Abstract
It has been suggested that exercise (or physical activity) might have the potential to have an impact on multiple sclerosis (MS) pathology and thereby slow down the disease process in MS patients. The objective of this literature review was to identify the literature linking physical exercise (or activity) and MS disease progression. A systematic literature search was conducted in the following databases: PubMed, SweMed+, Embase, Cochrane Library, PEDro, SPORTDiscus and ISI Web of Science. Different methodological approaches to the problem have been applied including (1) longitudinal exercise studies evaluating the effects on clinical outcome measures, (2) cross-sectional studies evaluating the relationship between fitness status and MRI findings, (3) cross-sectional and longitudinal studies evaluating the relationship between exercise/physical activity and disability/relapse rate and, finally, (4) longitudinal exercise studies applying the experimental autoimmune encephalomyelitis (EAE) animal model of MS. Data from intervention studies evaluating disease progression by clinical measures (1) do not support a disease-modifying effect of exercise; however, MRI data (2), patient-reported data (3) and data from the EAE model (4) indicate a possible disease-modifying effect of exercise, but the strength of the evidence limits definite conclusions. It was concluded that some evidence supports the possibility of a disease-modifying potential of exercise (or physical activity) in MS patients, but future studies using better methodologies are needed to confirm this.
Keywords: disease activity, exercise therapy, physical activity, training
Introduction
Multiple sclerosis (MS) is a clinically and pathologically complex and heterogeneous disease of unknown etiology [Kantarci, 2008]. In 28 European countries with a total population of 466 million people, it is estimated that 380,000 individuals are affected with MS [Sobocki et al. 2007]. The disorder is progressive but more than 80% of all MS patients have the disease for more than 35 years [Koch-Henriksen et al. 1998], the number of years of life lost to the disease being 5 to 10 [Ragonese et al. 2008]. The fact that MS is a chronic, long-lasting and disabling disease makes MS rehabilitation an important discipline in maintaining an independent lifestyle and the associated level of quality of life [Takemasa, 1998]. Despite the fact that MS patients for many years were advised not to participate in physical exercise because it was reported to lead to worsening of symptoms or fatigue, it has become generally accepted to recommend physical exercise for MS patients during the last two decades [Sutherland and Andersen, 2001]. Exercise is well tolerated and induces relevant improvements in both physical and mental functioning of persons with MS [Dalgas et al. 2008]. It is an open question whether exercise can reverse impairments caused by the disease per se, or whether exercise simply reverses the effects caused by inactivity secondary to the disease. However, most likely exercise may reverse the effects of an inactive lifestyle adopted by many patients [Garner and Widrick, 2003; Kent-Braun et al. 1997; Ng and Kent-Braun, 1997; Stuifbergen, 1997]. Nonetheless, it has been suggested that exercise might have the potential to have an impact on MS disease progression by slowing down the disease process itself [Heesen et al. 2006; Le-Page et al. 1994; White and Castellano, 2008b]. In other disorders exercise has been shown to pose the potential to have an impact on brain function and, as recently summarized by Motl and colleagues, exercise in older adults with or without dementia leads to cognitive improvement relative to a control condition [Motl et al. 2011b]. Based on this and the few existing findings in MS patients, Motl and colleagues suggested that exercise may similarly improve cognitive functioning in MS patients. However, in MS it has not been reviewed whether physical exercise has a more general disease-modifying effect.
To gain more insight on this important topic, we therefore conducted a systematic literature search aiming at identifying studies linking exercise (or physical activity) to disease progression in MS patients or in the experimental autoimmune encephalomyelitis (EAE) animal model of MS. A secondary purpose of the review was to discuss possible mechanisms explaining this link if it does exist and to discuss future study directions within this field.
Methods
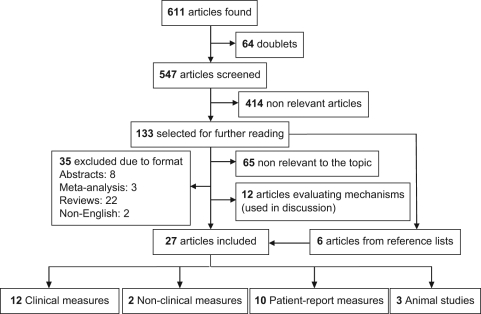
The included literature was identified through a comprehensive literature search (PubMed, SweMed+, Embase, Cochrane Library, PEDro, SPORTDiscus and ISI Web of Science) that was performed in order to identify relevant articles regarding MS and exercise up to 4 September 2011. The search was performed using the subject headings ‘exercise’, ‘exercise therapy’, ‘physical education and training’, ‘physical fitness’, ‘motor activity’ or ‘training’ in combination with ‘multiple sclerosis’ or ‘experimental autoimmune encephalomyelitis’. No limitations regarding publication year and age of subjects were entered. If possible, abstracts, comments and book chapters were excluded when performing the search in the different databases. This search yielded 547 publications. A screening of these publications based on title and abstract revealed 133 publications relevant for further reading. The reference lists of these 133 publications were checked for further relevant publications that were not captured by the search. This resulted in further six publications and in a total of 139 closely read publications. Studies that turned out to be nonrelevant (n = 65), meta-analyses (n = 3), reviews (n = 22), conference abstracts (n = 8) and articles not written in English (n = 2) were excluded from the final analysis (see Figure 1). Relevant cross- sectional and longitudinal studies were included.
Figure 1.
Flowchart showing literature search
According to Goldman and colleagues measures thought to reflect disease progression (or activity) in MS can be evaluated with objective or subjective outcome measures [Goldman et al. 2010]. Objective measures include (1) clinical outcome measures such as the Expanded Disability Status Scale (EDSS) and Multiple Sclerosis Functional Composite (MSFC) and (2) nonclinical measures such as MRI. The subjective measures include (3) patient-reported measures thought to reflect disease progression or disability such as the Late-Life Function and Disability Inventory. Studies applying patient-reported measures that included a measure of physical activity were also included in this category. Furthermore, we added a category containing studies applying (4) the EAE animal model of MS as study population. Based on this framework the localized articles were divided into the following four groups (see Table 1):
Table 1.
Studies included in the review.
