Abstract
Blood flow restoration to ischemic tissue is affected by various risk factors. The aim of this study was to examine gender effects on arteriogenesis and angiogenesis in a mouse ischemic hindlimb model. C57BL/6J mice were subjected to unilateral hindlimb ischemia. Flow recovery was less and hindlimb use impairment was greater in females. No gender difference in vessel number was found at baseline, although 7 days postsurgery females had fewer α-smooth muscle actin-positive vessels in the midpoint of the adductor region. Females had higher hindlimb vascular resistance, were less responsive to vasodilators, and were more sensitive to vasoconstrictors postligation. Western blotting showed that females had higher baseline levels of vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) in the calf, while 7 days postligation males had higher levels of VEGF, eNOS, and phosphorylated vasodilator stimulated phosphoprotein. Females had less angiogenesis in a Matrigel plug assay and less endothelial cell proliferation in vitro. Females have impaired recovery of flow, a finding presumably caused by multiple factors including decreased collateral remodeling, less angiogenesis, impaired vasodilator response, and increased vasoconstrictor activity; our results also suggest the possibility that new collateral formation, from capillaries, is impaired in females.
Keywords: angiogenesis, arteriogenesis, vasoreactivity
the incidence and outcome of peripheral artery disease (PAD) have been examined in both men and women. Interestingly, Hooi et al. (8) reported that the incidence of PAD is 1.5-fold higher in females than males, and Bevetti et. al. (2) found that women with PAD are more likely to develop critical limb ischemia than men.
The most important mechanism by which the body compensates for obstructive arterial disease is through flow supplied by collateral circulation. With the onset of obstruction, collateral vessels (which connect an adjacent or proximal arterial tree to the circulation downstream of the obstruction) provide perfusion to the ischemic tissue. The magnitude and severity of ischemic injury will depend on the number and diameter of preexisting collaterals and their capacity to positively remodel (enlarge) in response to the increased shear. This process is referred to as arteriogenesis, and the enlarging collateral requires the proliferation of endothelial cells, a process that is independent of ischemia (17). The presence of ischemia in tissue downstream to the obstruction also results in proliferation of capillaries, a process referred to as angiogenesis, and also requires EC proliferation. In addition to the arteriogenic and angiogenic processes, circulating, neurogenic, and endothelial cell-derived factors regulate tissue blood flow through their vasodilator and vasoconstrictor activities, which also influence arterial remodeling.
Although gender differences in the capacity to restore blood flow recovery to the lower limb following arterial obstruction have been reported, as have differences in vascular function, the results are controversial (11, 14, 18, 19). However, it appears well documented that women are less likely to survive a myocardial infarction and are known to have more complications in the presence of PAD (5, 7, 20). Therefore, the objective of this study is to test our hypothesis that female mice have impaired flow recovery following femoral artery occlusion and, if this hypothesis proves valid, to identify potential mechanisms responsible for the differences.
METHODS
All protocols were approved by the Institutional Animal Care and Use Committee of MedStar Health Research Institute. Male and female mice (C57BL/6J,12-wk-old) were obtained from the Jackson Laboratory (Bar Harbor, ME). Estrus cycle standardization in females was not performed in this study.
Mouse Hindlimb Ischemia Surgery
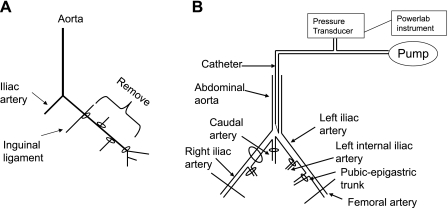
Mice were anesthetized by intraperitoneal administration of ketamine (30 mg/kg) and xylazine (6 mg/kg). The left femoral artery was exposed aseptically and ligated distal to inguinal ligament and proximal to the saphenous-popliteal bifurcation with 7–0 suture. The artery and all the branches were carefully dissected free from veins and nerves and excised (Fig. 1A).
Fig. 1.
A: diagram of surgery. Left femoral artery was ligated between the inguinal ligament and the bifurcation, which divides the artery into saphenous and popliteal arteries. Branching vessels between these ligatures were tied off, and the femoral artery was removed. B: diagram of in vivo perfused hindlimb preparation. To create a circuit solely perfusing the left femoral artery, the right iliac, caudal, left internal iliac, and pubic-epigastric arteries were ligated.
