Summary
Setting
Recent studies suggest that the prevalence of drug-resistant TB in sub-Saharan Africa may be rising. This is of concern since HIV co-infection in MDR- and XDR-TB has been associated with exceedingly rates of mortality.
Objective
To identify risk factors associated with mortality in MDR- and XDR-TB patients co-infected with HIV in South Africa.
Design
Case-control study of patients who died of all causes within two years of diagnosis with MDR- or XDR-TB.
Results
Among 123 MDR-TB patients, 78 (63%) died following diagnosis. CD4 count less than 50 (HR 4.64, p=0.01) and 51-200 cells/mm3 (HR 4.17, p=0.008) were the strongest independent risk factors for mortality. Among 139 XDR-TB patients, 111 (80%) died. CD4 count less than 50 cells/mm3 (HR 4.46, p=0.01) and resistance to all six drugs tested (HR 2.54, p=0.04) were the principal risk factors. Use of antiretroviral therapy (ART) was protective (HR 0.34, p=0.009).
Conclusions
Mortality in MDR- and XDR-TB was associated with greater degree of immunosuppression and drug resistance. Efforts to reduce mortality must focus on preventing the amplification of resistance by strengthening TB treatment programs, as well as, reducing the pool of immunosuppressed HIV-infected patients through aggressive HIV testing and ART initiation.
Keywords: multidrug-resistant tuberculosis, extensively drug-resistant tuberculosis, risk factors, mortality, HIV/TB Co-infection
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) have emerged as severe global public health threats.1,2 At least one country in every region of the world has reported a high prevalence of MDR-TB (more than 3% of all new TB cases), and XDR-TB has been diagnosed in more than 57 countries.1 Recent reports suggest that the prevalence of drug-resistant TB in sub-Saharan Africa may be increasing,3-5 raising concerns for a disastrous convergence with the continent's generalized HIV/AIDS epidemic.
Drug-resistant TB cases are more difficult to treat because they require at least 18-24 months of treatment with second-line TB medications (e.g., aminoglycosides, fluoroquinolones, ethionamide, cycloserine, etc.), which are less potent, more toxic, and more expensive than drug-susceptible TB medications. Although cure rates of 60-70% for MDR-TB and 40-50% for XDR-TB have been reported from low-HIV-prevalence settings,6,7 these have not been replicated in high-HIV-prevalence settings.8-10 MDR- and XDR-TB patients with HIV co-infection have exceedingly high and rapid mortality,11,12 however reasons for this are not yet clear.
In the past decade, the epidemic of drug-resistant TB has exploded in South Africa,4,13 a country with among the highest burden of HIV/AIDS in the world. In KwaZulu-Natal province, MDR-TB prevalence has risen from 14 cases per 100,000 in 2002 to nearly 30 cases per 100,000 in 2007.4,14,15 Tugela Ferry, located in KwaZulu-Natal province, has been the site of a well-characterized epidemic of MDR- and XDR-TB. MDR-TB incidence exceeds 70 cases per 100,000 population and a recent study found that the median time to death for MDR- and XDR-TB, respectively, was 60 days and 28.5 days.12 These findings mirror those from the MDR-TB outbreaks among HIV-infected patients in industrialized countries in the 1990s,11 although those outbreaks predated the availability of combination antiretroviral therapy (ART).
Although both HIV and drug-resistant TB are, each, now recognized to be treatable diseases, limited data exist evaluating the impact of important covariates – such as HIV status, CD4 count, and antiretroviral therapy – on MDR- and XDR-TB outcomes. Thus, we conducted a case-control study to identify clinical risk factors associated with mortality among MDR- and XDR-TB patients co-infected with HIV in South Africa.
METHODS
Setting and Standardized TB treatment
Tugela Ferry is a resource-limited, rural area in KwaZulu-Natal province, South Africa. In 2007, the incidences of drug-susceptible TB, MDR-TB, and XDR-TB were approximately 1100, 119, and 73 cases per 100,000 population respectively.12 More than 80% of all TB cases are HIV co-infected.
All patients presenting with signs and symptoms of TB were evaluated clinically and had sputum sent for culture and drug-susceptibility testing (DST). Mycobacterial culture was performed using both liquid (BACTEC mycobacterial growth indicator tube [MGIT]-960 system [BD, Franklin Lakes, NJ]) and solid media (Middlebrook 7H10; Difco Laboratories, Detroit, MI). DST for isoniazid (H), rifampicin (R), ethambutol (E), streptomycin (S), ciprofloxacin (Cx), and kanamycin (Km) was performed on all positive cultures by the 1% proportional method on Middlebrook 7H10 agar.