| Study | Design | Intervention | Sample size | Disability (EDSS) | Type of MS | Outcome measures | Main findings |
|---|---|---|---|---|---|---|---|
| Clinical outcome measures (n = 12) | |||||||
| Pilutti et al. [2011] | Noncontrolled | 12w of ET, 3d/w | ET: 6 | 5-8 | PP, SP | EDSS MSFC | EDSS: NS MSFC: NS |
| Fimland et al. [2010] | RCT | 3w of RT, 5d/w | RT: 7 C: 7 | 3-6.5 | NR | EDSS | EDSS: NS |
| Golzari et al. [2010] | RCT | 8w of CT, 3d/w | CT: 10 C: 10 | <4 | RR | EDSS | EDSS: Improved |
| Bjarnadottir et al. [2007] | RCT | 5w of CT, 3d/w | CT: 6 C: 10 | <4 | NR | EDSS | EDSS: NS |
| Romberg et al. [2004, 2005] | RCT | 26w of CT, 3-4d/w | CT: 47 C: 48 | 1-5.5 | All | EDSS MSFC FIM | EDSS: NS MSFC: Improved FIM: NS |
| Dalgas et al. [2009] | RCT | 12w of RT, 2d/w | RT: 19 C: 19 | 3-5.5 | RR | EDSS | EDSS: NS |
| White et al. [2004] | Noncontrolled | 8w of RT, 2d/w | RT: 8 | 1-5 (SR) | RR | EDSS (SR) | EDSS (SR): NS |
| van den Berg et al. [2006] | RCOT | 4w of ET, 3d/w | ET: 10 C: 9 | NR | NR | GNDS | GNDS: NS |
| Kileff and Ashburn [2005] | Noncontrolled | 12w of ET, 2d/w | ET: 8 | 4-6 | NR | GNDS | GNDS: Improved |
| Rodgers et al. [1999] | Noncontrolled | 24w of ET, 3d/w | ET: 18 | 1-6.5 | NR | EDSS | EDSS: NS |
| Petajan et al. [1996] | RCT | 15w of ET, 3d/w | ET: 21 C: 25 | <6 | NR | EDSS | EDSS: NS |
| Nonclinical measures (n = 2) | |||||||
| Prakash et al. [2009] | Cross-sectional | N/A | MS: 21 HC: 15 | <6.5 | RR | MRI VO2-prak | Association between VO2 peak and regional gray matter volumes and higher focal fractional anisotropy |
| Prakash et al. [2007] | Cross-sectional | N/A | MS: 24 | <6.5 | RR | MRI VO2-peak | VO2 peak was associated with activity of IFG/MFC and ACC |
| Patient-reported measures (n = 10) | |||||||
| Tallner et al. [2011] | Cross-sectional | N/A | MS: 632 | Mean: 3.0 | NR | Relapse rate BQ |
No association between sports activity and clinical relapses. |
| Motl et al. [2011] | Descriptive longitudinal study | N/A (MS patients followed for 6 months) | MS: 269 | NR (PDSS: 0-6) | RR | GLTEQ | Change in physical activity was associated with residual change in walking impairment. |
| IPAQ | |||||||
| MSWS-12 | |||||||
| Motl and McAuley [2009, 2011] | Descriptive longitudinal study | N/A (MS patients followed for 6 months) | MS: 292 | NR (PDSS: 0-6) | All | GLTEQ | Association between change in physical activity and change in disability progression. Change in physical activity was associated with residual change in function and change in function was associated with residual change in disability. |
| ACCEL | |||||||
| MSRSC | |||||||
| LL-FDI | |||||||
| SI | |||||||
| PPDS | |||||||
| Motl et al. [2008c] | Cross-sectional | N/A | MS: 80 | <7 | RR, PP, SP | ACCEL | Physical activity was associated with reduced neurological impairment and disability |
| SI | |||||||
| PS | |||||||
| EDSS (SR) | |||||||
| Motl et al. [2008a] | Descriptive longitudinal study | N/A (MS patients followed for 3-5years) | MS: 51 | NR | All | IPAQ Symptom- Interview |
Worsening of symptoms is associated with lower levels of physical activity. |
| Motl et al. [2007, 2008b] | Cross-sectional | N/A | MS: 133 | NR | All | GLTEQ | Physical activity was negatively correlated with symptoms and positively to function. |
| ACCEL | |||||||
| MSRSC | |||||||
| LL-FDI | |||||||
| SI | |||||||
| BLEF | |||||||
| ALEF | |||||||
| Motl et al. [2006] | Cross-sectional | N/A | MS: 196 | Capacity for ambulation with minimal assistance | All | GLTEQ | Inverse correlation between physical activity and symptoms |
| ACCEL | |||||||
| MSRSC | |||||||
| Stuifbergen et al. [2006] | Descriptive longitudinal survey study. | N/A (MS patients followed for 5years) | MS: 611 | All | All | ISSHPLP-II | Increasing rates of changes in functional limitations correlated with decreasing rates of change in exercise behaviors |
| Animal studies (n = 3) | |||||||
| Rossi et al. [2009] | RCT | 50d of access to running wheel in cage. | ET: (CR-EAE): 10 C: (CR-EAE): 10 | N/A | CR-EAE | CEMS | Exercise attenuated clinical disturbances in both acute and chronic phases of EAE compared with control. |
| DO | |||||||
| MXS | |||||||
| Le-Page et al. [1996] | RCT | Four different experiments | ET: (MO-EAE) + C: (MO-EAE):85 | N/A | MO-EAE | CEMS | Exercise performed just after injection had a lowering effect on the clinical signs and delayed the onset of the disease and the day of maximal severity. |
| DO | |||||||
| DOR | |||||||
| MXS | |||||||
| Le-Page et al. [1994] | RCT | 10d of daily ET | ET:(CR-EAE):11 ET: (AC-EAE):9 ET: (MO-EAE):24 C: (CR-EAE): 18 C: (AC-EAE):4 C: (MO-EAE):15 | N/A | CR-EAE AC-EAE MO-EAE | CEMS | Onset of the disease was delayed and the duration of the first relapse in CR-EAE rats compared with control. |
| AC-EAE | DO | ||||||
| MO-EAE | DOR | ||||||
AC, acute; ACC, anterior cingulate cortex; ACCEL, 7-day accelerometer data; ALEF, advanced lower extremity function; BLEF, basic lower extremity function; BQ, Baecke questionnaire; C, control; CEMS, clinical EAE mobility score; CT, combined training; CR, chronic relapsing; d, days; DO, disease onset; DOR, duration of relapse; EDSS, Expanded Disability Status Scale; ET, endurance training; FIM, Functional Independence Measure; GLTEQ, Godin Leisure-Time Exercise Questionnaire; GNDS, Guys Neurological Disability Scale; HC, healthy control group; HPLP-II, Health Promoting Lifestyle Profile II; IFG/MFC, right inferior frontal gyrus/middle frontal gyrus; IPAQ, International Physical Activity Questionnaire; ISS, Incapacity Status Scale; LL-FDI, Late-Life Function and Disability Inventory; MO, monophasic; MS, multiple sclerosis; MSFC, Multiple Sclerosis Functional Composite; MSRSC, MS-Related Symptom Checklist; MSWS-12, Multiple Sclerosis Walking Scale-12; MXS, maximal severity; N/A, not applicable; NR, not reported; NS, not significant; PP, primary progressive; PS, performance scales; RCT, randomized controlled trial; RCOT, randomised crossover trial; RR, relapsing–remitting; RT, resistance training; SP, secondary progressive; SR, self-reported; SI, Symptom Inventory; w, weeks.
disease progression evaluated with clinical outcome measures (n = 12);
disease progression evaluated with nonclinical measures (n = 2);
disease progression evaluated with patient-reported measures (n = 10);
disease progression evaluated in animal studies (n = 3).