Laser Doppler Blood Flow Analysis
Scanning laser-Doppler perfusion imaging (Moor Instruments, Wilmington, DE) was used to record hindlimb perfusion before ligation and on postoperative days 0, 3, 7, 14, 21, and 28. Hair was removed by depilatory cream before imaging, and mice were placed on a heating pad at 37°C to minimize temperature variation. Average perfusion was recorded from the plantar surface, which indexes overall leg blood flow, and expressed as the ischemic-to-nonischemic ratio to account for variables, including ambient light and temperature (3).
Hindlimb Ischemia and Use
Seven days after ligation, hindlimb ischemia and use were assessed by assigning clinical-like appearance and muscle function scores, as previously described (3).
In Vivo Matrigel Plug Assay for Angiogenesis
Basic FGF was added to growth factor reduced Matrigel (BD Biosciences; 500 ng/ml), and plugs were injected subcutaneously into male (n = 5) and female (n = 5) mice. After 5 days, plugs were dissected and fixed in 10% formalin then transferred to 70% ethanol. Paraffin-embedded plugs were sectioned and stained with hematoxylin-eosin and examined by light microscopy. A blood vessel was defined as a vessel lumen with an identifiable cell layer lining the lumen and red blood cells inside. The number of vessels was counted by a blinded observer at ×200 magnification. For immunohistochemistry, antigen retrieval was achieved by boiling slides in antigen retrieval buffer (10 mM Tris-sodium citrate, pH 6.0) for 20 min. After blocking with 5% normal horse serum, slides were incubated overnight with primary antibody (rabbit anti-mouse CD31; 1:50; Abcam), biotinylated secondary antibody (Vector Labs, Burlingame, CA; 1:250) was added and developed using an ABC kit (Vector Labs). Images were analyzed (4 sections per mouse) using an Olympus light microscope at ×200 magnification.
Aortic Endothelial Cell Isolation and Proliferation Assay
Aortas were collected from male and female mice (n = 3 per group). Fat and connective tissue were rapidly removed, and aortas were cut into 2- to 4-mm rings and incubated in 0.25% trypsin + EDTA for 30 min at 37°C. The medium was collected and centrifuged at room temperature for 10 min at 1,000 g. The cell pellet was resuspended in 5 ml EGM-2 medium (Lonza) and cultured for 2 days. Unattached cells were discarded, and EGM-2 medium was added. After growing to confluence, the cells were split (1:2) into a 75-cm2 cell culture flask. Passage 4–6 cells were used for all experiments. Endothelial cell proliferation was assessed by MTT assay (ATCC) as previously described (22). The assays were run in triplicate and repeated three times.
α-Actin Staining
Seven days after ligation mice were anesthetized and perfused with vasodilators [papaverine (40 mg/l) and sodium nitroprusside (10−4 M)] and fixed with 2% paraformaldehyde. Thigh muscles were embedded in paraffin, sectioned, and stained for α-actin (mouse anti-human SMA; Dako), using a MOM kit (Vector Labs). The number of α-smooth muscle actin-positive vessels crossing the midpoint of the semimembranosus muscle (a collateral-rich region) were counted (3, 4).
Western Blot
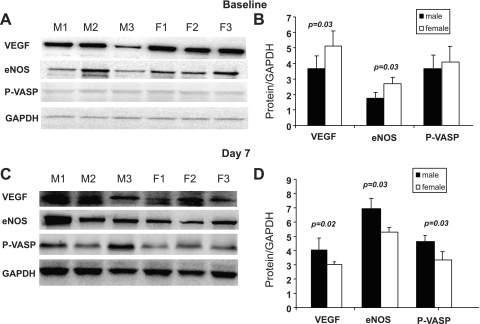
Calf muscle was collected at baseline (before ligation) and on day 7 after ligation from different groups of animals. The calf muscle was homogenized in lysis buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EDTA, and 1% NP40). Total protein was measured using a BCA kit (Pierce). Eighty-micrograms of protein was resolved using a 4–20% Tris-glycine gel and blotted onto a nitrocellulose membrane. Membranes were blocked with 5% milk and then incubated with primary antibody against VEGF (1:1,000; Santa Cruz), endothelial nitric oxide synthase (eNOS; 1:1,000; BD Biosciences), phosphorylated vasodilator stimulated phosphoprotein (p-VASP; 1:500; Calbiochem pSer239 Mouse-16C2), and GAPDH (1:1,000; Santa Cruz) at 4°C overnight and with corresponding secondary antibody for 1 h at room temperature. The signal was detected using enhanced chemiluminescence substrate (Pierce). Bands were quantified using Kodak Molecular Imaging Software–Standard Edition.