Patients diagnosed with TB received standard first-line TB therapy (2 months HREZ followed by 4 months HR) while awaiting results of culture and DST (typically 6–8 weeks). At the time of this study, patients with confirmed MDR- or XDR-TB were referred to a single specialty hospital in Durban, where MDR-TB patients received a standardized regimen of kanamycin, ofloxacin, ethionamide, ethambutol (if susceptible), pyrazinamide, and either terizidone or cycloserine (if ethambutol resistant) for 6 months (intensive phase) and continued on this regimen, without kanamycin, for an additional 18 months. XDR-TB cases received similar treatment, except that ciprofloxacin and kanamycin were replaced by para-amino salicylic acid (PAS) and capreomycin (available since early 2007). Patients did not routinely receive third-line TB drugs or surgical treatment.
All TB patients were offered HIV testing and, if HIV-infected, referred to a comprehensive HIV program, which provided free ART. Patients newly diagnosed with HIV who had CD4 counts less than 200 cells/mm3 were typically started on ART 1-2 months after initiating empiric first-line TB therapy.
Study Population and Drug-Resistance Classification
We performed a case-control study to determine risk factors for mortality among patients diagnosed with MDR- and XDR-TB from the district hospital in Tugela Ferry. Patients were identified using the drug-resistant TB register maintained by the TB treatment program and were enrolled if their diagnosis was made between January 2005 and December 2006. Cases were defined as patients who died of any cause within two years of diagnosis, whereas controls were patients who were alive at last follow-up. For the purpose of this study, patients with XDR-TB were analyzed separately from those with MDR-TB (who did not meet criteria for XDR-TB), to determine if the risk factors for mortality differ between these two drug resistance groups. Patients were classified as having MDR- or XDR-TB using standard definitions.16
Medical Record Review
We reviewed medical records for a convenience sample of patients with MDR-TB or XDR-TB. Variables collected included: patient demographics; sputum smear status, DST pattern, TB treatment history; HIV history (HIV status, CD4 count, and ART use); hospitalization history; clinical signs, symptoms and laboratory findings at the time of MDR- or XDR-TB diagnosis; treatment and vital status, as of last follow-up; and date of death (if applicable).
Statistical Analysis
We used frequencies and proportions to describe patients’ historical and clinical characteristics. Within each drug-resistance group (i.e., MDR-TB, XDR-TB), we performed bivariate analysis using product limit estimates and log-rank tests to identify variables associated with mortality. Variables with a p-value <0.1 on bivariate analysis were selected for inclusion in the multivariate model. We used Cox Proportional Hazards regression to determine the correlation between potential risk factors and mortality. Patients were censored at the time of last patient contact. For both bivariate and multivariate analysis, ART was analyzed as a time-dependent variable. Significance was declared at a two-sided level of 0.05 and all analyses were conducted using SAS, version 9.2 (Cary, NC).
As a sensitivity analysis, we performed multiple imputation simulation to assess the impact of missing CD4 count on the relationship between covariates and survival. We applied a Markov Chain Monte Carlo method to draw randomly imputed CD4 counts separately for missing observations in each drug resistance group. In doing so, both time to event and censoring indicators were also used to construct a posterior distribution of missing CD4 counts and after the initial 100,000 burn-in simulations, every 1000-th simulated CD4 counts were used for the imputation. This process was performed ten times to generate ten sets of imputed data. We then fit the Cox-model for MDR and XDR survival with each of the 10 imputed data sets and combined the results into a single result based on a pooling method proposed by Rubin.17 Models using only the observed data were compared with models computed following multiple imputation.
The study protocol was approved by the Ethics Committees of the University of KwaZulu-Natal, Albert Einstein College of Medicine, Yale University, and by the KwaZulu-Natal Department of Health.
RESULTS
Between 1 January 2005 and 31 December 2006, there were a total of 186 MDR TB and 234 XDR TB cases diagnosed. Of these, medical records were available for review for 123 (66%) MDR-TB and 139 (60%) XDR-TB patients. Patients included in this study did not differ significantly, in terms of demographics or survival, when compared to other MDR- and XDR-TB patients diagnosed during this period whose records were not available for review (data not shown).