Results
1. Disease progression evaluated with clinical measures
A number of studies evaluating structured exercise interventions lasting from 3 to 26 weeks have included clinical scales reflecting disease progression as an outcome measure. The applied clinical scales include the EDSS [Bjarnadottir et al. 2007; Dalgas et al. 2009; Fimland et al. 2010; Golzari et al. 2010; Petajan et al. 1996; Pilutti et al. 2011; Rodgers et al. 1999; Romberg et al. 2004; White et al. 2004], the MSFC [Pilutti et al. 2011; Romberg et al. 2005], the Guys Neurological Disability Scale (GNDS) [Kileff and Ashburn, 2005; van den Berg et al. 2006] and the Functional Independence Measure (FIM) [Romberg et al. 2005]. Studies applying the EDSS have generally not found any change after either endurance training [Petajan et al. 1996; Pilutti et al. 2011; Rodgers et al. 1999], resistance training [Dalgas et al. 2009; Fimland et al. 2010; White et al. 2004] or combined training interventions [Bjarnadottir et al. 2007; Romberg et al. 2004]. Only one study by Golzari and colleagues evaluating the effects of 8 weeks of combined training (3 days/week) reported an improvement in EDSS score [Golzari et al. 2010]. This finding was not confirmed in a long-term study (26 weeks) [Romberg et al. 2005] also evaluating the effects of combined training. In the study by Romberg and colleagues no effect on EDSS and FIM were found, but a small positive effect was seen in the MSFC. A few studies applied the GNDS with one reporting an improvement after 12 weeks of biweekly endurance training [Kileff and Ashburn, 2005] and one reporting no effects of 4 weeks endurance training completed 3 days a week [van den Berg et al. 2006].
In summary, structured exercise intervention studies of different exercise modalities lasting 3–26 weeks have generally found no effects on EDSS scores. A few exercise studies have shown positive effects when applying other clinical scales (MSFC and GNDS).
2. Disease progression evaluated with non-clinical measures
Two studies by Prakash and colleagues have evaluated the effects of cardiorespiratory fitness on brain function and structure by applying (functional) MRI [Prakash et al. 2007, 2009]. One study [Prakash et al. 2007] investigated the impact of cardiorespiratory fitness on cerebrovascular functioning of MS patients. Twenty-four female participants with relapsing–remitting MS were recruited for the study and all participants went through fitness assessment (VO2 peak) and were scanned in a 3-T MRI system while performing the Paced Visual Serial Addition Test (PVSAT). Higher fitness levels were associated with faster performance during the PVSAT that could be related to greater recruitment of a specific region of the cerebral cortex (right inferior frontal gyrus [IFG] and middle frontal gyrus [MFG]) known to be recruited by MS patients during performance of PVSAT to purportedly compensate for the cognitive deterioration attributable to MS. In contrast, lower levels of fitness were associated with enhanced activity in the anterior cingulate cortex (ACC), thought to reflect the presence of a larger amount of conflict increasing the potential for error in lower fit MS participants. The authors interpreted the results as supporting aerobic training as an intervention to support the development of additional cortical resources in an attempt to counter the cognitive decline resulting from MS. Among a number of cognitive tests, only the Paced Auditory Serial Addition Test (PASAT) showed a weak correlation (p = 0.42) to VO2 peak leading the authors to suggest that fitness does not have an influence on measures of general cognitive functioning.
In another study by Prakash and colleagues the relationship between cardiorespiratory fitness (VO2 max) and measures of gray matter atrophy and white matter integrity (both of which have been associated with the disease process) were studied [Prakash et al. 2009]. A voxel-based approach to analysis of gray matter and white matter was applied on brainscans from a 3-T MRI system. More specifically it was examined whether higher levels of fitness in 21 female MS patients were associated with preserved gray matter volume and integrity of white matter. A positive association between cardiorespiratory fitness and regional gray matter volumes and higher focal fractional anisotropy values were reported. Both preserved gray matter volume and white matter tract integrity were associated with better performance on measures of processing speed. Recognizing the cross-sectional nature of the data, the authors suggested that fitness exerts a prophylactic influence on the structural decline observed early on, preserving neuronal integrity in MS, thereby reducing long-term disability.
In summary, (f)MRI studies suggesting a protective effect of cardiorespiratory fitness on brain function and structure in MS patients have started to emerge. However, the cross-sectional nature of the few existing studies limit conclusions regarding the existence of a causal relationship.
3. Disease progression evaluated with patient-reported measures
A number of studies have addressed the relationship between exercise or physical activity and disease progression in large-scale questionnaire studies applying patient-reported measures.
In a large descriptive longitudinal survey study, Stuifbergen and colleagues examined the correlations between the change in functional limitations, exercise behaviors and quality of life [Stuifbergen et al. 2006]. More than 600 MS patients completed a number of questionnaires every year for a period of 5 years. The self-reported longitudinal measures were analyzed by applying latent curve modeling. The Incapacity Status Scale provided a measure of functional limitations due to MS, whereas the Health Promoting Lifestyle Profile II provided a measure of exercise behavior. At the first test point (baseline test) cross-sectional data showed a significant negative correlation (r = −0.34) between functional limitations and exercise behaviors, suggesting that at the start of the study higher levels of functional limitations were associated with lower levels of exercise. Longitudinal data from the study showed that increasing rates of changes in functional limitations correlated with decreasing rates of change in exercise behaviors (r = −0.25). In other words these findings are suggesting that increases in exercise behaviors correspond with decreased rates of change in functional limitations. No correlation between the initial degree of limitation and continuing rate of exercise was found which led the authors to suggest that persons with MS with varied levels of limitations might slow the trajectory of increasing limitations over the long term with consistent exercise participation.
A series of studies from Motl and colleagues have addressed the relationship between physical activity, symptoms, functional limitations and disability in MS patients. In a cross-sectional study [Motl et al. 2006] in 196 MS patients, the number of symptoms within 30 days (MS-related Symptom Checklist) and physical activity (Godin Leisure-Time Exercise Questionnaire and 7-day accelerometer data) were collected. After modeling data a direct relationship between symptoms and physical activity were found (r = −0.24) indicating that a greater number of symptoms resulted in lower amounts of physical activity. However, the authors noted that the cross-sectional design precludes inferences about the direction of causality, and physical activity might affect symptoms as symptoms affect physical activity participation. When modeled this way a moderate inverse correlation between physical activity and symptoms was found (r = −0.42) indicating fewer symptoms when the physical activity level is high. This led the authors to suggest the existence of a bi-directional relationship between physical activity and symptoms.
In a following questionnaire study Motl and colleagues examined physical activity (Godin Leisure-Time Exercise Questionnaire and 7 day accelerometer data) and symptoms (Symptom Inventory and MS-related Symptom Checklist) as correlates of functional limitations and disability (Late-Life Function and Disability Inventory) in 133 MS patients [Motl et al. 2007, 2008b]. A model based on the disablement model proposed by Nagi (1976) was tested as the primary model and this showed that physical activity and symptoms were negatively correlated (r = −0.59) and those who were more physically active had better function (r = 0.4). Furthermore, those with better function had less disability (r = 0.63) which led the authors to conclude that the findings indicate that physical activity is associated with reduced disability (through an association with function) consistent with Nagi’s disablement model (Nagi 1976), but again the cross-sectional design limited definite conclusions on the direction of the relationships.
Motl and colleagues then published a longitudinal (case report) study examining the relationship between worsening of symptoms and the level of physical activity throughout a 3- to 5-year period [Motl et al. 2008a]. The study showed that worsening of symptoms (interview) was significantly associated with lower levels of self-reported physical activity (International Physical Activity Questionnaire [IPAQ]) in a group of 51 subjects with MS. The study supports symptoms as a possible explanation for the rate of physical inactivity among MS patients but the direction of the cause and effect relationship could still not be established. Based on the results the authors suggest that managing symptoms might be important for the promotion of physical activity, but also that symptoms may be both an antecedent and consequence of physical activity.