Pressure Measurement and Vasoreactivity: In Vivo Perfused Hindlimb Preparation
Experimental preparation.
Mice were anesthetized and injected with heparin (100 U/ml). The abdominal aorta and iliac artery were dissected free from veins. To create a circuit solely perfusing the left femoral artery, the right iliac, caudal, and left internal iliac arteries were ligated (Fig. 1B). For these experiments, the femoral artery was ligated and removed, as described above. The vena cava and femoral vein were cut before measurements were made. A 24-G catheter was inserted into the abdominal aorta and fixed by two 5–0 sutures. The other end of the catheter was connected to a three-way stopcock, one end of which was connected to a pump providing constant flow. A pressure transducer connected to the stopcock was connected to a Powerlab system to record pressure variation. The left leg was perfused and equilibrated for 20 min with physiological saline solution (PSS: 119 mM NaCl, 4.7 mM KCl, 1.17 mM MgSO4, 2.0 mM CaCl2, 25 mM NaHCO3, 1.2 mM NaH2PO4, 0.02 mM EDTA, and 5.0 mM glucose) containing 5 mg/ml albumin (Sigma) at a constant flow of 1 ml/min. To test responsiveness of the in vivo perfused hindlimb preparation, vessels were perfused with 5 uM phenylephrine (PE) and with 10 uM acetylcholine (ACh) using the infusion pump. Microfil dye was then injected to test the integrity of the flow system. If there was no response to PE or ACh, if the dye did not appear in the left toe, or if the dye appeared in the vessels of right hindlimb or pelvis, the animal was excluded from the experiment.
Experimental design.
Pressure measurements were obtained immediately following and at 10 days postfemoral artery ligation. To investigate gender differences in vasoconstrictor and vasodilator responsiveness of the vessel wall, we administered in the perfusate ACh, nitroglycerin (NTG), and PE separately in a series of concentrations (10−9M—10−4M) (6, 13, 21). To further investigate signaling mechanisms, we used 100 uM of the endothelial nitric oxide synthase blocker NG-nitro-l-arginine methyl ester (l-NAME). The vessels were perfused with PSS and hindlimb perfusion pressure was allowed to return to resting levels between each experimental test. At the end of the series of studies, the vessels were perfused with calcium-free PSS to attain maximal vasorelaxation. The pressure was automatically recorded using the Powerlab system. Resistance was calculated as resistance = pressure/flow. Since flow is constant throughout the experiment, changes in arterial pressure reflect changes in arterial resistance.
Data analysis.
Response to the vasodilators ACh and NTG was expressed as a percentage of maximal relaxation according to the formula: relaxation (%) = (Pb − Ps)/(Pb − Pm). Pb is the steady-state pressure recorded after the vessels were perfused with PSS. Ps is the pressure recorded after each addition of each drug. Pm is the pressure recorded during perfusion with calcium-free PSS. The response to PE was expressed as fold change of baseline pressure using the following formula: constriction (%) = (Ps − Pb)/(Pm − Pb) (13). Two-way ANOVA was used to detect differences between gender at different drug concentrations.
Statistical Analysis
All data are presented as means ± SE. Groups were compared by repeated-measures ANOVA. For differences between the two groups, a Students t-test was used. Differences were considered statistically significant at a value of P < 0.05. Sample size and power calculations were performed for this study. These data show that there was enough power to detect the difference between male and female mice for most measurements except for the preischemia NTG vasoreactivity experiment. Given the nature of biological studies and the data (see Fig. 5B), we are confident that there is no biological difference between male and female mice when NTG is used under preischemic conditions in this model.
