273: 13578–13582, 1998 [PubMed] [Google Scholar]
- 14. Janero DR, Hreniuk D, Sharif HM. Hydroperoxide-induced oxidative stress impairs heart muscle cell carbohydrate metabolism. Am J Physiol Cell Physiol 266: C179–C188, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Kagaya Y, Weinberg EO, Ito N, Mochizuki T, Barry WH, Lorell BH. Glycolytic inhibition: effects on diastolic relaxation and intracellular calcium handling in hypertrophied rat ventricular myocytes. J Clin Invest 95: 2766–2776, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy DJ, Vetteth S, Xie M, Periyasamy SM, Xie Z, Han C, Basrur V, Mutgi K, Fedorov V, Malhotra D, Shapiro JI. Ouabain decreases sarco(endo)plasmic reticulum calcium ATPase activity in rat hearts by a process involving protein oxidation. Am J Physiol Heart Circ Physiol 291: H3003–H3011, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kim YH, Garfinkel A, Ikeda T, Wu TJ, Athill CA, Weiss JN, Karagueuzian HS, Chen PS. Spatiotemporal complexity of ventricular fibrillation revealed by tissue mass reduction in isolated swine right ventricle. Further evidence for the quasiperiodic route to chaos hypothesis. J Clin Invest 100: 2486–2500, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King LM, Opie LH. Glucose and glycogen utilisation in myocardial ischemia–changes in metabolism and consequences for the myocyte. Mol Cell Biochem 180: 3–26, 1998 [PubMed] [Google Scholar]
- 19. Kobayashi K, Neely JR. Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res 44: 166–175, 1979 [DOI] [PubMed] [Google Scholar]
- 20. Kockskamper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol 545: 65–79, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kockskamper J, Zima AV, Blatter LA. Modulation of sarcoplasmic reticulum Ca2+ release by glycolysis in cat atrial myocytes. J Physiol 564: 697–714, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SY, Lee CO. Inhibition of Na+-K+ pump and l-type Ca2+ channel by glibenclamide in Guinea pig ventricular myocytes. J Pharmacol Exp Ther 312: 61–68, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Sato T, Seharaseyon J, Szewczyk A, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels. Viable candidate effectors of ischemic preconditioning. Ann N Y Acad Sci 874: 27–37, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol 34: 919–939, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Manly BFJ. Randomization, bootstrap, and Monte Carlo methods in biology. Boca Raton, FL: Chapman & Hall/CRC, 2007 [Google Scholar]
- 26. Milberg P, Pott C, Fink M, Frommeyer G, Matsuda T, Baba A, Osada N, Breithardt G, Noble D, Eckardt L. Inhibition of the Na+/Ca2+ exchanger suppresses torsades de pointes in an intact heart model of long QT syndrome-2 and long QT syndrome-3. Heart Rhythm 5: 1444–1452, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Miura T, Liu Y, Goto M, Tsuchida A, Miki T, Nakano A, Nishino Y, Ohnuma Y, Shimamoto K. Mitochondrial ATP-sensitive K+ channels play a role in cardioprotection by Na+-H+ exchange inhibition against ischemia/reperfusion injury. J Am Coll Cardiol 37: 957–963, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Miyauchi M, Qu Z, Miyauchi Y, Zhou SM, Pak H, Mandel WJ, Fishbein MC, Chen PS, Karagueuzian HS. Chronic nicotine in hearts with healed ventricular myocardial infarction promotes atrial flutter that resembles typical human atrial flutter. Am J Physiol Heart Circ Physiol 288: H2878–H2886, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Miyauchi Y, Hayashi H, Miyauchi M, Okuyama Y, Mandel WJ, Chen PS, Karagueuzian HS. Heterogeneous pulmonary vein myocardial cell repolarization implications for reentry and triggered activity. Heart Rhythm 2: 1339–1345, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Miyauchi Y, Zhou S, Okuyama Y, Miyauchi M, Hayashi H, Hamabe A, Fishbein MC, Mandel WJ, Chen LS, Chen PS, Karagueuzian HS. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation 108: 360–366, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Morita N, Lee JH, Xie Y, Sovari A, Qu Z, Weiss JN, Karagueuzian HS. Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol 57: 366–375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z, Weiss JN, Karagueuzian HS. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol 297: H1594–H1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novotny MV, Yancey MF, Stuart R, Wiesler D, Peterson RG. Inhibition of glycolytic enzymes by endogenous aldehydes: a possible relation to diabetic neuropathies. Biochim Biophys Acta 1226: 145–150, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Ogawa M, Morita N, Tang L, Karagueuzian HS, Weiss JN, Lin SF, Chen PS. Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm 6: 784–792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohara K, Miyauchi Y, Ohara T, Fishbein MC, Zhou S, Lee MH, Mandel WJ, Chen PS, Karagueuzian HS. Downregulation of immunodetectable atrial connexin40 in a canine model of chronic left ventricular myocardial infarction: implications to atrial fibrillation. J Cardiovasc Pharmacol Ther 7: 89–94, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Ono N, Hayashi H, Kawase A, Lin SF, Li H, Weiss JN, Chen PS, Karagueuzian H. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am J Physiol Heart Circ Physiol 292: H639–H648, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Opie LH, Nathan D, Lubbe WF. Biochemical aspects of arrhythmogenesis and ventricular fibrillation. Am J Cardiol 43: 131–148, 1979 [DOI] [PubMed] [Google Scholar]
- 38. Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins 1. J Am Coll Cardiol 47: 1196–1206, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318: 214–222, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res 58: 356–371, 1986 [DOI] [PubMed] [Google Scholar]
- 42. Van Emous JG, Vleggeert-Lankamp CL, Nederhoff MG, Ruigrok TJ, Van Echteld CJ. Postischemic Na+-K+-ATPase reactivation is delayed in the absence of glycolytic ATP in isolated rat hearts. Am J Physiol Heart Circ Physiol 280: H2189–H2195, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Van Wagoner DR. Electrophysiological remodeling in human atrial fibrillation. Pacing Clin Electrophysiol 26: 1572–1575, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res 46: 376–392, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 500: 631–642, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest 75: 436–447, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weiss JN, Lamp ST. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science 238: 67–69, 1987 [DOI] [PubMed] [Google Scholar]
- 49. Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac l-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol Heart Circ Physiol 276: H2168–H2178, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104: 79–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J 99: 1408–1415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu KY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res 77: 88–97, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Yang X, Salas PJ, Pham TV, Wasserlauf BJ, Smets MJ, Myerburg RJ, Gelband H, Hoffman BF, Bassett AL. Cytoskeletal actin microfilaments and the transient outward potassium current in hypertrophied rat ventriculocytes. J Physiol 541: 411–421, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yitzhaki S, Shneyvays V, Jacobson KA, Shainberg A. Involvement of uracil nucleotides in protection of cardiomyocytes from hypoxic stress. Biochem Pharmacol 69: 1215–1223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zaniboni M, Pollard AE, Yang L, Spitzer KW. Beat-to-beat repolarization variability in ventricular myocytes and its suppression by electrical coupling. Am J Physiol Heart Circ Physiol 278: H677–H687, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J 68: 949–964, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]