After that Motl and colleagues published a cross-sectional study examining the correlation between physical activity and neurological impairment and disability in a group of 80 MS patients [Motl et al. 2008c]. Physical activity (7-day accelerometer day), impairment and disability (Symptom Inventory and self-reported EDSS) was measured and significant correlations were found between physical activity and both EDSS (r = −0.60) and Symptom Inventory (r = −0.56). The authors concluded that physical activity was associated with reduced neurological impairment and disability, but also stated that no causal relationship could be established due to the cross-sectional nature of the study.
Motl and McAuley then published a large-scale longitudinal questionnaire study examining the changes in physical activity (Godin Leisure-Time Exercise Questionnaire and 7-day accelerometer data) and symptoms (Symptom Inventory and MS-related Symptom Checklist) as correlates of changes in functional limitations and disability (Late-Life Function and Disability Inventory) [Motl and McAuley, 2009]. A total of 292 MS patients were followed for 6 months. Again a model based on the disablement model proposed by Nagi (1976) was tested as the primary model and this showed that change in physical activity was associated with residual change in function (r = 0.22) and change in function was associated with residual change in disability (r = 0.20). This led the authors to conclude that the findings indicate that change in physical activity is associated with change in disability (through an association with function) consistent with Nagi’s disablement model, but other models may be applied during analysis and a causal interpretation, therefore, still could not be adopted.
In a 6-month longitudinal study Motl and colleagues then tested the hypothesis that a change in physical activity (Godin Leisure-Time Exercise Questionnaire and International Physical Activity Questionnaire) would be inversely associated with a change in walking impairment (Multiple Sclerosis Walking Scale-12) in patients with relapsing–remitting MS [Motl et al. 2011a]. Data from 263 MS patients were analyzed using linear panel analysis and covariance modeling. Findings showed that a standard deviation unit change of 1 in physical activity was associated with a standard deviation unit residual change of 0.16 in walking impairment. These findings, therefore, support physical activity as an important approach, when trying to avoid walking impairments.
Finally, Motl and McAuley published a paper on longitudinal data (6 months) from 292 MS patients evaluating the relationship between a change in physical activity (7-day accelerometer data) and change in disability progression (Patient Determined Disease Steps Scale) [Motl and McAuley, 2011]. Panel analysis showed that a change in physical activity was associated with a change in disability progression (path coefficient: –0.09). This led the authors to conclude that a reduction in physical activity is a behavioral correlate (but not necessarily a cause) of short-term disability progression in persons with MS.
Recently, Tallner and colleagues evaluated the relationship between sports activity (Baecke Questionnaire – sports index) and MS relapses during the last 2 years (based on self-reports) in 632 German MS patients [Tallner et al. 2011]. Patients were divided into four groups based on their sports index. The study showed no overall differences between the four groups concerning the number of relapses within the last 2 years. However, the most active group had the lowermost mean and standard deviation of all groups. Consequently, these data suggest that exercise does not negatively influence relapse rate and the data further indicate that exercise actually reduce relapse rate.
In summary, patient-reported measures of the association between exercise or physical activity and disease progression (expressed as symptoms, functional limitations or disability) or activity (relapse rate) provide evidence of an association with more physical activity providing protection. However, due to the nature of the studies the causality of this association has not been established.
4. Disease progression evaluated in animal studies
Some obvious methodological difficulties exists in designing a human study clarifying whether or not exercise has an impact on disease progression in MS patients. Therefore, the question has been addressed in the EAE animal model of MS.
In a preliminary study by Le-Page and colleagues four groups of EAE rats were followed from day 1 to day 10 after injection with an agent inducing EAE [Le-Page et al. 1994]. The injection resulted in three different disease courses in the rats, namely acute (rats rapidly developed serious clinical signs and died without signs of recovery), monophasic (rats developed only one bout of disease followed by complete recovery) and chronic relapsing (CR-EAE, more than one bout of disease followed by remission). The CR-EAE disease course is characterized by the development of an initial acute paralytic attack 10–20 days after immunization with neuroantigens and the development of spontaneous relapses thereafter. A female and a male group of rats exercised and a female and male group served as control. Exercise consisted of running on a treadmill from day 1 to day 10 after injection. The protocol was progressively adjusted with the duration increasing from 60 min towards 120 min and the running speed increasing from 15 to 30 m/min. The study showed that in the exercised CR-EAE rats of both sexes the onset of the disease was significantly delayed compared with the onset in control CR-EAE rats. Also, the duration of the first relapse was significantly reduced in exercised CR-EAE rats compared with control rats whereas no effect was seen on the peak severity of the disease. No effects of exercise were observed in the acute and monophasic EAE rats. The authors concluded that endurance exercise during the phase of induction of EAE diminished lightly one type of EAE (CR-EAE) but also that exercise did not exacerbate the disease.
In a complementary study Le-Page and colleagues conducted further four experiments in the monophasic EAE model [Le-Page et al. 1996]. Experiments 1 and 2 showed that 2 consecutive days of intensive exercise (250–300 min/day) performed just after injection had a lowering effect on the course of the clinical signs of disease as compared with control rats. Also, the onset of the disease and the day of maximal severity were both delayed in the exercising rats, whereas no change was observed in disease duration. When the 2 consecutive days of exercise were performed before injection no effects were observed. In experiments 3 and 4 it was tested how 5 days of more moderate exercise at either constant (15–25 m/min for 2 hours) or variable speed (3 min at 2 m/min and then 2 min at 35 m/min for a total of 1 hour) affected the course of the disease and the clinical parameters. No effects were observed on the disease course and on the clinical parameters. The authors concluded that severe exercise contrary to more moderate exercise slightly influenced the effector phase of monophasic EAE, and confirmed that physical exercise performed before onset of EAE did not exacerbate the clinical signs.
More recently, Rossi and colleagues further explored the effects of physical activity on disease progression in the CR-EAE mice model [Rossi et al. 2009]. In this study one group of mice had their cage equipped with a running wheel on the day of immunization, while the control group had no running wheel. The amount of physical activity was not controlled and it was therefore the amount of voluntary physical activity in the running wheel that constituted the intervention. In a further experiment EAE mice in standard cages were compared with EAE mice in cages equipped with a blocked wheel. This was done to dissect the role of physical activity from that of sensory enrichment caused by the wheel itself, and showed not to influence the clinical course of the disease. During the initial phase (13 days after injection) of the disease the exercising mice ran spontaneously an average of 760 turns/day in the running wheel which dropped to 18 turns/day when motor impairment peaked (20–25 days after injection). The study showed that the severity of EAE-induced clinical disturbances was attenuated in both acute and chronic phases of EAE in the physically active mice, who consistently exhibited less severe neurological deficits compared with control EAE animals during a time period of 50 days after EAE induction. Furthermore, it was shown that both synaptic and dendritic defects caused by EAE were attenuated by physical activity.
In summary, aerobic exercise (or voluntary physical activity) has the potential to influence the clinical course of the disease in the EAE animal model of MS.
Discussion
Recent evidence from studies applying nonclinical and patient-reported measures as well as from studies applying the EAE animal model of MS indicate a possible disease-modifying effect of exercise (or physical activity) but the strength of the evidence limits definite conclusions. Furthermore, these findings are not confirmed in intervention studies evaluating disease progression by clinical outcome measures. Despite the obvious associated difficulties future long-term exercise intervention studies in a large group of MS patients are needed within this field.