Multidrug-resistant Tuberculosis
Among 123 patients with MDR-TB, 53 (43%) were women and the median age was 34 years (Table 1). Sputum smear was positive in 77 (65%) patients. Twenty-five (20%) patients had resistance to HR only, while 80 (65%) and 13 (11%) had resistance to HRS and HRES, respectively. An additional five patients (4%) also had resistance to either ciprofloxacin or kanamycin (HRCx: n=1; HRSCx: n=1; HRSKm: n=1; and HRESCx: n=2). Given the small numbers, these groups were collapsed into the larger groups based on their first-line resistance pattern (e.g., HRCx was included in the HR group; HRSCx and HRSKm included in HRS group; HRESCx included in HRES group).
Table 1.
Demographics and Clinical Characteristics of Patients with Multidrug-resistant Tuberculosis (MDR-TB)
| Characteristic | Total | Died | Alive | P value§ |
|---|---|---|---|---|
| Total N | 123 | 78 (63%) | 45 (37%) | |
| Female sex: n (%) | 53 (43) | 31 (40) | 22 (49) | 0.26 |
| Median age, years: (IQR) | 34 (29-43) | 34 (29-41) | 33 (27-43) | 0.65 |
| Positive sputum smear: n (%) | 77 (65) | 55 (73) | 22 (51) | 0.009 |
| DST pattern: HR resistant* | 26 (21) | 11 (14) | 15 (33) | 0.003 |
| HRE resistant | 0 | 0 | 0 | – |
| HRS resistant† | 82 (67) | 57 (73) | 25 (56) | 0.008 |
| HRES resistant‡ | 15 (12) | 10 (13) | 5 (11) | 0.77 |
| Tested for HIV: n (%) | 92 (75) | 61 (78) | 31 (69) | – |
| HIV Positive: n (% tested) | 85 (92) | 57 (93) | 28 (90) | 0.46 |
| CD4 Available at Diagnosis: n (% of HIV+) | 56 (66) | 35 (61) | 21 (75) | – |
| Median CD4, cells/mm3: (IQR) | 94 (44-220) | 79 (35-144) | 171 (46-417) | 0.002 |
| CD4 <50 cells/mm3: n (% CD4 available) | 16 (29) | 10 (29) | 6 (29) | – |
| CD4 51-200 cells/mm3: n (% CD4 available) | 25 (45) | 19 (54) | 6 (29) | – |
| CD4 >200 cells/mm3: n (% CD4 available) | 15 (27) | 6 (17) | 9 (43) | – |
| Received ART (time dependent): n (% of HIV+) | 24 (28) | 12 (21) | 12 (43) | 0.63 |
| Extrapulmonary MDR-TB: n (%) | 34 (28) | 29 (37) | 5 (11) | 0.003 |
| Previous TB treatment, Any: n (%) | 93 (75) | 59 (76) | 34 (76) | 0.29 |
| Hospitalization within past year: n (%) | 56 (46) | 39 (50) | 17 (38) | 0.01 |
DST: Drug-susceptibility test; H: isoniazid; R: rifampin; E: ethambutol; S: streptomycin; Cx: ciprofloxacin; Km: kanamycin; IQR: interquartile range; ART: antiretroviral therapy
p values determined based on product limit estimates and log rank tests
includes one patient with HRCx resistance pattern
includes one patient with HRSCx and one patient with HRSKm resistance patterns
includes two patients with HRESCx resistance pattern
Among patients whose HIV status was known (n=92, 75%), 85 (92%) were HIV co-infected, with a median CD4 count of 94 cells/mm3 (IQR 44-220). Twenty-four (28%) patients co-infected with MDR-TB and HIV received ART; 12 patients were already receiving ART at the time of their MDR-TB diagnosis, whereas the remaining 12 patients initiated ART after their MDR-TB diagnosis (median time to ART initiation 68 days [IQR 40-145]). Thirty-four (28%) had both pulmonary and extrapulmonary disease. Ninety-three (75%) patients had been previously treated with first-line TB drugs, of whom 17 (18%) were previously cured, 43 (46%) failed treatment, and 10 (11%) defaulted.