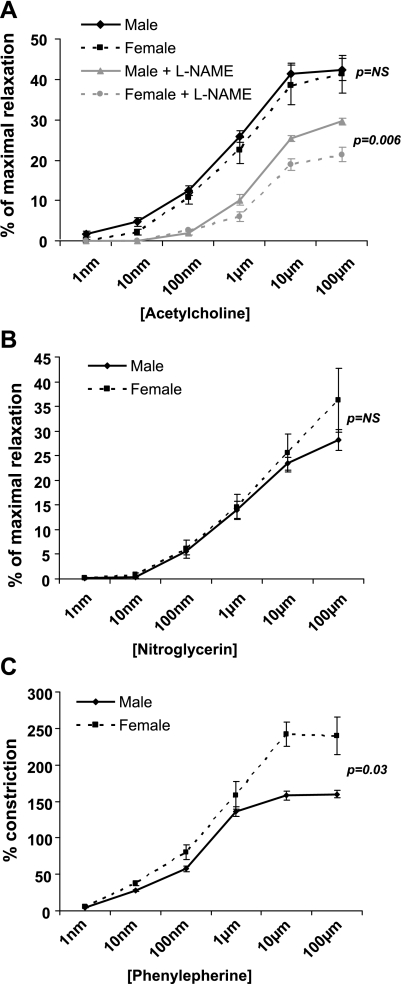
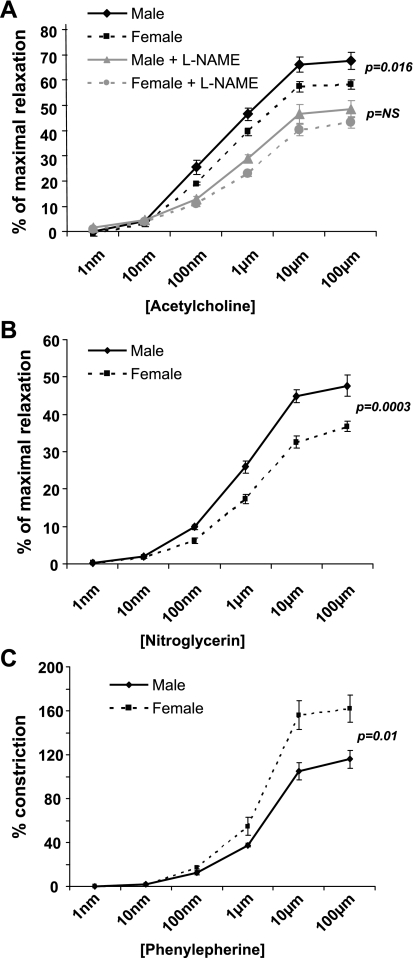
Fig. 5.
Vascular reactivity immediately following femoral artery ligation. A: vasoreactivity to acetylcholine (ACh). There is no difference between genders unless NG-nitro-l-arginine methyl ester (l-NAME) is used to block eNOS activity, and then males exhibit greater vasodilatation (P = 0.006). B: there is no difference in baseline vasoreactivity to nitroglycerin. C: females have a greater %vasoconstriction to phenylephrine (P = 0.03).
RESULTS
Hindlimb Blood Flow Recovery After Femoral Artery Ligation
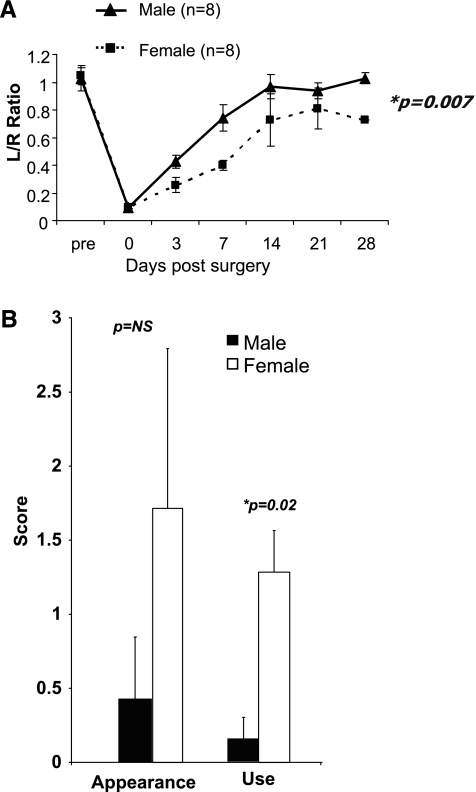
Immediately after ligation of the femoral artery (day 0), male and female C57BL/6J mice had similar blood flow, although during the 28-day follow-up period females had less blood flow recovery than males (Fig. 2; P = 0.007). Females had impaired hindlimb use on day 7 after hindlimb femoral artery ligation (P = 0.02), but there was no difference between males and females in the appearance score.
Fig. 2.
A: flow recovery in females is less compared with male mice (P = 0.007). L/R ratio, left (ligated) leg perfusion/right (nonligated) leg perfusion. B: while there was no significant difference in average appearance score, females had a higher average use score, indicating impaired hindlimb function (P = 0.02). Foot appearance score (index of ischemia): 0, normal; 1–5, cyanosis or loss of nail(s), where the score is dependent on number of nails affected; 6–10, partial or complete atrophy of digit(s), where the score reflects number of digits affected; and 11, partial atrophy of forefoot. Hindlimb use score (index of muscle function): 0, normal; 1, no toe flexion; 2, no plantar flexion; and 3, dragging foot. A higher score indicates impaired use or appearance.