MS disease progression
Some major methodological problems arise when trying to measure MS disease progression. The ideal MS outcome measure would quantify irreversible sustained disease progression, but in MS this has proven difficult. The pleiotropic expression of MS makes it challenging to measure all facets of the disease and it may be necessary to focus on specific symptoms. Furthermore, great patient heterogeneity, population variability in the disease course and tempo of progression, subclinical MRI changes of uncertain impact on delayed disability progression, multifaceted neurological deficits with varied abilities for individual patients to compensate and patient comorbidities complicate things further [Goldman et al. 2010].
Clinical outcome measures
EDSS, MSFC and relapse rate are the standard clinical outcome measures for MS therapeutic trials and the most widely used measure of disease progression is the EDSS [Goldman et al. 2010]. Our literature review shows that exercise studies (resistance, endurance and combined training) applying EDSS generally do not report any change after an exercise intervention. In medical studies applying EDSS, large sample sizes and interventions lasting 2–3 years are typically required to measure changes in exacerbation rates between treatment and placebo [Bates, 2011]. This corresponds poorly to the short intervention periods (3–26 weeks) and the small sample sizes applied in most exercise studies. This is due to the overall low responsiveness and sensitivity to change of the EDSS as reported in a number of studies (for references see Goldman et al. [2010]). Also, the EDSS have been criticized for its noninterval scaling, emphasis on ambulation status and absence of adequate cognitive and visual components [Balcer, 2001]. Despite the emphasis on ambulation and that a recent meta-analysis concluded that exercise impacts walking positively [Snook and Motl, 2009], no changes were seen in the EDSS in most of the reviewed studies, indicating low scale responsiveness towards exercise interventions. In clinical trials the MSFC is claimed to be more sensitive to change than the EDSS [Goldman et al. 2010]. This suggestion is supported by the finding from one exercise study applying both the EDSS and the MSFC. In this long-term study (26 weeks) [Romberg et al. 2005] the effects of combined training on EDSS and MSFC were evaluated. Only the MSFC showed a significant effect which led the authors to conclude that the MSFC was more sensitive than the EDSS in the detection of improvement of functional impairment as a result of combined exercise. In future exercise studies evaluating disease progression it should therefore be considered to add the MSFC as a clinical outcome measure.
In addition to low scale responsiveness, short-term interventions and small sample sizes other explanations for the general lack of effects on clinical outcome measures can be hypothesized. Despite no clear pattern in the existing data, the type of exercise (e.g. endurance versus resistance training) may influence the effect captured by clinical scales. Also, most studies have evaluated mild to moderately impaired (EDSS <6) MS patients. Perhaps the clinical scales would be more sensitive to change in more severely impaired patients. Finally, findings can be biased if it is generally more physically fit patients that accept to be enrolled in exercise studies. If so, the baseline fitness level may be above average in these patients further lowering the possibility of a change on clinical scales with low responsiveness.
Only a few studies [Bjarnadottir et al. 2007; Petajan et al. 1996; Romberg et al. 2004; White et al. 2004] present clear data on relapse rate but due to the short intervention periods and the small sample sizes in most studies changes in the relapse rate, would not be expected to be evident. However, Romberg and colleagues found a total of 11 relapses (five in the combined training group and six in the control group) during a 6-month intervention period [Romberg et al. 2004]. Similarly, Petajan and colleagues (endurance training group four relapses and control group three relapses) [Petajan et al. 1996] and Bjarnadottir and colleagues (combined training group one relapse and control group one relapse) [Bjarnadottir et al. 2007] reported identical relapse rates in exercise and control groups. In the study by White and colleagues no participants experienced relapses during the 8-week intervention evaluating resistance training [White et al. 2004]. Recently, Tallner and colleagues collected self-report questionnaires on relapse rates and physical activity from MS patients to examine the relationship of different levels of sports activity and relapses [Tallner et al. 2011]. Based on these data the authors concluded that exercise had no significant influence on clinical disease activity. Taken together the few existing data do not indicate that any type of exercise increases relapse rate among MS patients. However, these data should be interpreted with caution due to the small number of participants (not stratified according to disease type or severity) and the short intervention periods in most studies. Consequently, future long-term studies with a large number of participants should, therefore, include relapse rate as an outcome measure.
Nonclinical measures
Application of MRI has revolutionized the diagnosis and management of patients with MS [Bar-Zohar et al. 2008]. In regard to clinical trials, MRI offers several advantages over the accepted clinical outcome measures for MS, including an increased sensitivity to disease activity and a better association with histopathology findings. Also, MRI provides highly reproducible measures on ordinal scales, and the assessment of MRI can be performed at the highest degree of blinding [Bar-Zohar et al. 2008]. Consequently, a surrogate MRI measure reflecting disease progression such as lesion activity (gadolinium-enhanced lesions and new or enlarged T2-hyperintense lesions) or disease severity (total T2-hyperintense lesion volume, total T1-hypointense lesion volume and whole-brain atrophy) [Bermel et al. 2008] may reduce the required sample sizes needed to evaluate the effects of exercise therapy on disease progression considerably. Until now only two cross-sectional studies have evaluated the effects of exercise (expressed as the current cardiorespiratory fitness level) on different MRI measures limiting the conclusions that can be drawn from this type of study. However, the promising findings do encourage the inclusion of MRI as an outcome measure, in future longitudinal trials evaluating the effects of exercise on disease progression.
Patient-reported measures
Patient-reported measures of the association between exercise or physical activity and disease progression (expressed as symptoms, functional limitations or disability) provide evidence of an association with more physical activity providing protection. However, the nature of the studies does not allow conclusions on the causality of this association. In the group of studies applying patient-reported measures we decided to include not only measures of exercise, but also measures of physical activity. It is acknowledged that a measure of physical activity is not necessarily a surrogate measure of exercise, but the many interesting findings from particularly the group of Motl and colleagues caused this. In a recent paper, based on their own studies, Motl and colleagues concludes that recent research has identified physical activity as a behavioral correlate of disability in MS. This made the authors suggest, that physical activity might attenuate the progression of what they call ‘mobility disability’ by improving physiological function in persons with MS, particularly those who have achieved a benchmark of irreversible disability (EDSS >4) [Motl, 2010]. It might be more cost effective to offer the more disabled (EDSS >4) MS patients exercise therapy, but it must be noted that most exercise studies do not indicate that a relationship between the degree of training adaptation and neurological disability exist. In fact, studies indicate that MS patients with an EDSS score below 4.5 experience the largest improvements after a period of endurance training as compared with more disabled MS patients [Ponichtera-Mulcare et al. 1997; Schapiro et al. 1988] or that no differences exists [Petajan et al. 1996]. It must be noted that none of these studies were powered to evaluate the effects of exercise in MS patients with different levels of disability. However, a recent study by Filipi and colleagues specifically evaluated whether 6 months of resistance training improves strength in MS patients with different levels of disability (EDSS 1–8) and concluded that all individuals with MS, despite different disability levels, showed parallel improvement in muscle strength [Filipi et al. 2011]. This leads to the suggestion, that exercise may be equally important during the early phases of the disease, also in regard to impact on disease progression.
An important advantage of applying patient-reported measures is the opportunity to collect data from large sample sizes in longitudinal studies. Furthermore, it seems important to collect data on patient perspective when evaluating the effects of exercise on disease progression. Future studies including patient-reported measures should also include clinical and/or nonclinical outcome measures if possible.