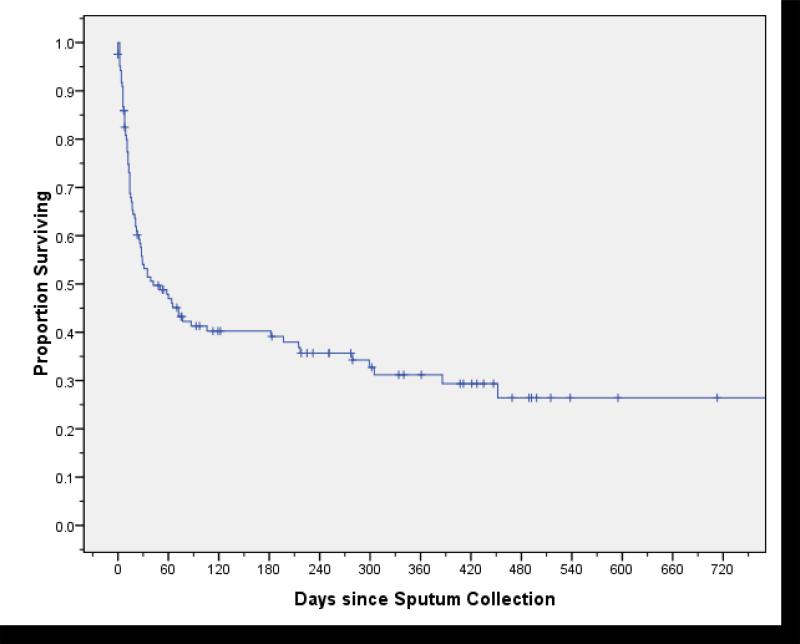
Within two years of their diagnosis, 78 (63%) MDR-TB patients died, with a median survival of 42 days (95% CI, 25–106) (Figure 1). On bivariate analysis, patients who died were more likely to have sputum smear-positive disease (p=0.009), resistance to HRS (p=0.008), extrapulmonary TB (p=0.003), hospitalization within the past year (p=0.01), or lower baseline CD4 cell count (p=0.002) (Table 1). Notably, receiving ART was not associated with survival on bivariate analysis (p=0.63).
Figure 1.
Survival among patients with multidrug-resistant tuberculosis from the time of sputum collection.
In multivariate analysis, CD4 count less than 50 cells/mm3 (HR 4.08, p=0.02) and CD4 count of 51-200 cells/mm3 (HR 3.87, p=0.01) were independently associated with higher risk of mortality (Table 2). Positive sputum smear and higher degree of drug resistance (i.e., resistance to HRS) showed a borderline association with mortality, but this did not reach statistical significance.
Table 2.
Characteristics Associated with Mortality in Patients with Multidrug-resistant Tuberculosis (MDR-TB)
| Unadjusted Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio | p-value | Hazard Ratio | p-value |
| Female sex | 0.77 | 0.26 | 1.15 | 0.73 |
| Positive sputum smear | 1.99 | 0.009 | 2.10* | 0.08* |
| DST: Resistance to HR† | Ref | Ref | Ref | Ref |
| Resistance to HRS‡ | 1.98 | 0.008 | 2.45∥ | 0.07∥ |
| Resistance to HRES§ | 1.11 | 0.77 | 1.55¶ | 0.53¶ |
| CD4 cell count: <50 cells/mm3 | 1.27 | 0.53 | 4.64 | 0.01 |
| 51-200 cells/mm3 | 2.10 | 0.03 | 4.17 | 0.008 |
| >200 cells/mm3 | Ref | Ref | Ref | Ref |
| Received ART (time dependent) | 0.86 | 0.63 | 0.67 | 0.35 |
| Extrapulmonary MDR-TB | 2.02 | 0.003 | 1.39# | 0.42# |
| Hospitalization within past year | 1.80 | 0.01 | 1.12 | 0.78 |
H: isoniazid; R: rifampin; E: ethambutol; S: streptomycin; Cx: ciprofloxacin; Km: kanamycin
Bold denotes p-value<0.05
Positive sputum smear: HR=2.54, p=0.002: in multivariable model following imputation
Includes one patient with HRCx resistance pattern
Includes one patient with HRSCx and one patient with HRSKm resistance patterns
Includes two patients with HRESCx resistance pattern
Resistance to HRS: HR=2.53, p=0.01: in multivariable model following imputation
Resistance to HRES: HR=2.97, p=0.02: in multivariable model following imputation
Extrapulmonary TB: HR=1.75, p=0.04: in multivariable model following imputation
Following multiple imputation for missing CD4 counts, the multivariable models demonstrated the association between low CD4 count and mortality, as well as a statistically significant association for positive sputum smear, higher degree of drug resistance and extrapulmonary TB (footnote in Table 2).
