To the Editor:
In a recent issue of The Journal of Experimental Medicine, Griffin and Rothstein described two subsets within the human B1 cell compartment: a major CD11b− subset (9/10th of the population) and a minor CD11b+ subset (1/10th of the population), with the latter being increased in lupus (Griffin and Rothstein, 2011).
Griffin and Rothstein (2011) analyzed these two B1 cell subsets using gene profiling and compared these gene profiles with those of naive and memory blood B cells. A heat map is shown in Fig. 2 of their paper. This figure highlights a distinct expression profile for each of the CD11b+ and CD11b− subsets compared with naive and memory B cells.
Using the data deposited in the National Center for Biotechnology Information GEO database (GSE29717), we performed the same comparison and found that the gene profile of each of these subsets argues in favor of a different conclusion.
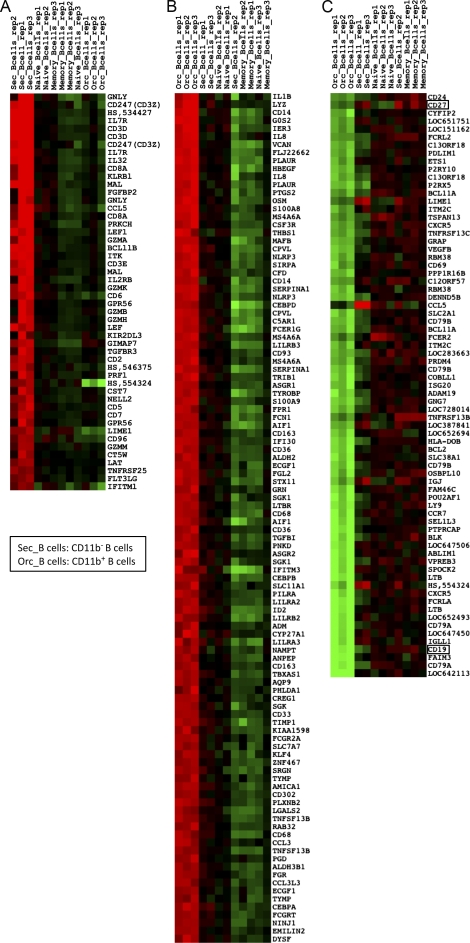
For CD11b− B1 cells, the 50 most discriminating genes (comparing this subset to CD11b+ B1 cells, naive cells, and memory B cells) include those encoding CD8α; CD3ζ; CD3δ; CD2; CD5; CD7; CD96; LAT; MAL; granzymes A, B, H, and K; and granulolysin (Fig. 1 A). This profile resembles that of cytotoxic CD8+ T cells. This subset also expresses CD19, CD22, and other B cell markers, which does not discriminate them from naive and memory B cells. Thus CD11b− B1 cells most likely consist of CD8+ T cell–B cell doublets.
Figure 1.
Gene expression profiles of human CD11b+ and CD11b− B1 cell subsets. (A) Top 50 overexpressed genes discriminating CD11b− B1 cells from CD11b+, naïve, and memory B cells. Sec_B cells, CD11b− B1 cells. Orc_B cells, CD11b+ B1 cells. (B) Top 106 overexpressed genes discriminating CD11b+ B1 cells from CD11b−, naïve, and memory B cells. (C) Top 73 underexpressed genes discriminating CD11b+ B1 cells from CD11b−, naïve, and memory B cells. The total number of genes displayed, which represent an unbiased list of genes according to their ranking order in the comparison, was selected to include most of the discriminating genes selected by Griffin and Rothstein in the heat map in Fig. 2 of their paper. A classical red/green heat map display is used for overexpressed/under-expressed genes.
For CD11b+ B1 cells, overexpressed genes include those encoding SIRPα, CD33, CD68, CD163, CD302, IL-1β, APRIL (TNFSF13), and CD14 (Fig. 1 B). These genes correspond to a myeloid profile. Underexpressed genes include those encoding CD79A and CD79B, and other B cell markers, including TACI, BAFF-R (TNFRSF13b and TNFRSF13c), BLK, CD19, and CD27 (Fig. 1 C). The latter two markers were among the criteria used by Griffin and Rothstein to isolate these cells. This subset thus represents cells of the monocyte lineage that were sorted by unspecific (possibly Fc-mediated) staining as CD19+CD27+ cells. The CD11b and CD14 phenotype is thus not surprising.
There probably exists a human B cell subset with a spontaneous capacity to secrete IgM, as reported in the two papers by Griffin and colleagues on human B1 cells (Griffin and Rothstein, 2011; Griffin et al., 2011). However, the characteristics described by these authors thus far pertain to cells that appear to represent two different types of cell sorting artifacts.
Acknowledgments
We thank Nicolas Cagnard (Bioinformatics platform, Paris Descartes) for performing the microarray analysis.
References
- Griffin D.O., Rothstein T.L.. 2011. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 208:2591–2598. 10.1084/jem.20110978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.O., Holodick N.E., Rothstein T.L.. 2011. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70-. J. Exp. Med. 208:67–80. 10.1084/jem.20101499 [DOI] [PMC free article] [PubMed] [Google Scholar]