Animal studies
Our review showed that aerobic exercise (or activities) has the potential to influence the clinical course of the disease in the EAE animal model of MS. The obvious question is whether or not the findings from the EAE animal model of MS can be extrapolated to humans. At the moment no clear answer can be given to this question. A recent review summarized whether the current disease-modifying treatments are justified on the basis of the results of EAE studies. Here it was concluded that although EAE is certainly an imperfect mirror of MS, many clinical, immunopathological and histological findings are impressively replicated by animal models, making EAE invaluable in elucidating the basic immunopathological mechanisms of MS and providing a testing ground for novel therapies [Farooqi et al. 2010]. Consequently, a direct transfer of findings into human subjects cannot be made, but testing of difficult hypotheses can start here. Also, it should be noted that in EAE you cannot control the relative exercise intensity since no maximal exercise test (such as a VO2 max test) can be performed. As a consequence the applied relative exercise intensity may differ between animals. This is also why it is very difficult to evaluate the effects of aerobic exercise on aerobic capacity in EAE. Nonetheless, the EAE model offers a number of advantages compared to human studies. In addition lower costs, easy control with adherence to the intervention and controlled environmental and genetic factors the EAE model also allows evaluation of possible mechanisms located in the central nervous system (CNS), which should have attention in future studies. Another review stated that the genetic heterogeneity, which is so critical in the MS population, is only reflected when multiple different models of EAE are studied in parallel [Gold et al. 2006]. This aspect should also be incorporated in future studies.
Possible mechanisms
Several mechanisms have been proposed as a possible link between exercise and disease status in MS. Some of the most promising candidates include cytokines and neurotrophic factors [White and Castellano, 2008a].
Cytokines
Cytokines play an important role in the pathogenesis of MS and are a major target for treatment interventions. In particular, interleukin (IL)-6, interferon (IFN)-γ and tumor necrosis factor (TNF)-α have a prominent role in the process of demyelination and axonal damage experienced by persons with MS [Compston and Coles, 2008].
Changes in the concentrations of certain cytokines, in particular IFN-γ and TNF-α, have been associated with changes in disease status in MS, and elevated concentrations of pro-inflammatory Th-1 cytokines (such as TNF-α, IFN-γ, IL-2 and IL-12) may contribute to neurodegeneration and disability [Ozenci et al. 2002]. This has led to the suggestion that exercise may counteract imbalances between the pro-inflammatory Th1 cytokines and the anti-inflammatory Th2 cytokines (such as IL-4 and IL-10) by enhancing anti-inflammatory mechanisms, and thereby potentially be able to alter the disease activity in MS patients [White and Castellano, 2008b].
In MS both the acute and/or chronic effects of resistance [White et al. 2006], endurance [Castellano et al. 2008; Heesen et al. 2003; Schulz et al. 2004] and combined training [Golzari et al. 2010] on several cytokines have been evaluated. A study by White and colleagues reported that resting levels of IL-4, IL-10, C-reactive protein (CRP) and IFN-γ were reduced, while TNF-α, IL-2 and IL-6 levels remained unchanged after 8 weeks of biweekly resistance training [White et al. 2006]. These results suggest that progressive resistance training may have an impact on resting cytokine concentrations and, thus, could have an impact on overall immune function and disease course in individuals with MS. However, the study was not controlled and only 10 participants were included obviously limiting the strength of the evidence. Heesen and colleagues evaluated the acute effects of 8 weeks of endurance training on IFN-γ, TNF-α and IL-10 and compared this to both a waitlist MS control group and a group of matched healthy subjects [Heesen et al. 2003]. After completing 30 minutes of endurance training (cycling) an increase in IFN-γ were induced similarly in all groups while trends towards smaller increases in TNF-α and IL-10 were observed in the two groups of MS patients. Based on these data the authors concluded, that no deviation in pro-inflammatory immune response to physical stress could be demonstrated in MS patients. These findings, therefore, supports that a single bout of endurance training can influence the cytokine profile at least for a period of time in MS patients. In another publication from the same study Schulz and colleagues were not able to demonstrate any differences between the resting level or the acute IL-6 response after 30 minutes of endurance exercise in the MS training group (8 weeks of bicycling) and the MS control group [Schulz et al. 2004].
A study by Castellano and colleagues evaluated the effects of 8 weeks of endurance training (cycling, 3 days/week) on IL-6, TNF-α and IFN-γ in 11 MS patients and 11 healthy matched controls. In MS patients both resting IFN-γ and TNF-α was elevated after endurance training whereas no changes were observed in healthy controls [Castellano et al. 2008]. Like in the study by Heesen and colleagues [Heesen et al. 2003], Castellano and colleagues also studied the acute effects of a single bout of endurance training and similarly found no differences when compared to the healthy controls, but in this study no increase in IFN-γ and TNF-α were observed in any of the groups contrasting the findings by Heesen and colleagues.
In the most recent study Golzari and colleagues performed a randomized controlled trial (RCT) evaluating the effects of 8 weeks of combined endurance and resistance training on IFN-γ, IL-4 and IL-17 [Golzari et al. 2010]. The study showed significant reductions in the resting concentrations of IFN-γ and IL-17 in the exercise group, whereas no changes were seen in the control group, but no group comparisons were made.
In summary, no clear pattern can be seen in the reported cytokine responses to exercise probably reflecting large methodological differences between the studies (study type, type of exercise intervention, time of measurements, standardizations, etc.) and a low statistical power which is critical due to the great variation in this type of measurements. Nonetheless, a single bout of exercise have been reported to influence a number of (pro-inflammatory) cytokines in MS patients and also chronic changes in the resting concentration of several cytokines have been reported after a training period. Furthermore, the response seems to be comparable to that of healthy subjects. Cytokines, therefore, may link exercise and disease progression in MS, but large-scale future RCTs have to evaluate this further.
Neurotrophic factors
Neurotrophic factors are a family of proteins that are thought to play a role in preventing neural death and in favoring the recovery process, neural regeneration and remyelination throughout life [Ebadi et al. 1997]. Some of the more well-characterized neurotrophic factors include brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) [White and Castellano, 2008b].
Gold and colleagues evaluated the acute effects of a single exercise bout (30 min cycling at 60% VO2 max) on NGF and BDNF in 25 MS patients and compared this with a group of matched healthy controls [Gold et al. 2003]. The study showed that baseline concentrations of NGF were significantly higher in MS patients compared with controls. Thirty minutes after exercise a significant increase was observed in BDNF while a trend towards an increase in NGF was observed. However, the changes did not differ from the changes observed in the healthy subjects. This made the authors conclude that moderate exercise can be used to induce neutrophin production in subjects with MS possibly mediating the beneficial effects of physical exercise. In a study from the same group Schulz and colleagues evaluated the effects of biweekly cycling for 8 weeks on BDNF and NGF in a RCT in MS patients [Schulz et al. 2004]. The study showed no effects on the resting concentration and on the response to acute exercise after the intervention period, and only a trend towards lower resting NGF levels was found. Castellano and White also evaluated whether 8 weeks of cycling (three times a week), would affect serum concentrations of BDNF in MS patients and in healthy controls [Castellano and White, 2008]. In contrast to the findings of Gold and colleagues, resting BDNF was lower at baseline in MS patients as compared with controls, but no difference (a trend) between groups was found after 8 weeks. In MS patients BDNF concentration at rest was significantly elevated between weeks 0 and 4 and then tended to decrease between weeks 4 and 8, whereas resting BDNF concentration remained unchanged at 4 and 8 weeks of training in controls. Also, the response to a single bout of exercise was evaluated showing a significant reduction in BDNF 2 and 3 hours after exercise in both groups again contrasting with the findings by Gold and colleagues. The authors concluded that their findings provided preliminary evidence showing that exercise may influence BDNF regulation in humans.