Extensively Drug-resistant Tuberculosis
Among 139 patients with XDR-TB, 78 (56%) were women and the median age was 34 years (Table 3). Sputum smear was positive in 84 (63%) patients. Thirty-three (24%) patients had resistance to only HRCxKm, while 77 (55%) had resistance to HRESCxKm (all drugs tested). Among patients whose HIV status was known (n=117, 84%), 115 (98%) were HIV co-infected, with a median CD4 count of 78 cells/mm3 (IQR 30-169). Twenty-eight (24%) patients co-infected with XDR-TB and HIV received ART; 25 patients were already on ART at the time of their XDR-TB diagnosis, whereas two patients started ART after their XDR-TB diagnosis (time to ART initiation 42 and 70 days, respectively). Forty-one (30%) had both pulmonary and extrapulmonary disease. Ninety-seven (70%) patients had been previously treated with first-line anti-TB drugs, of whom 15 (15%) were previously cured, 59 (60%) failed treatment, and 4 (4%) defaulted (prior TB treatment outcome was not available for 19 (20%) patients). No XDR-TB patients had previously received treatment for MDR-TB with second-line drugs.
Table 3.
Demographics and Clinical Characteristics of Patients with Extensively Drug-resistant Tuberculosis (XDR-TB)
| Characteristic | Total | Died | Alive | P value§ |
|---|---|---|---|---|
| Total N | 139 | 111 (80%) | 28 (20%) | |
| Female sex: n (%) | 78 (56) | 57 (51) | 21 (75) | 0.06 |
| Median age, years: (IQR) | 34 (29-42) | 35 (29-43) | 32 (26-37) | 0.80 |
| Positive sputum smear: n (%) | 84 (63) | 73 (68) | 11 (41) | 0.009 |
| DST pattern: HRCxKm resistant | 33 (24) | 23 (21) | 10 (36) | 0.21 |
| HRECxKm resistant | 6 (4) | 5 (5) | 1 (4) | 0.48 |
| HRSCxKm resistant | 23 (17) | 15 (14) | 8 (29) | 0.28 |
| HRESCxKm resistant | 77 (55) | 68 (61) | 9 (32) | 0.02 |
| Tested for HIV: n (%) | 117 (84) | 93 (84) | 24 (86) | – |
| HIV Positive: n (% tested) | 115 (98) | 92 (99) | 23 (96) | 0.13 |
| CD4 Available at Diagnosis: n (% of HIV+) | 59 (51) | 44 (48) | 15 (65) | – |
| Median CD4, cells/mm3: (IQR) | 78 (30-169) | 61 (28-150) | 158 (53-221) | 0.03 |
| CD4 <50 cells/mm3: n (% CD4 available) | 20 (34) | 17 (39) | 3 (20) | – |
| CD4 51-200 cells/mm3: n (% CD4 available) | 28 (47) | 22 (50) | 6 (40) | – |
| CD4 >200 cells/mm3: n (% CD4 available) | 11 (19) | 5 (11) | 6 (40) | – |
| Received ART (time dependent): n (% of HIV+) | 28 (24) | 20 (22) | 8 (35) | 0.005 |
| Extrapulmonary XDR-TB: n (%) | 41 (30) | 35 (32) | 6 (21) | 0.64 |
| Previous TB treatment, Any: n (%) | 97 (70) | 82 (74) | 15 (56) | 0.33 |
| Hospitalization within past year: n (%) | 73 (53) | 64 (58) | 9 (32) | 0.02 |
DST: Drug-susceptibility test; H: isoniazid; R: rifampin; E: ethambutol; S: streptomycin; Cx: ciprofloxacin; Km: kanamycin; IQR: interquartile range; ART: antiretroviral therapy
p values determined based on product limit estimates and log rank tests
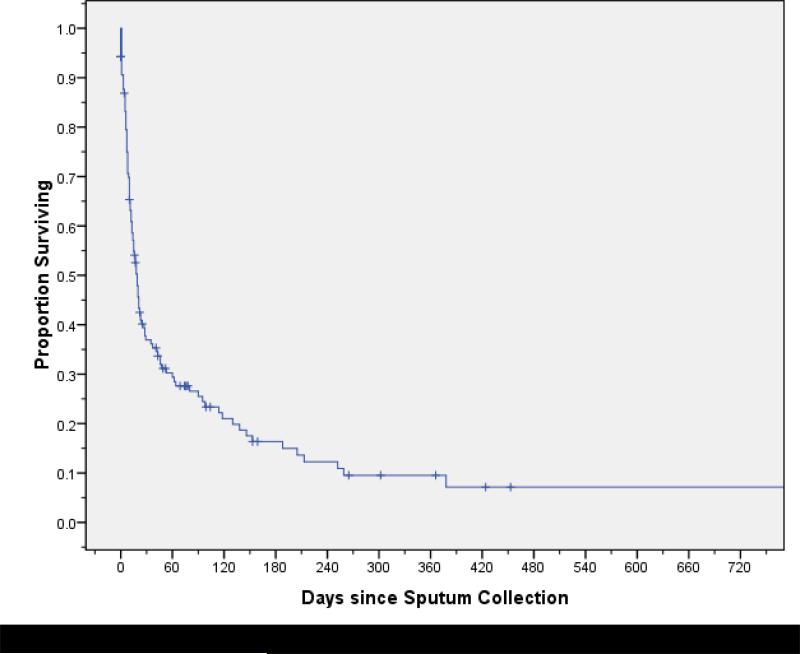
Within two years of diagnosis, 111 (80%) XDR-TB patients died, with a median survival of 19 days (95% CI, 13–23) (Figure 2). In bivariate analysis, female sex (p=0.06), sputum smear positivity (p=0.009), resistance to HRESCxKm (p=0.02), lower baseline CD4 cell count (p=0.03), and hospitalization within one year of TB diagnosis (p=0.02) were associated with mortality (Table 3). Treatment with ART had a protective association (p=0.006).