In vivo Matrigel plug assay and in vitro proliferation assay.
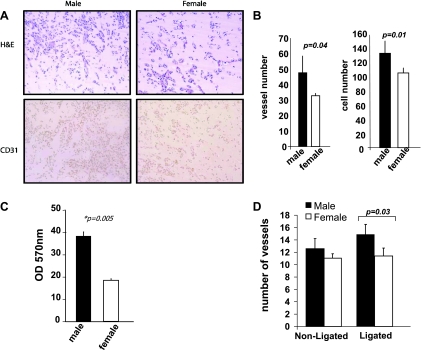
There were fewer red blood cell-containing vessels present in the Matrigel plug sections from female mice (33.2 ± 7.1 vs. 44.7 ± 10.9; P = 0.04) and fewer CD31-positive cells (101 ± 7.8 vs. 138 ± 10.9; P = 0.01) compared with the plugs from the male animals (Fig. 3, A and B).
Fig. 3.
A: representative images of cellular infiltration and CD31 staining in Matrigel plugs collected from male and female mice. B: males had a greater number of red blood cell-containing vessels (P = 0.04), and higher cellular infiltration (P = 0.01) compared with females. C: aortic endothelial cells (AECs) isolated from male mice showed a greater proliferative capacity as measured by MTT assay than female AECs (P = 0.005). D: 7 days postligation, male mice had a greater number of α-actin-positive vessels in the semimembranousus muscle compared with females (P = 0.03).
Aortic endothelial cells from female animals showed less proliferative capacity using an MTT assay compared with endothelial cells isolated from male mice (P = 0.005; Fig. 3C).
α-Smooth muscle actin staining.
The number of α-smooth muscle actin-positive vessels in the nonligated semimembranosus region was similar in female vs. male mice, although 7 days postsurgery females had fewer α-smooth muscle actin-positive vessels compared with males (11.4 ± 0.72 vs. 14.7 ± 0.97; P = 0.03; Fig. 3D).
VEGF and eNOS expression in hindlimb.
Under baseline conditions (before ligation), female mice had higher levels of VEGF and eNOS protein in calf muscle compared with males (P = 0.03) when normalized to an endogenous control (Fig. 4, A and B). p-VASP levels were similar between female and male mice. Interestingly, 7 days postsurgery, the levels of VEGF, eNOS, and p-VASP were lower in female calf muscle than male (P = 0.02, 0.035 and 0.02, respectively; Fig. 4, C and D).
Fig. 4.
A and B: Western blot of VEGF, eNOS, and phosphorylated vasodilator stimulated phosphoprotein (p-VASP) in calf muscle at baseline. Females had higher expression of VEGF and eNOS (P = 0.03 and 0.0,3 respectively). C and D: Western blots on day 7 show that males have higher levels of VEGF, eNOS, and p-VASP postischemia (P = 0.02, 0.035, and 0.02, respectively).
Pressure Measurement and Vasoreactivity: In Vivo Perfused Hindlimb Preparation
The pressure measurements for female and male mice immediately and 10 days after femoral artery ligation are shown in Table 1. Immediately after ligation there were no gender differences in baseline pressure and pressure with maximal dilation. On day 10 postligation, the pressure (which under the constant flow conditions we used is proportional to resistance) was higher in both genders compared with immediately postligation. However, absolute pressures in the runoff vessels (which include collaterals) were greater in female vs. male mice.
Table 1.
Characteristics of hindlimb vessels immediately after and 10 days postligation in male and female mice
| Immediate Postligation |
10 Days Postligation |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Spontaneous pressure, mmHg | 43.23 ± 3.18 | 45.78 ± 4.21 | 69.70 ± 5.11 | 84.78 ± 6.22* |
| Maximal dilated pressure | 24.64 ± 1.69 | 25.14 ± 1.95 | 45.29 ± 4.23 | 49.20 ± 4.82 |
Values are means ± SE. Immediately postligation there is no gender difference in pressure. Ten days postligation, females have a higher pressure compared with males.
P < 0.05.
Vasoreactivity studies.