In summary contrasting findings on the effects of exercise on neurotrophic factors exists in MS patients, making more studies warranted. However, findings do imply that exercise may influence several neurotrophic factors known to be involved in neuroprotective processes.
Conclusions
It cannot be clearly stated whether exercise has a disease-modifying effect or not in MS patients but studies indicating this do exist. Future long-term intervention studies in a large group of MS patients are therefore needed to address this important question.
Acknowledgments
The authors would like to thank research Librarian Edith Clausen for a substantial contribution to the comprehensive literature search.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
UD has received travel grants and/or honorary from Biogen Idec, Merck Serono and Sanofi Aventis. ES has received research support and travel grants from Biogen Idec, Merck Serono and Bayer Schering and travel grants from Sanofi Aventis.
References
- Balcer L.J. (2001) Clinical outcome measures for research in multiple sclerosis. J Neuroophthalmol 21: 296–301 [DOI] [PubMed] [Google Scholar]
- Bar-Zohar D., Agosta F., Goldstaub D., Filippi M. (2008) Magnetic resonance imaging metrics and their correlation with clinical outcomes in multiple sclerosis: a review of the literature and future perspectives. Mult Scler 14: 719–727 [DOI] [PubMed] [Google Scholar]
- Bates D. (2011) Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials. Neurology 76: S14–S25 [DOI] [PubMed] [Google Scholar]
- Bermel R.A., Fisher E., Cohen J.A. (2008) The use of MR imaging as an outcome measure in multiple sclerosis clinical trials. Neuroimaging Clin N Am 18: 687–701, xi [DOI] [PubMed] [Google Scholar]
- Bjarnadottir O.H., Konradsdottir A.D., Reynisdottir K., Olafsson E. (2007) Multiple sclerosis and brief moderate exercise. A randomised study. Mult Scler 13: 776–782 [DOI] [PubMed] [Google Scholar]
- Castellano V., Patel D.I., White L.J. (2008) Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol 104(6): 1697–1702 [DOI] [PubMed] [Google Scholar]
- Castellano V., White L.J. (2008) Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J Neurol Sci 269: 85–91 [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008) Multiple sclerosis. Lancet 372: 1502–1517 [DOI] [PubMed] [Google Scholar]
- Dalgas U., Stenager E., Ingemann-Hansen T. (2008) Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler 14: 35–53 [DOI] [PubMed] [Google Scholar]
- Dalgas U., Stenager E., Jakobsen J., Petersen T., Hansen H., Knudsen C., et al. (2009) Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 73: 1478–1484 [DOI] [PubMed] [Google Scholar]
- Ebadi M., Bashir R.M., Heidrick M.L., Hamada F.M., Refaey H.E., Hamed A., et al. (1997) Neurotrophins and their receptors in nerve injury and repair. Neurochem Int 30: 347–374 [DOI] [PubMed] [Google Scholar]
- Farooqi N., Gran B., Constantinescu C.S. (2010) Are current disease-modifying therapeutics in multiple sclerosis justified on the basis of studies in experimental autoimmune encephalomyelitis? J Neurochem 115: 829–844 [DOI] [PubMed] [Google Scholar]
- Filipi M.L., Kucera D.L., Filipi E.O., Ridpath A.C., Leuschen M.P. (2011) Improvement in strength following resistance training in MS patients despite varied disability levels. NeuroRehabilitation 28: 373–382 [DOI] [PubMed] [Google Scholar]
- Fimland M.S., Helgerud J., Gruber M., Leivseth G., Hoff J. (2010) Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur J Appl Physiol 110: 435–443 [DOI] [PubMed] [Google Scholar]
- Garner D.J., Widrick J.J. (2003) Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 27: 456–464 [DOI] [PubMed] [Google Scholar]
- Gold R., Linington C., Lassmann H. (2006) Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129: 1953–1971 [DOI] [PubMed] [Google Scholar]
- Gold S.M., Schulz K.H., Hartmann S., Mladek M., Lang U.E., Hellweg R., et al. (2003) Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol 138: 99–105 [DOI] [PubMed] [Google Scholar]
- Goldman M.D., Motl R.W., Rudick R.A. (2010) Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. Ther Adv Neurol Disord 3: 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzari Z., Shabkhiz F., Soudi S., Kordi M.R., Hashemi S.M. (2010) Combined exercise training reduces IFN-gamma and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int Immunopharmacol 10: 1415–1419 [DOI] [PubMed] [Google Scholar]
- Heesen C., Gold S.M., Hartmann S., Mladek M., Reer R., Braumann K.M., et al. (2003) Endocrine and cytokine responses to standardized physical stress in multiple sclerosis. Brain Behav Immun 17: 473–481 [DOI] [PubMed] [Google Scholar]
- Heesen C., Romberg A., Gold S., Schulz K.H. (2006) Physical exercise in multiple sclerosis: supportive care or a putative disease-modifying treatment. Expert Rev Neurother 6: 347–355 [DOI] [PubMed] [Google Scholar]
- Kantarci O.H. (2008) Genetics and natural history of multiple sclerosis. Semin Neurol 28: 7–16 [DOI] [PubMed] [Google Scholar]
- Kent-Braun J.A., Ng A.V., Castro M., Weiner M.W., Gelinas D., Dudley G.A., et al. (1997) Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol 83: 1998–2004 [DOI] [PubMed] [Google Scholar]
- Kileff J., Ashburn A. (2005) A pilot study of the effect of aerobic exercise on people with moderate disability multiple sclerosis. Clin Rehabil 19: 165–169 [DOI] [PubMed] [Google Scholar]
- Koch-Henriksen N., Bronnum-Hansen H., Stenager E. (1998) Underlying cause of death in Danish patients with multiple sclerosis: results from the Danish Multiple Sclerosis Registry. J Neurol Neurosurg Psychiatry 65: 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Page C., Bourdoulous S., Beraud E., Couraud P.O., Rieu M., Ferry A. (1996) Effect of physical exercise on adoptive experimental auto-immune encephalomyelitis in rats. Eur J Appl Physiol Occup Physiol 73: 130–135 [DOI] [PubMed] [Google Scholar]
- Le-Page C., Ferry A., Rieu M. (1994) Effect of muscular exercise on chronic relapsing experimental autoimmune encephalomyelitis. J Appl Physiol 77: 2341–2347 [DOI] [PubMed] [Google Scholar]
- Motl R.W. (2010) Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev 38: 186–191 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Arnett P.A., Smith M.M., Barwick F.H., Ahlstrom B., Stover E.J. (2008a) Worsening of symptoms is associated with lower physical activity levels in individuals with multiple sclerosis. Mult Scler 14: 140–142 [DOI] [PubMed] [Google Scholar]
- Motl R.W., McAuley E. (2009) Longitudinal analysis of physical activity and symptoms as predictors of change in functional limitations and disability in multiple sclerosis. Rehabil Psychol 54: 204–210 [DOI] [PubMed] [Google Scholar]