Figure 2.
Survival among patients with extensively drug-resistant tuberculosis from the time of sputum collection.
In multivariate analysis, receiving ART (HR 0.34, p=0.009) was protective, while CD4 cell count less than 50 cells/mm3 (HR 4.46, p=0.01), and resistance to all six drugs tested (HRESCxKm; HR 2.54, p=0.04) were independently associated with higher mortality (Table 4). CD4 count between 51 and 200 cells/mm3 (HR 2.34, p=0.15) and hospitalization within the past year (HR 1.89, p=0.08) had a borderline association with mortality, although they did not reach statistically significance.
Table 4.
Characteristics Associated with Mortality in Patients with Extensively Drug-resistant Tuberculosis (XDR-TB)
| Unadjusted Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio | p-value | Hazard Ratio | p-value |
| Female sex | 0.70 | 0.06 | 1.12 | 0.74 |
| Positive sputum smear | 1.73 | 0.009 | 0.91 | 0.80 |
| DST: Resistance to HRCxKm | Ref | Ref | Ref | Ref |
| Resistance to HRECxKm or HRSCxKm | 0.69 | 0.14 | 1.39 | 0.57 |
| Resistance to HRESCxKm | 1.56 | 0.02 | 2.54 | 0.04 |
| CD4 cell count: < 50 cells/mm3 | 2.47 | 0.008 | 4.46 | 0.01 |
| 51-200 cells/mm3 | 0.82 | 0.51 | 2.34 | 0.15 |
| >200 cells/mm3 | Ref | Ref | Ref | Ref |
| Received ART (time dependent) | 0.49 | 0.005 | 0.34 | 0.009 |
| Hospitalization within past year | 1.58 | 0.02 | 1.89§ | 0.08§ |
H: isoniazid; R: rifampin; E: ethambutol; S: streptomycin; Cx: ciprofloxacin; Km: kanamycin; ART: antiretroviral therapy.
Bold denotes p-value<0.05
Hospitalization within past year: HR = 2.04, p=0.002: in multivariable model following imputation
Following multiple imputation for missing CD4 counts, the multivariable models were similar to the non-imputed models, although the association between hospitalization and mortality did reach statistical significance (p=0.002, footnote of Table 4).
DISCUSSION
The very high and rapid mortality associated with HIV and drug-resistant TB co-infection has been demonstrated over the past two decades.11,12 In this study, we sought to identify risk factors to identify patients at greatest risk for death. We found that low CD4 cell count and greater degree of drug-resistance were the principal risk factors for mortality in both MDR-TB and XDR-TB patients. Although the remaining risk factors differed, the most notable was a protective effect of ART use among XDR-TB patients, but not those with MDR-TB.