Immediately after femoral artery ligation, ACh-induced vasodilation was not different between genders (Fig. 5A). Adding l-NAME reduced the ACh-induced vasodilation in both female and male animals, but the reduction in vasodilatation was greater in females, resulting in a significantly lower percentage of maximal relaxation than males (Fig. 5A; P = 0.006). This suggests that shortly after ligation eNOS-related mechanisms are more dominant in achieving ACh-induced vasodilatation in females than in males (a finding compatible with the gender-related differences in eNOS expression-see below). There was no difference in the vasodilation response to the NO donor NTG between females and males immediately after ligation (Fig. 5B). This suggests that the greater role of eNOS-related mechanisms in ACh-induced vasodilatation in females is not due to greater sensitivity to the vasodilator effects of NO.
Female mice had greater vasoconstrictor response to PE than males (P = 0.03; Fig. 5C).
Ten days postligation, in contrast to the findings immediately postligation, there were significant differences in ACh-induced vasodilatation, with females showing less relaxation than males (P = 0.016; Fig. 6A). Although l-NAME inhibited ACh-induced relaxation in both genders, this effect was less in females. Interestingly, l-NAME eliminated the differences in the response to ACh between genders. This suggests that over time, as collaterals remodel, males develop a more robust eNOS-mediated vasodilatation than females in response to ACh.
Fig. 6.
A: day 10 vasoreactivity to ACh. Males exhibit greater collateral vasodilatation in response to ACh compared with females (P = 0.016), but this difference is obliterated in the presence of l-NAME. B: males have a greater collateral vasodilation response to nitroglycerin following ischemia (P = 0.0003). C: females have greater %vasoconstriction to phenylephrine postischemia (P = 0.01).
Ten days postligation gender differences were also observed in the smooth muscle cell-dependent vasodilatation following NTG treatment, with females exhibiting less vasodilatation than males (P = 0.0003; Fig. 6B).
Similar to the findings immediately after femoral artery ligation, 10 days postocclusion PE-induced vasoconstriction was greater in females than males (P = 0.01; Fig. 6C).
DISCUSSION
In this study, we investigated the potential role of gender on the capacity of collaterals to augment blood flow recovery following the induction of hindlimb ischemia in a mouse model. We found that female mice have impaired recovery compared with males. As stated in methods section, we did not standardize the estrus cycle. In mice, the estrus cycle is ∼4–5 days in length. The flow studies were performed over a 28-day period. During this time, if the animals are cycling randomly, they will each have experienced multiple estrus cycles. We reasoned that any possible effects of cycle stages would be averaged out over the entire follow-up period. We expected that performing these studies in the absence of cycle standardization, there may be an increase in variability in results. Despite this limitation, we still found a significant difference between male and female flow recovery. Although we recognize that one of the benefits of using an animal model is the ability to standardize such potential confounders as the estrus cycle, we still believe our findings are valid, and perhaps more applicable to gender differences in humans, since the issue of variation in female hormonal cycles exists in the general population.
Immediately following ligation of the femoral artery, no gender-related differences were observed in the perfusion of the foot, as measured by laser Doppler imaging (Fig. 2A). This finding is consistent with two additional independent measurements we made. First, to measure vasoreactivity differences between genders, we surgically created a constant flow circuit draining solely into the left femoral artery (Fig. 1); pressures that develop during constant perfusion reflect resistance of the runoff vessels (which includes collaterals). We found that perfusion pressure was identical in males and females (Table 1).
Second, cross sections of the semimembranosus muscle (a collateral-rich zone; Refs. 3, 4) were stained, and the number of α-smooth muscle actin vessels was counted. There was no difference in the number of α-actin-positive vessels between female and male mice in the nonligated limbs (Fig. 3D), findings compatible with the results of Kyriakides et al. (11) who measured capillary and arteriole density in a rabbit model of hindlimb ischemia; they found no differences between genders in angiogenesis and arteriogenesis.
However, by day 7 postligation, although there was no change from baseline in the number of α-smooth muscle actin-positive vessels in the female hindlimbs, there was a significant increase in males. This is an intriguing observation. It cannot be explained by angiogenesis, as capillaries do not have a smooth muscle lining. It is possible that this difference reflects a transformation of preexisting capillaries into collaterals by recruitment of pericytes, a mechanism suggested by several investigators (12, 23) as a means of increasing the collateral circulation. Further studies would be required to definitively determine whether this mechanism does in fact occur.