- Motl R.W., McAuley E. (2011) Association between change in physical activity and short-term disability progression in multiple sclerosis. J Rehabil Med 43: 305–310 [DOI] [PubMed] [Google Scholar]
- Motl R.W., McAuley E., Wynn D., Vollmer T. (2011a) Lifestyle physical activity and walking impairment over time in relapsing-remitting multiple sclerosis: results from a panel study. Am J Phys Med Rehabil 90: 372–379 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Sandroff B.M., Benedict R.H. (2011b) Cognitive dysfunction and multiple sclerosis: developing a rationale for considering the efficacy of exercise training. Mult Scler 17: 1034–1040 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Snook E.M., McAuley E., Gliottoni R.C. (2006) Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Res Nurs Health 29: 597–606 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Snook E.M., McAuley E., Scott J.A., Gliottoni R.C. (2007) Are physical activity and symptoms correlates of functional limitations and disability in multiple sclerosis? Rehabil Psychol 52: 463–469 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Snook E.M., Schapiro R.T. (2008b) Symptoms and physical activity behavior in individuals with multiple sclerosis. Res Nurs Health 31: 466–475 [DOI] [PubMed] [Google Scholar]
- Motl R.W., Snook E.M., Wynn D.R., Vollmer T. (2008c) Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis 196: 492–495 [DOI] [PubMed] [Google Scholar]
- Nagi S. Z. (1976) An epidemiology of disability among adults in the United States. Milbank Quarterly 54: 439–467 [PubMed] [Google Scholar]
- Ng A.V., Kent-Braun J.A. (1997) Quantitation of lower physical activity in persons with multiple sclerosis. Med Sci Sports Exerc 29: 517–523 [DOI] [PubMed] [Google Scholar]
- Ozenci V., Kouwenhoven M., Link H. (2002) Cytokines in multiple sclerosis: methodological aspects and pathogenic implications. Mult Scler 8: 396–404 [DOI] [PubMed] [Google Scholar]
- Petajan J.H., Gappmaier E., White A.T., Spencer M.K., Mino L., Hicks R.W. (1996) Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 39: 432–441 [DOI] [PubMed] [Google Scholar]
- Pilutti L.A., Lelli D.A., Paulseth J.E., Crome M., Jiang S., Rathbone M.P., et al. (2011) Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil 92: 31–36 [DOI] [PubMed] [Google Scholar]
- Ponichtera-Mulcare J.A., Mathews T., Barret P.J., Gupta S.C. (1997) Change in aerobic fitness of patients with multiple sclerosis during a 6 month training program. Sports Med Train Rehabil 7: 265–272 [Google Scholar]
- Prakash R.S., Snook E.M., Erickson K.I., Colcombe S.J., Voss M.W., Motl R.W., et al. (2007) Cardiorespiratory fitness: A predictor of cortical plasticity in multiple sclerosis. Neuroimage 34: 1238–1244 [DOI] [PubMed] [Google Scholar]
- Prakash R.S., Snook E.M., Motl R.W., Kramer A.F. (2009) Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res 23: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragonese P., Aridon P., Salemi G., D’Amelio M., Savettieri G. (2008) Mortality in multiple sclerosis: a review. Eur J Neurol 15: 123–127 [DOI] [PubMed] [Google Scholar]
- Rodgers M.M., Mulcare J.A., King D.L., Mathews T., Gupta S.C., Glaser R.M. (1999) Gait characteristics of individuals with multiple sclerosis before and after a 6-month aerobic training program. J Rehabil Res Dev 36: 183–188 [PubMed] [Google Scholar]
- Romberg A., Virtanen A., Ruutiainen J. (2005) Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol 252: 839–845 [DOI] [PubMed] [Google Scholar]
- Romberg A., Virtanen A., Ruutiainen J., Aunola S., Karppi S.L., Vaara M., et al. (2004) Effects of a 6-month exercise program on patients with multiple sclerosis: a randomized study. Neurology 63: 2034–2038 [DOI] [PubMed] [Google Scholar]
- Rossi S., Furlan R., De-Chiara V., Musella A., Lo G.T., Mataluni G., et al. (2009) Exercise attenuates the clinical, synaptic and dendritic abnormalities of experimental autoimmune encephalomyelitis. Neurobiol Dis 36: 51–59 [DOI] [PubMed] [Google Scholar]
- Schapiro R.T., Petajan J.H., Kosich D. (1988) Role of cardiovascular fitness in multiple sclerosis: a pilot study. J Neurol Rehabil 2: 43–49 [Google Scholar]
- Schulz K.H., Gold S.M., Witte J., Bartsch K., Lang U.E., Hellweg R., et al. (2004) Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci 225: 11–18 [DOI] [PubMed] [Google Scholar]
- Snook E.M., Motl R.W. (2009) Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair 23: 108–116 [DOI] [PubMed] [Google Scholar]
- Sobocki P., Pugliatti M., Lauer K., Kobelt G. (2007) Estimation of the cost of MS in Europe: extrapolations from a multinational cost study. Mult Scler 13: 1054–1064 [DOI] [PubMed] [Google Scholar]
- Stuifbergen A.K. (1997) Physical activity and perceived health status in persons with multiple sclerosis. J Neurosci Nurs 29: 238–243 [DOI] [PubMed] [Google Scholar]
- Stuifbergen A.K., Blozis S.A., Harrison T.C., Becker H.A. (2006) Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil 87: 935–943 [DOI] [PubMed] [Google Scholar]
- Sutherland G., Andersen M.B. (2001) Exercise and multiple sclerosis: physiological, psychological, and quality of life issues. J Sports Med Phys Fitness 41: 421–432 [PubMed] [Google Scholar]
- Takemasa S. (1998) Factors affecting QOL of the home-bound elderly disabled. Kobe J Med Sci 44: 99–114 [PubMed] [Google Scholar]
- Tallner A., Waschbisch A., Wenny I., Schwab S., Hentschke C., Pfeifer K., et al. (2011) Multiple sclerosis relapses are not associated with exercise. Mult Scler, epub ahead of print. [DOI] [PubMed] [Google Scholar]
- van den Berg M., Dawes H., Wade D.T., Newman M., Burridge J., Izadi H., et al. (2006) Treadmill training for individuals with multiple sclerosis: a pilot randomised trial. J Neurol Neurosurg Psychiatry 77: 531–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L.J., Castellano V. (2008a) Exercise and brain health - implications for multiple sclerosis: part 1 - neuronal growth factors. Sports Med 38: 91–100 [DOI] [PubMed] [Google Scholar]
- White L.J., Castellano V. (2008b) Exercise and brain health–implications for multiple sclerosis: part II - immune factors and stress hormones. Sports Med 38: 179–186 [DOI] [PubMed] [Google Scholar]
- White L.J., Castellano V., McCoy S.C. (2006) Cytokine responses to resistance training in people with multiple sclerosis. J Sports Sci 24: 911–914 [DOI] [PubMed] [Google Scholar]
- White L.J., McCoy S.C., Castellano V., Gutierrez G., Stevens J.E., Walter G.A., et al. (2004) Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult Scler 10: 668–674 [DOI] [PubMed] [Google Scholar]