That low baseline CD4 cell count and greater degree of drug-resistance were strongly associated with death in both MDR-TB and XDR-TB suggests that two key factors are necessary for survival: sufficient immune defenses and effective anti-TB therapy. In stepwise fashion, the likelihood of mortality was greater with lower CD4 cell counts. Similarly, the risk of death was greatest with the highest degree of drug resistance, underscoring the notion that fewer active drugs available for treatment are associated with worse survival. The association between CD4 count and mortality has been previously demonstrated in patients with susceptible TB/HIV co-infection,18 while the association between increasing drug resistance and poorer outcomes, including death, is consistent with studies from HIV-negative drug-resistant TB patients.19,20 In settings such as South Africa, the concern is that the impact of low CD4 count and higher drug resistance may be additive in individual patients, resulting in the dramatic mortality seen in both MDR- and XDR-TB with HIV co-infection.12
Our current study provides some hope for treatment of XDR-TB by demonstrating a substantial protective effect of ART. The anti-TB treatment options are severely limited in patients with XDR-TB, and three-quarters of the patients in our study had additional resistance to at least one other drug (ethambutol and/or streptomycin). With such profound drug resistance, it becomes difficult to construct an adequate XDR-TB treatment regimen (i.e., containing the recommended 4-5 active medications).21,22 In these scenarios, the effect of bolstering the immune system with ART may provide an important and independent effect in preventing death. The protective effect of ART in XDR-TB treatment has now been shown in at least three retrospective studies of XDR-TB/HIV co-infected patients, warranting consideration of early ART as an essential component of XDR-TB treatment in HIV co-infected patients.9,23,24
In addition to providing support for ART use in treatment of XDR-TB, the findings in our study add to the growing TB/HIV literature supporting earlier ART treatment to prevent TB disease.25 In our study, the median baseline CD4 cell count was less than 100 cells/mm3 in both the MDR- and XDR-TB groups, but fewer than 25% of patients were receiving ART. Yet, nearly 75% of these patients had been previously treated for TB and half had been hospitalized in the past year. These represent missed opportunities for diagnosing HIV co-infection and initiating ART. Prior studies have shown that TB incidence and all-cause mortality are both higher with low CD4 cell counts and that risk diminishes as the CD4 cell count improves on ART.18,25 Routine or opt-out testing for all patients entering the healthcare system, or at a minimum those treated for TB, may enable HIV diagnosis at higher CD4 cell counts and earlier initiation of ART; these interventions, in turn, may reduce the number of drug-resistant TB cases, as well as the mortality among these cases.
A surprising finding in our study was that receiving ART was not associated with improved survival in HIV-infected MDR-TB patients. Although MDR-TB treatment success rates were better than that for XDR-TB, we would have expected that ART would nonetheless have provided an additive benefit on survival. Although the proportion of patients who received ART was small in both groups, a key difference between MDR-TB and XDR-TB patients was that nearly all of the XDR-TB patients who received ART initiated treatment before their XDR-TB diagnosis; in contrast, 50% of MDR-TB patients who received ART initiated treatment after a median of 68 days of MDR-TB treatment. This delay in ART initiation may have attenuated the impact of ART in the MDR-TB patients, particularly given the small proportion overall who received ART.
As with all retrospective studies, this analysis has several limitations. First, we were limited by the availability, quality and completeness of data in patient records included in this study. This may have introduced biases that were not measured or adjusted for in this analysis. For instance, baseline CD4 cell count was only available in two-thirds of MDR-TB-patients and half of XDR-TB patients. To minimize bias for this key variable, we performed sensitivity analysis using multiple imputation. The major findings of this study did not change following imputation, although it did allow for additional risk factors to achieve statistical significance. Next, while we examined several host factors associated with mortality, other important variables, such as diabetes mellitus, tobacco and alcohol use, were not available. In addition, we were unable to evaluate the impact of second-line TB drug treatment on patient survival, given the centralized drug-resistant TB treatment program's requirement for documented drug resistance before second-line treatment initiation. As a result, all patients in this study received empiric first-line TB treatment at initial presentation; patients were not referred for second-line TB drugs until their DST results were available, typically 6-12 weeks later and after many patients had already died. Thus, the risk factors reported here reflect those which influence survival early in drug-resistant TB disease before patients are initiated on appropriate second-line anti-TB drug treatment. Earlier second-line treatment would likely improve survival in these patients; however, given the nature of the treatment program in our province, we were not able to evaluate it. Finally, we did not evaluate the impact of MDR- or XDR-TB strain genotype or phenotype on mortality. Further animal and molecular studies are needed to better understand the virulence of the LAM4/KZN and other endemic TB strains.
CONCLUSION
The TB/HIV epidemic in South Africa has resulted in exceedingly high morbidity and mortality, now amplified by the growing drug-resistant TB epidemic. The key factors to successfully combating this epidemic include earlier diagnosis of MDR- and XDR-TB, earlier initiation of appropriate second-line TB treatment, and more aggressive HIV testing and ART initiation. There is growing data to support this coordinated response, which will require health systems strengthening and greater collaboration of TB and HIV programs. Indeed, “turning off the spigot”26 begins with not only eliminating circulating MDR- and XDR-TB strains through case detection, infection control, and effective treatment, but also by eliminating the pool of vulnerable hosts through HIV testing and earlier ART. As drug-resistant TB and HIV converge in other parts of Africa and Eastern Europe, immediate, intensive efforts to combat this dual epidemic are critical for averting its further expansion and its associated high mortality.