An alternative explanation is that males do have a greater number of preexisting collaterals; however, immediately following ligation of the conduit artery they are too small to be detected by histology and are not yet functional. Over time these vessels remodel, more robustly in males vs. females, making them easier to recognize by histology. Gender differences in preexisting collateral number, accompanied by positive remodeling in the days following femoral artery occlusion, would explain the greater increase in blood flow recovery and improvement in limb function exhibited by males (Fig. 2, A-C). The functional relevance of the increased number of α-smooth muscle actin-positive vessels in male vs. female hindlimbs may further be reflected by the lower perfusion pressure (i.e., lower resistance) present in males vs. females by day 10 postfemoral artery ligation (Table 1). It is important to highlight that the observed gender differences in perfusion pressure may also result from gender-dependent disparities in the production of metabolic vasodilators, such as adenosine (10), or from variation in vascular permeability (9), and not only from differences in collateralization.
We also hypothesized that there are gender-based differences in vasoreactivity that may contribute to the large differences in flow recovery. In our constant flow perfusion circuit, we measured pressure (and therefore resistance) both immediately after femoral artery ligation and 10 days later. Although there was no gender difference in pressure detected immediately postligation (further supporting the blood flow recovery measurements immediately postligation that showed no difference), the 10-day measurement demonstrates that the runoff vessels of females have higher pressure, and thus resistance, than males. An important limitation to our studies is that the pressure measurements cannot distinguish between whether the increased resistance observed in the females is due to the presence of fewer vessels, or to an increased constriction in the existing arteries, or both.
Vascular resistance and function are also critically dependent on the hemostatic balance modulated by vasoconstrictors and vasodilators. We found that immediately after femoral artery ligation there was no gender difference in ACh-mediated vasodilation. However, when we administered l-NAME (1) to interrupt the NO/cGMP pathway in endothelial cells, females had an impaired response to ACh compared with males (Fig. 5A). Western blotting results indicate that female mice have higher levels of eNOS protein under baseline conditions (Fig. 4, A and B). It therefore would be expected that blocking the activity of eNOS with l-NAME immediately after femoral artery ligation would have a greater negative impact on vasodilation in female vessels (Fig. 5A).
By 7 days postligation, Western blot results indicate that a switch has occurred: males now have higher levels of eNOS protein. This finding may explain, at least in part, why males, 10 days after femoral artery occlusion, have a greater percent relaxation in response to ACh. Compatible with this interpretation is the finding that the gender difference in percent relaxation in response to ACh 10 days postligation was abolished in the presence of l-NAME.
Smooth muscle cell-dependent vasoreactivity differences were also found in response to PE and to NTG, a direct NO donor. Female mice showed greater vasoconstriction to PE vs. males both at baseline and 10 days following ligation. Females also exhibited less vasodilatation in response to NTG 10 days (but not immediately) postligation. Taken together, these data suggest that a greater sensitivity to vasoconstrictors and a less robust eNOS pathway, as well as an impaired sensitivity to the direct vasodilator activity of the eNOS pathway, probably contribute to the impaired blood flow recovery present in females following femoral artery occlusion. Additional independent results support this concept. p-VASP (15, 21) is a downstream product of NO/cGMP, and changes in p-VASP levels reflect the bioavailability of NO and of cGMP in smooth muscle cells. We found that p-VASP is lower in the calf muscles of females following ligation.
In this study, the selective α1-adrenergic receptor agonist PE was used to assess responsiveness to vasoconstrictors. Thus it is unclear whether the increased female sensitivity to PE can be extrapolated to all vasoconstrictors or if it is specific for α1-adrenergic receptor signaling. Passmore et. al. (16) have found that renal blood vessels isolated from female rats have higher expression of α1-adrenergic receptors compared with males. If the observed increased vasoconstriction in females is in fact specific for PE, it would be interesting to determine if differences in receptor expression might offer a mechanistic explanation.
In parallel to the inferred decreased arteriogenesis capability of female mice, females also appear to have less angiogenesis capacity. It is possible that the growth medium used for the in vitro studies was not optimized for female cells, and if an alternative medium were utilized, the results may have lead to a different conclusion. With this limitation in mind, however, angiogenesis was assessed using the in vivo Matrigel plug assay. Female mice developed fewer red blood cell-containing vessels and less endothelial cell infiltration in response to basic FGF impregnated into the Matrigel plug. These findings were further supported by the results of the MTT assay in which endothelial cells isolated from female aortas had less of a proliferative response compared with endothelial cells isolated from male aortas (Fig. 3, A-C). Thus differences in angiogenic capacity of endothelial cells between females and males may contribute to the observed disparity in blood flow recovery and to the greater impairment of hindlimb function in females.