ACKNOWLEDGEMENTS
We are grateful to the dedicated, courageous, and inspiring staff of the Church of Scotland Hospital, the Umzinyathi district Department of Health, and the KwaZulu-Natal province TB control program. We thank the Inkosi Albert Luthuli Central Hospital / National Health and Laboratory Services for performing all TB culture and drug-susceptibility testing for patients from Tugela Ferry. This work would not have been possible without the medical students, residents, research assistants, and data capturers who contributed to data collection and data cleaning. NRG and NSS are both recipients of the Doris Duke Charitable Foundation Clinical Scientist Development Award (NRG 2007070, NSS 2007071). GF is also supported by the Doris Duke Charitable Foundation (2007018), the Gilead Foundation, and The Irene Diamond Fund (R05130). Statistical support was provided by the Center for AIDS Research (CFAR) of Albert Einstein College of Medicine / Montefiore Medical Center. We also thank Mr. Xuan Li for his helpful comments during the analysis of these data. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.World Health Organization Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva. 2010 [Google Scholar]
- 2.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 3.Chirenda J, Menzies H, Moalosi G, et al. The trend of resistance to anti-tuberculosis drugs in Botswana: results from the 4th national anti-tuberculosis drug resistance survey.. Programs and Abstracts from the 40th Union World Conference on Lung Health; Cancun, Mexico. Dec 3-7, 2009.2009. [Google Scholar]
- 4.Buthelezi SSS. Situational Analysis of TB Drug Resistance in KwaZulu-Natal Province: Republic of South Africa.. 2nd Meeting of the Global XDR TB Task Force; Geneva, Switzerland. April 9, 2008. [Google Scholar]
- 5.Ben Amor Y, Nemser B, Singh A, Sankin A, Schluger N. Underreported threat of multidrug-resistant tuberculosis in Africa. Emerg Infect Dis. 2008;14:1345–52. doi: 10.3201/eid1409.061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 7.Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J. 2009;33:871–81. doi: 10.1183/09031936.00168008. [DOI] [PubMed] [Google Scholar]
- 8.Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000-2003. Int J Tuberc Lung Dis. 2010;14:413–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 375:1798–807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13:855–61. [PMC free article] [PubMed] [Google Scholar]
- 11.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 13.Cox HS, McDermid C, Azevedo V, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS One. 5:e13901. doi: 10.1371/journal.pone.0013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8:10. doi: 10.1186/1471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance . Anti-tuberculosis drug resistance in the world: third global report. World Health Organization; Geneva: 2004. [Google Scholar]
- 16.Shah NS, Wright A, Bai GH, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin D. Multiple imputation for Nonresponse in Surveys. Wiley and Sons.; Hoboken, NJ: 1987. [Google Scholar]
- 18.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. Aids. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtz TH, Sternberg M, Kammerer S, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Annals of internal medicine. 2006;144:650–9. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 20.Cox HS, Kalon S, Allamuratova S, et al. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: treatment complexity and XDR-TB among treatment failures. PLoS One. 2007;2:e1126. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NS, Richardson JR, Moodley P, et al. Second-line Drug Resistance Among Extensively Drug-Resistant Tuberculosis Patients in Rural South Africa. Emerg Infect Dis. 2011;17:510–13. doi: 10.3201/eid1703.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mphahlele M, Syre H, Valvatne H, et al. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol. 2008;46:3459–64. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell M, Padayatchi N, Grobler A, Master I, Horsburgh R. Long Term Treatment Outcomes of Patients with Extensively Drug Resistant Tuberculosis (XDR-TB) and HIV.. Programs and Abstracts from the 17th Conferences on Retroviruses and Opportunistic Infections; San Francisco, CA. Feb16-19, 2010; 2010. Abstract #787. [Google Scholar]
- 24.Kvasnovsky CL, Cegielski JP, Erasmus R, Siwisa NO, Thomas K, der Walt ML. Extensively Drug-Resistant TB in Eastern Cape, South Africa: High Mortality in HIV-Negative and HIV-Positive Patients. J Acquir Immune Defic Syndr. 2011;57:146–52. doi: 10.1097/QAI.0b013e31821190a3. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. Aids. 2009;23:1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nardell E, Dharmadhikari A. Turning off the spigot: reducing drug-resistant tuberculosis transmission in resource-limited settings. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14:1233–43. [PMC free article] [PubMed] [Google Scholar]