In conclusion, our results demonstrate important gender-based differences in collateral function. Females have less blood flow recovery in a mouse model of hindlimb ischemia, compared with males. Our results also demonstrate that multiple mechanisms likely contribute to this gender difference. The impaired increase in blood flow recovery, accompanied by a higher vascular resistance exhibited by females, could be due in part to a reduced capacity to remodel, to fewer preexisting collaterals, or to both. There are also major differences in runoff vessel vasoreactivity, as female vessels have diminished smooth muscle sensitivity to nitric oxide-induced vasodilation and a greater sensitivity to the vasoconstrictor PE, compared with males. Also, the contributions of the eNOS pathway to vasodilator capacity are lower in females. These important gender differences may provide insight as to why women are less likely to survive a myocardial infarction than men or have more serious complications if PAD is present (5, 7, 20). Hence, gender-specific therapies targeting vasoreactivity and endothelial function may provide useful clinical strategies in the future.
GRANTS
X. Peng was partially supported by a grant from the State Scholarship Fund from the China Scholarship Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Baumbach GL, Sigmund CD, Faraci FM. Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ Res 95: 822–829, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Brevetti G, Bucur R, Balbarini A, Melillo E, Novo S, Muratori I, Chiariello M. Women and peripheral arterial disease: same disease, different issues. J Cardiovasc Med (Hagerstown) 9: 382–388, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics 30: 179–191, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106: 1870–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiebach NH, Viscoli CM, Horwitz RI. Differences between women and men in survival after myocardial infarction. Biology or methodology? JAMA 263: 1092–1096, 1990 [PubMed] [Google Scholar]
- 6. Gonzales RJ, Bryant JM, Naik JS, Resta TC, Walker BR. Gender differences in mesenteric vasoconstrictor reactivity following chronic hypoxia. Microcirculation 15: 473–484, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Greenland P, Reicher-Reiss H, Goldbourt U, Behar S. In-hospital and 1-year mortality in 1,524 women after myocardial infarction. Comparison with 4,315 men. Circulation 83: 484–491, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol 153: 666–672, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Huxley VH, Wang J, Whitt SP. Sexual dimorphism in the permeability response of coronary microvessels to adenosine. Am J Physiol Heart Circ Physiol 288: H2006–H2013, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 59: 89–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyriakides ZS, Petinakis P, Kaklamanis L, Lyras T, Sbarouni E, Karayannakos P, Iliopoulos D, Kremastinos DT. Gender does not influence angiogenesis and arteriogenesis in a rabbit model of chronic hind limb ischemia. Int J Cardiol 92: 83–91, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Mac Gabhann F, Peirce SM. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation 17: 333–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Nguyen LL, Hevelone N, Rogers SO, Bandyk DF, Clowes AW, Moneta GL, Lipsitz S, Conte MS. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation 119: 123–130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 87: 999–1005, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Passmore JC, Joshua IG, Rowell PP, Tyagi SC, Falcone JC. Reduced alpha adrenergic mediated contraction of renal preglomerular blood vessels as a function of gender and aging. J Cell Biochem 96: 672–681, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 23: 1143–1151, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Sieveking DP, Lim P, Chow RW, Dunn LL, Bao S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS, Ng MK. A sex-specific role for androgens in angiogenesis. J Exp Med 207: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg 45: 1185–1191, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Tofler GH, Stone PH, Muller JE, Willich SN, Davis VG, Poole WK, Strauss HW, Willerson JT, Jaffe AS, Robertson T, Passamani ER, Braunwald E, for the MILIS Study Group Effects of gender and race on prognosis after myocardial infarction: adverse prognosis for women, particularly black women. J Am Coll Cardiol 9: 473–482, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Versteilen AM, Korstjens IJ, Musters RJ, Groeneveld AB, Sipkema P. Rho kinase regulates renal blood flow by modulating eNOS activity in ischemia-reperfusion of the rat kidney. Am J Physiol Renal Physiol 291: F606–F611, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Zbinden S, Wang J, Adenika R, Schmidt M, Tilan JU, Najafi AH, Peng X, Lassance-Soares RM, Iantorno M, Morsli H, Gercenshtein L, Jang GJ, Epstein SE, Burnett MS. Metallothionein enhances angiogenesis and arteriogenesis by modulating smooth muscle cell and macrophage function. Arterioscler Thromb Vasc Biol 30: 477–482 [DOI] [PubMed] [Google Scholar]
- 23. Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 30: 923–934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]